Abstract
Objective
In the 24-week, phase 3 LIBERTY 1 (L1) and LIBERTY 2 (L2) trials, relugolix combination therapy (relugolix-CT (relugolix 40 mg, estradiol 1 mg, norethisterone acetate 0.5 mg)) reduced uterine fibroid (UF)-associated symptoms. This post hoc analysis assessed safety and efficacy of relugolix-CT in European women from L1/L2.
Methods
Premenopausal women (aged 18–50 years) with UF-associated heavy menstrual bleeding (HMB) were randomized 1:1:1 in L1 (N = 388) and L2 (N = 382) to relugolix-CT or placebo for 24 weeks, or delayed relugolix-CT (relugolix 40 mg then relugolix-CT; 12 weeks each). Primary endpoint: proportion of responders (menstrual blood loss (MBL) <80 mL and reduction of ≥50% from baseline MBL volume) over the last 35 days of treatment. Secondary endpoints: MBL volume, amenorrhea, UF-associated pain, symptom severity, distress related to bleeding and pelvic discomfort, health-related quality of life (HRQoL). Safety endpoints included adverse event (AE) reporting and bone mineral density (BMD) assessment.
Results
In European women from L1/L2 (N = 124, 16%), a significantly greater proportion of treatment responders was observed with relugolix-CT vs. placebo (85.4% vs. 19.1%, respectively; nominal p < .0001). There were statistically significant improvements with relugolix-CT vs. placebo for several secondary endpoints: reduction in MBL volume, amenorrhea rate, proportion achieving mild-to-no pain, reduction in symptom severity and distress from bleeding and pelvic discomfort, and improvement in HRQoL. Incidence of AEs and percentage changes in BMD from baseline to week 24 were similar for relugolix-CT and placebo.
Conclusions
In European women with UF and HMB, once-daily relugolix-CT vs. placebo improved UF-associated symptoms and preserved BMD.
Introduction
Uterine fibroids (UFs), or leiomyomas, are common benign solid tumors with an estimated cumulative incidence of approximately 80% in black women and 70% in white women by 50 years of age [Citation1,Citation2]. Of women with UF, approximately 20–50% are symptomatic and may experience heavy menstrual bleeding (HMB), pain and reproductive issues that can impact quality of life (QoL) [Citation3,Citation4].
UF management includes surgical treatment, minimally invasive procedures, and medical treatment. Many women in Europe undergo surgery, typically myomectomies and hysterectomies [Citation5,Citation6]. However, surgery may be unsuitable for women wanting to preserve fertility or avoid ‘radical’ surgery [Citation7]. Gonadotropin-releasing hormone (GnRH) receptor agonists are approved for pre-surgical management of UF [Citation8], but are limited to short-term use due to hypoestrogenic effects, including bone mineral density (BMD) loss, hot flushes, and vaginal dryness [Citation9,Citation10]. Other interventions like oral contraceptives, progestin-containing intrauterine devices, and non-steroidal anti-inflammatory drugs have low-level evidence and are not specifically indicated for UF [Citation11].
Oral GnRH receptor antagonists combined with low-dose hormone therapy are a potential long-term UF treatment [Citation10]. GnRH antagonists reversibly block GnRH receptors, suppressing release of follicle-stimulating and luteinizing hormones from the anterior pituitary, leading to rapid and reversible reductions in estrogen and progesterone levels [Citation10,Citation12,Citation13]. Relugolix combination therapy (relugolix-CT (relugolix 40 mg, estradiol 1 mg, and norethisterone acetate 0.5 mg)) is a GnRH receptor antagonist designed to manage UF-associated symptoms by keeping estradiol levels similar to those in the menstrual cycle’s early follicular phase, and to minimize hypoestrogenic effects [Citation14]. It is approved in Europe and several other jurisdictions for managing moderate-to-severe symptoms of UF in adult women of reproductive age [Citation15], and in the USA for managing UF-associated HMB in premenopausal women and managing moderate-to-severe pain associated with endometriosis [Citation16].
In the phase 3 LIBERTY 1 (L1) and LIBERTY 2 (L2) trials, relugolix-CT led to improvements in UF-associated symptoms vs. placebo including reduction of menstrual blood loss (MBL) volume; additionally, BMD was generally preserved through 24 weeks [Citation14].
