Abstract
Objective
Neonatal outcomes in women with and without medically managed gestational diabetes mellitus (GDM) were compared after accounting for differences in maternal baseline characteristics using a propensity score (PS) analysis.
Methods
Women without preexisting diabetes, delivering singletons during 2010-2017 in a large hospital, were eligible for inclusion. Using nearest-neighbour PS matching, women with non-pharmacological managed GDM were matched with women whose GDM was medically managed. A conditional logistic regression consequently compared the neonatal adverse outcomes between the groups after adjusting for gestational age, induction of labor, birth type, and number of ultrasounds conducted during the pregnancy.
Results
Of the overall 10028 births, GDM was diagnosed in 930 (9.3%), of whom 710 (76.3%) were successfully matched. The conditional regressions found higher risk of neonatal adverse outcomes in neonates of women with non-pharmacological managed GDM compared to neonates of women with medically managed GDM. These included a higher risk of hypoglycemia (odds ratio (OR) 1.56, 95% confidence interval (CI) 1.03–2.38, p = 0.037), hypothermia (OR 2.29, 95%CI 1.05-5.00, p = 0.037), and birth injuries (OR 3.50, 95%CI 1.62–7.58, p = 0.001), and a higher risk of being small for gestational age (OR 2.06, 95%CI 1.01-4.18, p = 0.046) and being admitted to a special care unit (OR 2.04, 95%CI 1.29-3.21, p = 0.002).
Conclusions
The increased neonatal morbidity associated with non-medicated GDM identified in our study may indicate that diet and lifestyle changes alone are not sufficient to achieve glycaemic control in some women with GDM. Our findings indicate that gestational diabetes management approach is independently associated with neonatal outcomes.
Introduction
Gestational diabetes mellitus (GDM), defined as impaired glucose tolerance with onset or first detection during pregnancy [Citation1], is one of the most common complications of pregnancy affecting as many as one in seven births [Citation2]. Rising prevalence of obesity, increasing age of expectant mothers of reproductive age [Citation3,Citation4] and changes to diagnostic criteria [Citation5], have contributed to the increasing rates of GDM observed worldwide.
In addition to adverse outcomes for mothers, GDM is associated with adverse outcomes for infants. Infants born to mothers with GDM experience elevated risks of shoulder dystocia, birth injury, neonatal hypoglycemia, hyperbilirubinemia and have a higher risk of developing obesity and type 2 diabetes later in life [Citation6–8].
The aim of GDM treatment is to normalize maternal blood glucose values, whilst avoiding hypoglycemia [Citation9]. Appropriate management of GDM improves infant outcomes and lowers the risk of adverse events [Citation10]. The first-line approach to management is dietary and lifestyle education, comprising of dietary controlled management (i.e. medical nutrition therapy (MNT)), weight management, and increased physical activity [Citation11]. Eating healthy food and reducing consumption of simple sugars or using a formal low glycaemic index diet are some of the recommendations for the dietary management of GDM; however, there is limited evidence as to the most effective diet for GDM management [Citation12]. The American Diabetes Association reports that, depending on the population and diagnostic criteria used, 70–85% of women diagnosed with GDM can be appropriately managed with lifestyle modification alone [Citation11]. Although evidence shows that dietary and lifestyle modifications can improve perinatal outcomes in GDM in comparison to routine care [Citation13], there is debate about whether non-pharmacological methods are as effective as medication in the management of GDM, and it is not known at what point the management should transition to medication [Citation14]. Two retrospective studies of women with GDM found similar perinatal outcomes in those treated with diet alone and those treated with diet and medication [Citation15,Citation16]. However, in these studies, baseline characteristics of the women in each group differed, with the medication-treated women having a higher frequency of history of GDM, being multiparous, and having higher fasting blood glucose (FBG) values on diagnostic oral glucose tolerance test (OGTT). Another retrospective cohort of 801 women with GDM compared outcomes for diet-treated and medication-treated women and found that those treated with metformin had better infant outcomes than those treated with diet alone, with lower risks of macrosomia and neonatal hypoglycemia [Citation17]. However, there were significant differences in the baseline characteristics of participants potentially confounding the results, with the non-pharmacological treated women being younger, having lower body mass index (BMI) and lower fasting blood glucose values than medicated women with GDM [Citation17].