Because the L1/L2 studies were conducted in multiple countries, a broad range of regions, race, and ethnic backgrounds are represented. In these studies, most women were from North America (USA; ∼75%) and were primarily either Black or African American (∼50%) or White (∼45%), with other races accounting for approximately 5% of enrolled women [Citation14]. Considering that a small group of women (16%) were European, it was deemed reasonable to evaluate whether outcomes observed in the overall study population are applicable for European women enrolled. This post hoc analysis assessed the efficacy and safety of relugolix-CT through 24 weeks in the subpopulation of European women from L1/L2 and aimed to place findings in the context of outcomes in the rest of world (RoW; non-European) population and overall study population.
Materials and methods
Study design
Details of L1 (ClinicalTrials.gov registration: February 2017, NCT03049735) and L2 (ClinicalTrials.gov registration: April 2017, NCT03103087) have been previously published [Citation14]. Briefly, premenopausal women (aged 18–50 years) with ultrasound-confirmed UF and HMB (assessed by alkaline hematin method) were randomized 1:1:1 to receive once-daily relugolix-CT or placebo for 24 weeks, or delayed relugolix-CT (40 mg of relugolix monotherapy followed by relugolix-CT; 12 weeks each). HMB was defined as a MBL volume of ≥80 mL per cycle for two cycles, or a MBL volume of ≥160 mL during one cycle. Exclusion criteria included a Z-score of less than −2.0 for BMD [Citation14]. The delayed relugolix-CT regimen was included to evaluate the role of estradiol and norethisterone acetate in minimizing the risk of BMD loss and vasomotor symptoms associated with a hypoestrogenic state, and risk of endometrial hyperplasia associated with unopposed estrogen, respectively.
L1/L2 were Institutional Review Board-approved studies conducted in accordance with the Declaration of Helsinki, the International Council on Harmonisation guidelines, and the laws and regulations of the research countries. Participants provided written informed consent.
Efficacy and safety assessments
Primary endpoint: proportion of treatment responders at week 24 or the end of the treatment period (EOT; last 35 days of the treatment period). Treatment response: MBL volume <80 mL and reduction of ≥50% from baseline MBL volume over the last 35 days of treatment [Citation14].
Secondary endpoints: percentage reduction in MBL volume from baseline to week 24; proportion achieving amenorrhea; reduction in distress related to bleeding, passing of blood clots, and tightness or pressure in the pelvic area, measured by the Uterine Fibroid Symptom and Health-related QoL (UFS-QoL) Bleeding and Pelvic Discomfort (BPD) Scale score [Citation17]; change from baseline in QoL for symptom severity (SS) scale score; proportion with minimal-to-no fibroid-associated pain (maximum Numerical Rating Scale (NRS) score ≤1) at week 24 in the pain-evaluable population (i.e. women with moderate-to-severe pain (maximum NRS score ≥4) at baseline (on a scale where 0 reflects no pain and 10 reflects worst imaginable pain) and with at least 80% compliance to electronic diary over the last 35 days of treatment); health-related QoL (HRQoL) scale score change from baseline; and percentage change in uterine volume and volume of the largest measurable fibroid (assessed by transvaginal ultrasonography).
Safety assessments: adverse events (AEs) and percentage change in BMD (at the lumbar spine (L1–L4), total hip, and femoral neck) from baseline to week 24, assessed by dual-energy X-ray absorptiometry. Trial visits occurred at baseline and every 4 weeks up to 24 weeks.
Statistical analyses
This analysis used pooled L1/L2 data and evaluated women from Europe and RoW (North America, South America, and South Africa); statistical analyses and results for the overall population have been published elsewhere [Citation14]. The primary endpoint was analyzed using a Cochran–Mantel–Haenszel test for proportions stratified by baseline MBL volume. For repeated measures (e.g. MBL volume), a mixed model with autoregression covariance structure was used to estimate least squares mean percentage change. Treatment, visit, and treatment and visit interaction were included as fixed effects. Region and baseline MBL volume (stratification variable in the original L1/L2 trials) were not included in the regression model due to the small number of participants. Treatment comparisons were performed without multiplicity adjustment. All p values are considered nominal.