In observational studies, unless by chance, the characteristics of individuals constituting comparison groups of interest will be different, with some differences potentially being systematic and statistically significant [Citation18]. Propensity score (PS) analysis is a method developed by Rosenbaum and Rubin to allow researchers to control for confounding and selection bias in observational studies [Citation19]. This study evaluated neonatal outcomes for women with GDM managed with and without medication, after accounting for differences in maternal baseline covariates using propensity score analysis. This allowed us to compare treatment outcomes in the compared groups that were matched according to the baseline characteristics.
Material and methods
Study design
Retrospective cohort study with propensity score matching.
Study population
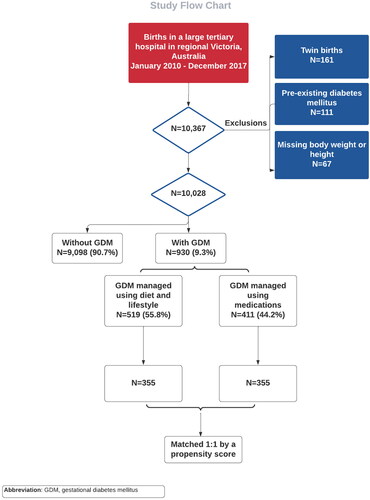
The sample has been described previously [Citation4,Citation20]. All women birthing at a large tertiary hospital in regional Victoria, Australia, between 1 January 2010 and 31 December 2017 were eligible for inclusion. Only singleton births were only included and women with known type 1 or type 2 diabetes were excluded. Similarly, women with a missing weight or height were also excluded (). Sociodemographic, antenatal, intrapartum, and postpartum information relating to each birth was extracted from the hospital Birthing Outcome System (BOS), which is an integrated data collection tool, whereby clinicians and administrative staff enter data on each birth. Missing information was not common, as all study variables were entered into compulsory data fields. The hospital has a catchment area of 58,957 km2, spanning 25.9% of the land mass of Victoria, and comprises ten local government areas.
Study variables included age, weight and height (measured at the first antenatal visit by a midwife), country of birth, residential postal code, comorbidities, smoking and substance use, parity and gravidity, antenatal, intrapartum, postpartum conditions and complications, GDM management, labor and delivery onset, delivery method, delivery outcome, infant birth weight, 1-min and 5-min Apgar scores, initiation of breastfeeding, and neonatal special care admission. Residential postcodes were matched with Australian Bureau of Statistic data to obtain the Socio-Economic Index for Areas – Index of Relative Socio-Economic Disadvantage (SEIFA-IRSD) [Citation21].
Infants were defined as being large for gestational age (LGA) when their weight, at birth, was above the 90th percentile of the distribution for the same gestational age and sex in the Australian population [Citation22]. Small for gestational age (SGA) was similarly defined, as below the 10th percentile of the distribution. The hospital’s Human Research Ethics Committee (LNR/16/BHCG/50) granted ethics clearance. The Ethics Committee waived informed consent.
Diagnostic criteria and GDM management
The diagnostic criteria for GDM changed during the study period [Citation23,Citation24]. The diagnosis of GDM was made by clinicians at the hospital following the recommended criteria for the time periods shown in Supplementary Table 1. In the participating hospital, universal screening for GDM is done between 24–28 weeks of gestation using a one-step diagnostic framework as recommended by the Royal Australian and New Zealand College of Obstetricians and Gynecologists (RANZCOG) [Citation25,Citation26]. However, since the diabetogenic effects of pregnancy increase with gestation, testing may be repeated in women with risk factors or with clinical features suggestive of hyperglycemia, such as excess fetal growth or polyhydramnios.