Results
Participants
In total, 124/770 (16.1%) women randomized in L1/L2 were European (relugolix-CT, N = 41; delayed relugolix-CT, N = 41; placebo, N = 42), participating from Belgium, Czech Republic, Hungary, Italy, Poland, or the United Kingdom. Of these, 106 (85.5%) completed treatment; demographics and baseline characteristics were similar across treatment groups (). When compared with the RoW population (Supplementary Table I), European women were leaner, included a lower proportion of women who identified as Black or African American, and had smaller baseline uterine and index UF volumes. Furthermore, at baseline, fewer European women had moderate-to-severe fibroid-associated pain, and had lower UFS-QoL symptom severity scores and higher UFS-QoL HRQoL total scores when compared with the RoW population (Supplementary Table I).
Table 1. Demographics and baseline characteristics of European women at baseline.
Efficacy endpoints
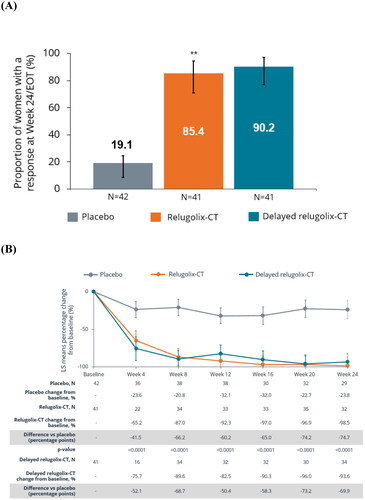
For the primary endpoint at week 24/EOT, a statistically significantly greater proportion of treatment responders was observed with relugolix-CT (35/41 (85.4%)) vs. placebo (8/42 (19.1%); nominal p < .0001; ); 90.2% of women in the delayed relugolix-CT group were responders (). Although the sample size was small, a trend toward higher responder rates was observed in European women compared with the RoW population (Supplementary Figure 1).
Figure 1. (A) Proportion of European women with reduction in MBL (treatment responders)a at week 24 and (B) percentage change in MBL volume in European women from baseline to week 24/EOT. **Nominal p < .0001 vs. placebo. Error bars show 95% confidence intervals. (A) p Values assessed using the Cochran–Mantel–Haenszel test for proportions stratified by baseline MBL volume. aTreatment responders were defined as women who achieved a MBL <80 mL and a ≥50% reduction from baseline in MBL volume over the last 35 days of treatment. Nominal p value based on pooled data not adjusted for multiplicity. MBL volume is calculated as sum of menstrual blood loss volume from all feminine products assessed by the alkaline hematin method at week 24/end of treatment visit. (B) LS means and 95% confidence intervals for MBL volume are from mixed model with visit, treatment and an interaction term of visit and treatment as fixed effect. The multiple visits for each patient were the repeated measures as random effect within each patient and an autoregressive covariance. Error bars show 95% confidence intervals. The lower limit of the 95% confidence interval was truncated to –100 in instances where values fell below –100%. CT: combination therapy; EOT: end of treatment; LS: least squares; MBL: menstrual blood loss.
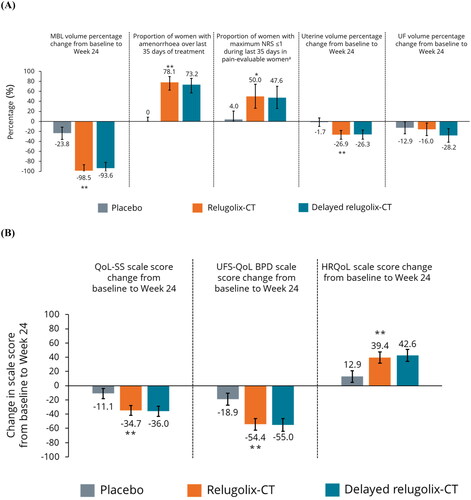
Relugolix-CT was statistically significantly more effective than placebo for several secondary efficacy endpoints, including mean reduction of MBL volume from baseline to week 24 (98.5% vs. 23.8%; nominal p < .0001; ), evident from week 4 of treatment (). A higher proportion of women receiving relugolix-CT (vs. placebo) reported achievement of amenorrhea (78.1% vs. 0%), and a higher proportion reported minimal-to-no pain over the last 35 days of treatment (50.0% vs. 4.0%) in the pain-evaluable population (; all nominal p < .001). Women receiving relugolix-CT reported statistically significant improvements in distress due to UF-associated symptoms and QoL, vs. placebo: SS scale score (–34.7 vs. −11.1), UFS-QoL BPD scale score (–54.4 vs. −18.9) and total HRQoL scale score (+39.4 vs. +12.9; all nominal p < .0001; ). There was a significant percentage reduction from baseline in uterine volume with relugolix-CT, vs. placebo (–26.9% vs. −1.7%; nominal p < .0001; ). Percentage change from baseline in UF volume for relugolix-CT vs. placebo was non-significant (–16.0% vs. −12.9%; nominal p = .72). Response to relugolix-CT for secondary endpoints in European women was comparable to the RoW population (Supplementary Figure 2).