To account for the change in the diagnostic criteria over time, we reported the results for the whole study period and then separately, by time periods relating to the different criteria used over that time. A woman with GDM was listed as medically managed if she was ever treated with an oral hypoglycemic agent (e.g. metformin, sulfonylureas) and/or subcutaneous insulin during her pregnancy. These were compared to women with GDM that was not managed pharmacologically during the pregnancy. In Australia, pharmacological management of GDM is usually considered when blood glucose concentrations exceed recommended targets on two or more occasions within one week [Citation27].
Construction of the propensity score
Logistic regression was used to generate a PS for each participant as the estimated probability of receiving non-pharmacological dietary/lifestyle management or medical management of diagnosed GDM after accounting for the following baseline characteristics: maternal age, BMI, SEIFA-IRSD score, country of birth, Indigenous status, smoking status, parity, polycystic ovarian syndrome, preexisting hypertension, concurrent pregnancy induced complications such as hypertension, preeclampsia or eclampsia, maternal pyrexia, and history of prior GDM. A balance between the treated and non-treated groups was achieved by splitting the data into quantiles of the propensity score until a balance in baseline characteristics was achieved in each quantile. The overall number of quantiles was incrementally increased by one until a balance on the propensity score was achieved between the compared groups within all quantiles [Citation19]. In this analysis, six PS quantiles were needed to achieve a balance between the compared groups.
PS balance diagnostics and matching strategy
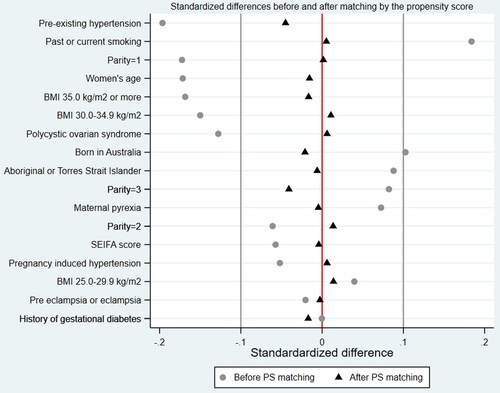
Standardized differences in covariates included in the PS construction model were estimated to assess covariate balance before and after propensity score matching and to quantify the difference in the prevalence of each characteristic between women who received dietary/lifestyle management and those who received medical management for their GDM. A standardized difference of less than the absolute value of 0.1 was considered negligible [Citation18].
After the PS construction, women with GDM in the non-pharmacological group were matched 1:1 to medically managed women using the constructed PS. Nearest-neighbour matching without replacement was used, utilizing the logit of the propensity score [Citation19]. Odds ratios (OR) were derived using bivariate logistic regression on the unmatched and matched samples to confirm covariate balance after PS matching.
Multivariable analysis
A conditional logistic regression model was subsequently used to investigate the odds of neonatal outcomes between the matched groups whilst also accounting for induction of labor, gestational age, birth type, and a number of ultrasounds conducted during the pregnancy, all of which were not accounted for in the PS model. The statistical analyses were conducted using Stata (version 16).
Results
During the study period, 10,028 births were included in the analysis (). Of these, GDM was diagnosed in 930 (9.3%) cases. GDM was detected between 24 and 28 weeks of pregnancy in 97.6% of the cases (n = 908) with the remaining 2.4% having their GDM detected in the third trimester of the pregnancy. Of the 930 diagnosed women, 519 (55.8%) were managed with non-pharmacological techniques, and 411 (44.2%) were managed medically. Of the latter, 133 (32.4%) received oral hypoglycemic agents (OHA), 226 (55.0%) were managed with insulin, and the remaining 52 (12.7%) were treated with both insulin and OHA. Compared to the medically managed group, those treated with the non-pharmacological techniques of dietary and lifestyle changes were younger, leaner, with lower rates of hypertension, but had higher rates of current or past smoking (). The imbalance in baseline characteristics is also demonstrated by the standardized difference for 7 of the 13 covariates (53.8%).