Figure 2. Secondary endpoints for efficacy (A) and QoL (B) in European women. *Nominal p < .001 vs. placebo; **nominal p < .0001 vs. placebo. Error bars show 95% confidence intervals. p Values for categorical outcomes are from the Cochran–Mantel–Haenszel test stratified by MBL volume. p Values (and LS means and 95% confidence intervals) for MBL are from mixed model with visit, treatment and an interaction term of visit and treatment as fixed effect. The multiple visits for each patient were the repeated measures as random effect within each patient and an autoregressive covariance. p Values (and LS means and 95% confidence intervals) for uterine volume change and UF volume change from baseline to week 24 are from generalized linear model with treatment and baseline value of uterine volume or UF volume as covariates. p Values (and LS means and 95% confidence intervals) for QoL-SS, UFS-QoL, and HRQoL are based on a mixed-effect model with treatment, visit, baseline MBL volume and treatment by visit interaction included as fixed effects. The multiple visits for each patient were the repeated measures as random effect within each patient and an unstructured covariance. The lower limit of the 95% confidence interval was truncated to –100 in instances where values fell below –100%. aA smaller number of women were included in the pain-evaluable population for each treatment arm: placebo, N = 25; relugolix-CT, N = 18; delayed relugolix-CT, N = 21. BPD: Bleeding and Pelvic Discomfort; CT: combination therapy; HRQoL: health-related quality of life; LS: least squares; MBL: menstrual blood loss; NRS: Numerical Rating Scale; QoL: quality of life; SS: symptom severity; UF: uterine fibroids; UFS-QoL: uterine fibroid symptom and quality of life.
Anemia was not assessed because few European women from L1/L2 had anemia (N = 17 (13.7%)); however, a significant improvement in hemoglobin levels was observed with relugolix-CT (vs. placebo) in the RoW population (proportion of women with hemoglobin ≤10.5 g/dL at baseline who achieved an increase in hemoglobin levels of >2 g/dL at week 24: 30/55 (54.6%) vs. 6/56 (10.7%), respectively; nominal: p < .0001).
Safety
The overall incidence of AEs was 70.7% with relugolix-CT, 80.5% with delayed relugolix-CT, and 61.9% with placebo. Women discontinuing treatment due to AEs: one (2.4%) for relugolix-CT, four (9.8%) for delayed relugolix-CT, and four (9.5%) for placebo. Incidence of serious AEs was <5% across treatment groups. No fatal AEs were reported. The most frequently reported AE was hot flush (12% with relugolix-CT, 34% with delayed relugolix-CT, and 7% with placebo; Supplementary Table II). These findings were consistent with what was observed in the RoW study population (Supplementary Table III).
Change from baseline to week 24 in lumbar spine BMD was −1.1% with relugolix-CT vs. −0.5% with placebo (Supplementary Figure 3). BMD measured at the total hip remained stable with relugolix-CT (0.5%). There were no clinically meaningful differences for change in BMD from baseline with relugolix-CT vs. placebo. Decreased BMD from baseline to week 24 was observed in women receiving delayed relugolix-CT (–3.5%), which included 12 weeks of treatment with relugolix monotherapy. BMD changes in European women were similar to those observed in the RoW population (Supplementary Figure 4). No cases of endometrial hyperplasia or endometrial cancer were observed in patients treated with relugolix-CT or delayed relugolix-CT; two women in the placebo group in L1 had endometrial findings of hyperplasia at week 24.
Discussion
The pathophysiology of UF has shown that mutations in MED12, part of a mediator complex that interacts with RNA polymerase II and impacts messenger RNA transcription, are present in approximately 70% of UF and are prevalent regardless of race/ethnicity and geography [Citation8,Citation18–22]. Because the cumulative incidence of UF by age 50 is approximately 70% and 80% among White and Black women, respectively [Citation2,Citation23], investigation into potential molecular mechanisms to explain the increased prevalence of UF in African American women in the USA compared with other racial backgrounds has not demonstrated a unifying hypothesis [Citation24]. Because the effectiveness of relugolix-CT at similar exposures was expected to be similar regardless of region, the LIBERTY pivotal studies were conducted globally (in North America, South America, Europe, and Africa). To address applicability of the overall study results to European women, several analyses were conducted, including a summary of demographic and other baseline characteristics, subgroup analyses for efficacy, and subgroup analyses for safety. Despite some differences in demographics between the European and RoW populations, particularly regarding the proportion of Black or African American women, BMI, and uterine and UF volume, there was not a regional difference in effect on efficacy of relugolix-CT for primary and secondary endpoints. Of note, these demographics and baseline clinical characteristics of European women were similar to European women in other UF trials with regards to age, BMI, race, and UF symptom severity (i.e. PEARL I–IV trials with ulipristal acetate) [Citation25–28]. For European women, responder rate for reduction in HMB with relugolix-CT was statistically significantly higher than placebo, with a trend toward a higher responder rate in European women compared with the RoW population, although the sample size of European women was small. Treatment effect on secondary endpoints was consistent with European, RoW, and overall populations, favoring relugolix-CT over placebo [Citation14].
Safety findings in European women were comparable to the RoW and overall study populations [Citation14], with no specific safety signals observed and a similar trend toward a higher proportion of women reporting hot flush and more BMD loss in the delayed relugolix-CT group. Minimal changes in BMD were observed with relugolix-CT at the lumbar spine in both European women and the RoW population, with no decline in BMD at the total hip. Interestingly, European women who received placebo experienced a minor BMD decline at week 24; as such, BMD changes between relugolix-CT and placebo groups were comparable in the European and the RoW populations. Decreases in BMD may indicate adaptation to a new estradiol steady-state, a consequence of equilibration from endogenous to exogenous estradiol in the context of GnRH antagonism. In the delayed relugolix-CT group, BMD loss at week 24 was likely due to hypoestrogenic effects following treatment with 12 weeks of relugolix monotherapy; reduction in BMD was consistent with results from relugolix monotherapy studies [Citation29,Citation30].
Unmet needs remain with existing medical interventions for UF [Citation31]. In Europe, ulipristal acetate is approved for intermittent treatment of UF in premenopausal women for whom surgery is unsuitable or has failed; however, hepatotoxicity issues and label changes in Europe limit its use [Citation12,Citation32]. Oral GnRH receptor antagonists in combination with estrogen/progestins may help address needs for long-term UF treatments. These include elagolix with estradiol and norethisterone acetate, which is approved in the USA [Citation33]; linzagolix choline, investigated with/without estradiol and norethisterone acetate [Citation34,Citation35], and approved in Europe for moderate-to-severe symptoms of UF [Citation36]; and relugolix-CT, which is approved in Europe, the USA, and several other jurisdictions [Citation14–16]. Relugolix-CT is the first one-pill, once-daily UF treatment [Citation15], and may provide an efficacious UF management option with preserved BMD. Long-term assessments of relugolix-CT through 52 weeks in the overall study population have been previously published that show maintenance of treatment effect [Citation37]; assessment of relugolix-CT through 104 weeks has been completed and will be described separately.
This analysis is limited by the relatively small number of women included (N = 124). Additionally, this was a subgroup post hoc analysis in which participants were not randomized within regions at baseline, and the analysis was not powered or adjusted for multiplicity.
In conclusion, clinical outcomes for European women in L1 and L2 demonstrated reduction of HMB and pain reduction, and overall safety that was consistent with RoW and the overall population. The benefit/risk profile in European women is consistent with that of the overall population, and the overall clinical data from L1 and L2 are applicable for European women.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (548.6 KB)Acknowledgements
The authors thank all patients who participated in the LIBERTY 1 and LIBERTY 2 studies, as well as the trial investigators and site staff who made these studies possible. The authors acknowledge the editorial support provided by AXON Communications (London, UK), funded by Myovant Sciences GmbH.