Table 1. Maternal characteristics by variables accounted for in the propensity score: before and after matching.
Of the 519 women with non-pharmacological managed GDM, 355 (68.4%) were matched 1:1 to women with GDM who were managed with medication, using the nearest-neighbour PS matching method. Matched women shared similar baseline characteristics for all baseline covariates as shown in and . Of the overall 710 women included in the final analysis, 345 (48.6%) had an unassisted vaginal birth, 106 (14.9%) had an assisted vaginal birth, 130 (18.3%) had a planned cesarean section, and the remaining 129 (18.2%) had an emergency cesarean. Induction of labor was done in 399 (56.2%) women.
Figure 2. Standardized differences before and after propensity score matching by study variables included in the construction of the propensity score.
Adjusted conditional logistic regressions constructed on the PS matched sample, also accounting for induction of labor, birth type, gestational age at birth, and a number of ultrasounds, showed that women with non-pharmacological managed GDM were significantly more likely than those who had their GDM managed medically to give birth to neonates with birth injuries (adjusted odds ratio (AOR) 3.50, 95% confidence interval (CI): 1.62–7.58, p = 0.001), hypoglycemia (AOR 1.56, 95%CI: 1.03–2.38, p = 0.037), hypothermia (AOR 2.29, 95%CI: 1.05–5.00, p = 0.037), and small for gestational age neonates (AOR 2.06, 95%CI: 1.01–4.18, p = 0.046) and neonates with significantly higher odds to being admitted to a special care unit (AOR 2.04, 95%CI: 1.29–3.21, p = 0.002) ().
Table 2. Conditional logistic regression! comparing neonatal outcomes in women with and without medically managed GDM (medicated group as reference) following PS matching.
Subgroup analyses by GDM diagnostic time periods were mostly underpowered due to smaller sample sizes within the categories. However, an increased risk of neonatal adverse outcomes among women with non-pharmacological managed GDM was noted for hypoglycemia, hypothermia, respiratory distress, and admission to a special care unit as compared with women whose GDM was medically managed (Supplementary Table 2).
Discussion
This retrospective cohort with propensity score matching study that was based on a population-based sample of pregnant women in a contemporary Australian population indicates that the management of GDM is independently associated with neonatal outcomes. Compared to neonates born to women with medically managed GDM, those born to women whose GDM was managed solely with non-pharmacological dietary measures and lifestyle changes were more likely to experience adverse outcomes including hypoglycemia, hypothermia, birth injury, and they were at greater risk of being born small for gestational age and being admitted to special care units.
The increased neonatal morbidity associated with non-medicated GDM identified in our study may indicate that diet alone is not sufficient to achieve glycaemic control in some women [Citation28]. Normalizing maternal glycaemic levels is effective in preventing neonatal morbidity, particularly that which is associated with excess fetal growth [Citation28–31]. However, even mild levels of maternal hyperglycemia have been associated with adverse pregnancy outcomes. The Hyperglycemia and Adverse Pregnancy Outcome Study (HAPO) demonstrated that morbidity risk related to hyperglycemia is a continuum and that a safe level of hyperglycemia cannot be presumed [Citation32].
Maintaining glycaemic control with diet alone is demanding for both the pregnant woman and the attending clinician [Citation29]. Trials and studies that utilized lifestyle and dietary interventions aiming at preventing GDM in pregnant women have demonstrated limited success in the past [Citation33–35]. Often program adherence is limited, with participation numbers dropping throughout the pregnancy and with pregnant women generally demonstrating low compliance with dietary and lifestyle interventions [Citation35].
The increased neonatal morbidity associated with non-medicated GDM may also indicate that the monitoring regime for women receiving dietary management may not have been effective at identifying those women whose glycaemic control was outside the target range. Various studies have found that between 19–44% of women managed with diet, progress to medication management in order to normalize their blood glucose [Citation16,Citation36]. The proportion of women progressing from dietary management to medication management has been found to increase as the intensity of glucose monitoring increases [Citation28,Citation37]. Improved neonatal outcomes have been associated with increased intensity of glucose monitoring. A prospective population-based study of 2461 women that compared conventional GDM therapy with intensive therapy found that those with intensive blood glucose monitoring were more likely to require insulin to manage their GDM than those in the conventional management group [Citation28]. They were also less likely than those in the conventional management group to experience serious neonatal adverse outcomes. Approximately two-thirds of the women in the intensive therapy group who were initially assigned dietary management subsequently required insulin as a result of poor glycaemic control compared to about one-quarter in the regular monitoring group.
Although a range of studies has demonstrated improved neonatal outcomes for dietary management of GDM in comparison to no treatment [Citation38], medication management strategies such as insulin therapy, or insulin therapy plus dietary measures, are associated with superior neonatal outcomes in comparison to diet alone, with outcomes approaching those of the control women, those without a GDM diagnosis [Citation15].
The decision to initiate drug therapy during pregnancy is a complex decision for women and their healthcare providers [Citation39] and there is debate about at what point in their non-pharmacological management they should transition to medical management [Citation9,Citation40]. A systematic review examining the criteria for commencing medical therapy in GDM identified seven different criteria for commencing medication subsequent to dietary management, [Citation14] concluding that “pharmacologic therapy should be considered in women with gestational diabetes when, despite an adequate diet and exercise, 1 or 2 blood glucose values are over the target values of 90 mg/dL fasting or 120 mg/dL 2-h postprandial over 1 or 2 weeks.” [Citation14]
The diagnosis and treatment of GDM results in an increased demand for medical services, with more antenatal visits, more lab tests, increased health care costs, as well as some evidence of maternal stress subsequent to the diagnosis [Citation41]. Changes in delivery management may also occur [Citation10]; however, there is little evidence of increased harm associated with the transition to medication management of GDM. A number of trials have confirmed the safety of both insulin [Citation39] and metformin [Citation42,Citation43] in pregnancy, and the positive impact they have on neonatal outcomes.
Limitations
Although our propensity score matching was able to balance known characteristics between the compared groups, unmeasured covariates were not balanced, and residual confounding could not be excluded. We had no access to pathology results and could not determine at what stage during the pregnancy a woman with GDM was prescribed insulin or metformin. We had no knowledge of which women achieved glycaemic control during labor and which women were compliant to clinical management. Although we adjusted for body mass index, as measured during the first trimester of the pregnancy, we could not account for gestational weight gain. Finally, clinician variation in the management of GDM was unknown to us. However, all clinicians worked in a single tertiary hospital which has standardized clinical guidelines that provided the components of diabetes care, general treatment goals, and tools to evaluate quality of care using the Royal Australian and New Zealand College of Obstetricians and Gynecologists guidelines.
Conclusions
This study suggests that infants born to women with diet/lifestyle-only controlled GDM are at greater risk of experiencing adverse outcomes than those born to women whose GDM was medically treated. Our findings suggest that women whose GDM is not medically managed should have their glycaemic control more closely monitored and that Medical Nutrition Therapy may be optimized together with medical management. Future trials are warranted to determine whether dietary measures to control GDM are as effective as medical management of the condition in avoiding preventable adverse neonatal outcomes.
Authors’ contribution
GM, MT, MW, HDM, RRH: research conception and design, and interpretation of results. GM, MT: statistical analysis. NY: data acquisition. GM, MT, FH: manuscript writing. All authors contributed to the critical revision of the manuscript and approved the final draft. GM is the guarantor of the study.
Ethical consent
This study was conducted in accordance with the Declaration of Helsinki. Ethics clearance was obtained from the Human Research Ethics Committee of the hospital (reference number LNR/16/BHCG/50) in April 2017 with amendments accepted in July 2020. No consent was required from the study participants. The Ethics Committee waived informed consent.