Disclosure statement
RV has received payment or honoraria for lectures, presentations, speakers bureaus, or educational events from Myovant Sciences, and is the owner of patents (unrelated to the topic of the present analysis). TR has received investigator fees, travel grants, payment or honoraria for lectures and support for attending meetings from Gedeon Richter, and has received speaker fees from Gedeon Richter and Astellas. JZ has no conflicts of interest to report. RBW and EZ are employees of Sumitomo Pharma America, Inc. VGR is a shareholder and employee of Myovant Sciences GmbH. FP served as the President of The Society of Endometriosis and Uterine Disorders (SEUD; 2018–2021) and has received support for attending the SEUD Congress Stockholm (2021).
Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
Additional information
Funding
References
- Laughlin-Tommaso SK, Stewart EA. Moving toward individualized medicine for uterine leiomyomas. Obstet Gynecol. 2018;132(4):1–7. doi: 10.1097/AOG.0000000000002785.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. doi: 10.1067/mob.2003.99.
- Marsh EE, Al-Hendy A, Kappus D, et al. Burden, prevalence, and treatment of uterine fibroids: a survey of U.S. women. J Womens Health. 2018;27(11):1359–1367. doi: 10.1089/jwh.2018.7076.
- Vilos GA, Allaire C, Laberge PY, et al. The management of uterine leiomyomas. J Obstet Gynaecol Can. 2015;37(2):157–178. doi: 10.1016/S1701-2163(15)30338-8.
- Chiumente M, De Rosa M, Messori A, et al. Burden of uterine fibroids in Italy: epidemiology, treatment outcomes, and consumption of health care resources in more than 5,000 women. Clinicoecon Outcomes Res. 2017;9:525–535. doi: 10.2147/CEOR.S139335.
- Zimmermann A, Bernuit D, Gerlinger C, et al. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. 2012;12:6. doi: 10.1186/1472-6874-12-6.
- Williams ARW. Uterine fibroids – what’s new? F1000Res. 2017;6:2109. doi: 10.12688/f1000research.12172.1.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35(6):473–480. doi: 10.1055/s-0037-1607264.
- Agency for Healthcare Research and Quality. Management of uterine fibroids. Comparative effectiveness review, no. 195. Rockville (MD): Agency for Healthcare Research and Quality (US) [Internet]; 2017 [cited 2021 Nov]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537742/
- Shon J, Zou P, Tran D, et al. Clinical pharmacology at the intersection of women’s health and regulation: drug development considerations for uterine fibroids. J Clin Pharmacol. 2020;60(Suppl. 2):S39–S48. doi: 10.1002/jcph.1736.
- Lewis TD, Malik M, Britten J, et al. A comprehensive review of the pharmacologic management of uterine leiomyoma. Biomed Res Int. 2018;2018:2414609. doi: 10.1155/2018/2414609.
- Donnez J, Dolmans MM. Fibroids and medical therapy: bridging the gap from selective progesterone receptor modulators to gonadotropin-releasing hormone antagonist. Fertil Steril. 2020;114(4):739–741. doi: 10.1016/j.fertnstert.2020.07.028.
- Nakata D, Masaki T, Tanaka A, et al. Suppression of the hypothalamic-pituitary-gonadal axis by TAK-385 (relugolix), a novel, investigational, orally active, small molecule gonadotropin-releasing hormone (GnRH) antagonist: studies in human GnRH receptor knock-in mice. Eur J Pharmacol. 2014;723:167–174. doi: 10.1016/j.ejphar.2013.12.001.
- Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384(7):630–642. doi: 10.1056/NEJMoa2008283.
- European Medicines Agency. Ryeqo (40 mg relugolix, 1 mg estradiol (as hemihydrate), and 0.5 mg norethisterone acetate). Summary of product characteristics; 2023 [cited 2023 Jul]. Available from: https://www.ema.europa.eu/en/documents/product-information/ryeqo-epar-product-information_en.pdf
- US Food and Drug Administration. Myfembree (relugolix, estradiol, norethindrone acetate) prescribing information; 2023 [updated 2023 Jul; cited Sep 2022]. Available from: https://www.myfembree.com/static/myfembree-prescribing-information.pdf
- Li J, Kang J, Hunsche E, et al. PIH57 the Bleeding and Pelvic Discomfort Scale: measuring patient-reported symptoms in uterine fibroids. Value Health. 2019;22:S637–S638. doi: 10.1016/j.jval.2019.09.1237.