Supplemental Material
Download MS Word (21.4 KB)Acknowledgement
Thanks to Ms Jessica Ciccosillo for proofreading the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Additional information
Funding
References
- American Diabetes Association Professional Practice Committee; 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Supplement_1):1–7. doi:10.2337/dc22-S002.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2020;33(9):1616–1624. doi:10.1080/14767058.2018.1519543.
- Poston L, Caleyachetty R, Cnattingius S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4(12):1025–1036. doi:10.1016/S2213-8587(16)30217-0.
- Mnatzaganian G, Woodward M, McIntyre HD, et al. Trends in percentages of gestational diabetes mellitus attributable to overweight, obesity, and morbid obesity in regional Victoria: an eight-year population-based panel study. BMC Pregnancy Childbirth. 2022;22(1):95. doi:10.1186/s12884-022-04420-9.
- International Association Of D, Pregnancy Study Groups Consensus P, Metzger BE, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682.
- Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi:10.1056/NEJMoa0707943.
- Lowe WL, Jr., Scholtens DM, Lowe LP, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320(10):1005–1016. doi:10.1001/jama.2018.11628.
- Blotsky AL, Rahme E, Dahhou M, et al. Gestational diabetes associated with incident diabetes in childhood and youth: a retrospective cohort study. CMAJ. 2019;191(15):E410–E417. doi:10.1503/cmaj.181001.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: what should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97(3):235–247. doi:10.1111/aogs.13257.
- Hartling L, Dryden DM, Guthrie A, et al. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive services task force and the national institutes of health office of medical applications of research. Ann Intern Med. 2013;159(2):123–129. doi:10.7326/0003-4819-159-2-201307160-00661.
- American Diabetes A. 14. Management of diabetes in pregnancy: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S165–S72.
- American Diabetes A. (12) Management of diabetes in pregnancy. Diabetes Care. 2015;38 (Supplement 1):S77–S9.
- Yamamoto JM, Kellett JE, Balsells M, et al. Gestational diabetes mellitus and diet: a systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight. Diabetes Care. 2018;41(7):1346–1361. doi:10.2337/dc18-0102.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32(17):2905–2914. doi:10.1080/14767058.2018.1449203.
- Koning SH, Hoogenberg K, Scheuneman KA, et al. Neonatal and obstetric outcomes in diet- and insulin-treated women with gestational diabetes mellitus: a retrospective study. BMC Endocr Disord. 2016;16(1):52. doi:10.1186/s12902-016-0136-4.
- Benhalima K, Robyns K, Van Crombrugge P, et al. Differences in pregnancy outcomes and characteristics between insulin- and diet-treated women with gestational diabetes. BMC Pregnancy Childbirth. 2015;15:271. doi:10.1186/s12884-015-0706-x.
- Bashir M, Aboulfotouh M, Dabbous Z, et al. Metformin-treated-GDM has lower risk of macrosomia compared to diet-treated GDM- a retrospective cohort study. J Matern Fetal Neonatal Med. 2020;33(14):2366–2371. doi:10.1080/14767058.2018.1550480.
- Mnatzaganian G, Davidson DC, Hiller JE, et al. Propensity score matching and randomization. J Clin Epidemiol. 2015;68(7):760–768. doi:10.1016/j.jclinepi.2015.01.002.
- Rosenbaum PR, Rubin DB. The Central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi:10.1093/biomet/70.1.41.
- Ward MC, Agarwal A, Bish M, et al. Trends in obesity and impact on obstetric outcomes in a regional hospital in Victoria, Australia. Aust N Z J Obstet Gynaecol. 2020;60(2):204–211. doi:10.1111/ajo.13035.
- Australian Bureau of Statistics. SEIFA: socio‐Economic Indexes for Areas. 2022. Available from: https://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa.
- Dobbins TA, Sullivan EA, Roberts CL, et al. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med J Aust. 2012;197(5):291–294. Erratum in: med J Aust. 2013;198:189. doi:10.5694/mja11.11331.
- Martin FIR. The diagnosis of gestational diabetes. Med J Aust. 1991;155(2):112.
- WHO. World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Geneva: WHO Press; 2013.
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG). Excellence in Women’s Health. 2020. https://ranzcog.edu.au.
- Department of Health. Clinical practice guidelines: pregnancy care. 2020. Canberra: Australian Government Department of Health.
- Hoffman L, Nolan C, Wilson JD, et al. Gestational diabetes mellitus – management guidelines. The australasian diabetes in pregnancy society. Med J Aust. 1998;169(2):93–97. doi:10.5694/j.1326-5377.1998.tb140192.x.
- Langer O, Rodriguez DA, Xenakis EM, et al. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol. 1994;170(4):1036–1046. discussion 46-7. doi:10.1016/s0002-9378(94)70097-4.
- McCance DR. Pregnancy and diabetes. Best Pract Res Clin Endocrinol Metab. 2011;25(6):945–958. doi:10.1016/j.beem.2011.07.009.
- Hillier TA, Vesco KK, Pedula KL, et al. Screening for gestational diabetes mellitus: a systematic review for the U.S. Preventive services task force. Ann Intern Med. 2008;148(10):766–775. doi:10.7326/0003-4819-148-10-200805200-00009.
- Mendez-Figueroa H, Schuster M, Maggio L, et al. Gestational diabetes mellitus and frequency of blood glucose monitoring: a randomized controlled trial. Obstet Gynecol. 2017;130(1):163–170. doi:10.1097/AOG.0000000000002101.
- Catalano PM, McIntyre HD, Cruickshank JK, et al. The hyperglycemia and adverse pregnancy outcome study. Associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35(4):780–786. doi:10.2337/dc11-1790.
- Brown J, Grzeskowiak L, Williamson K, et al. Insulin for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;11(11):CD012037. doi:10.1002/14651858.CD012037.pub2.
- Oostdam N, van Poppel MN, Wouters MG, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG. 2012;119(9):1098–1107. doi:10.1111/j.1471-0528.2012.03366.x.
- Egan AM, Simmons D. Lessons learned from lifestyle prevention trials in gestational diabetes mellitus. Diabet Med. 2019;36(2):142–150. doi:10.1111/dme.13772.
- Moss JR, Crowther CA, Hiller JE, et al. Costs and consequences of treatment for mild gestational diabetes mellitus - evaluation from the ACHOIS randomised trial. BMC Pregnancy Childbirth. 2007;7:27. doi:10.1186/1471-2393-7-27.
- Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. doi:10.1056/NEJMoa042973.
- Kapur K, Kapur A, Hod M. Nutrition management of gestational diabetes mellitus. Ann Nutr Metab. 2021;76(suppl 3):1–13. doi:10.1159/000509900.
- Simeonova-Krstevska S, Bogoev M, Bogoeva K, et al. Maternal and neonatal outcomes in pregnant women with gestational diabetes mellitus treated with diet, metformin or insulin. Open Access Maced J Med Sci. 2018;6(5):803–807. doi:10.3889/oamjms.2018.200.
- Brawerman GM, Dolinsky VW. Therapies for gestational diabetes and their implications for maternal and offspring health: evidence from human and animal studies. Pharmacol Res. Apr. 2018;130:52–73. doi:10.1016/j.phrs.2018.02.002.
- Langer O. Oral antidiabetic drugs in pregnancy: the other alternative. Diabetes Spectrum. 2007;20(2):101–105. doi:10.2337/diaspect.20.2.101.
- Hyer S, Balani J, Shehata H. Metformin in pregnancy: mechanisms and clinical applications. Int J Mol Sci. 2018;19:1954. doi:10.3390/ijms19071954.
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358(19):2003–2015. doi:10.1056/NEJMoa0707193.