- Mäkinen N, Mehine M, Tolvanen J, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334(6053):252–255. doi: 10.1126/science.1208930.
- Matsubara A, Sekine S, Yoshida M, et al. Prevalence of MED12 mutations in uterine and extrauterine smooth muscle tumours. Histopathology. 2013;62(4):657–661. doi: 10.1111/his.12039.
- Halder SK, Laknaur A, Miller J, et al. Novel MED12 gene somatic mutations in women from the Southern United States with symptomatic uterine fibroids. Mol Genet Genomics. 2015;290(2):505–511. doi: 10.1007/s00438-014-0938-x.
- Wang L, Zeng H, Wang Q, et al. MED12 methylation by CARM1 sensitizes human breast cancer cells to chemotherapy drugs. Sci Adv. 2015;1(9):e1500463. doi: 10.1126/sciadv.1500463.
- Baranov VS, Osinovskaya NS, Yarmolinskaya MI. Pathogenomics of uterine fibroids development. Int J Mol Sci. 2019;20(24):6151. doi: 10.3390/ijms20246151.
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59(1):2–24. doi: 10.1097/GRF.0000000000000164.
- Catherino WH, Eltoukhi HM, Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med. 2013;31(5):370–379. doi: 10.1055/s-0033-1348896.
- Donnez J, Donnez O, Matule D, et al. Long-term medical management of uterine fibroids with ulipristal acetate. Fertil Steril. 2016;105(1):165–173.e4. doi: 10.1016/j.fertnstert.2015.09.032.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409–420. doi: 10.1056/NEJMoa1103182.
- Donnez J, Tomaszewski J, Vázquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366(5):421–432. doi: 10.1056/NEJMoa1103180.
- Donnez J, Vázquez F, Tomaszewski J, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril. 2014;101(6):1565–1573. doi: 10.1016/j.fertnstert.2014.02.008.
- Osuga Y, Enya K, Kudou K, et al. Oral gonadotropin-releasing hormone antagonist relugolix compared with leuprorelin injections for uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2019;133(3):423–433. doi: 10.1097/AOG.0000000000003141.
- Hoshiai H, Seki Y, Kusumoto T, et al. Relugolix for oral treatment of uterine leiomyomas: a dose-finding, randomized, controlled trial. BMC Womens Health. 2021;21(1):375. doi: 10.1186/s12905-021-01475-2.
- Piecak K, Milart P, Woźniakowska E, et al. Ulipristal acetate as a treatment option for uterine fibroids. Prz Menopauzalny. 2017;16(4):133–136.
- European Medicines Agency. Ulipristal acetate (Esmya). Summary of product characteristics; 2023 [updated 2023 Jul; cited Aug 2021]. Available from: https://www.ema.europa.eu/en/documents/product-information/esmya-epar-product-information_en.pdf
- US Food and Drug Administration. Oriahnn (elagolix, estradiol, and norethindrone acetate capsules; elagolix capsules) prescribing information; 2023 [updated 2023 Jul; cited 2021 Oct]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213388s000lbl.pdf
- Pohl O, Marchand L, Fawkes N, et al. Gonadotropin-releasing hormone receptor antagonist mono- and combination therapy with estradiol/norethindrone acetate add-back: pharmacodynamics and safety of OBE2109. J Clin Endocrinol Metab. 2018;103(2):497–504. doi: 10.1210/jc.2017-01875.
- Stewart EA, Taylor HS, Taylor RN, et al. Efficacy and safety of linzagolix (lgx) for the treatment of heavy menstrual bleeding (HMB) due to uterine fibroids (UF): results from two phase 3 randomized clinical trials. Fertil Steril. 2020;114(3):e527. doi: 10.1016/j.fertnstert.2020.09.016.
- European Medicines Agency. Linzagolix choline (Yselty). Summary of product characteristics; 2022 [cited 2023 Jul]. Available from: https://www.ema.europa.eu/en/documents/product-information/yselty-epar-product-information_en.pdf
- Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140(6):920–930. doi: 10.1097/AOG.0000000000004988.
