Abstract
Objective
Premature ovarian failure (POF), also known as primary ovarian insufficiency, is a major cause of infertility in female worldwide. Excessive apoptosis and impaired autophagy in ovarian granulosa cells are the main pathological mechanisms of POF. The total flavonoids from semen cuscutae (TFSC) are often used in the treatment of gynecological endocrine disorders. In addition, low intensity pulsed ultrasound (LIPUS) is report as an effective method to improve ovarian function. This study aims to investigate the protective effect of POF by the combined use of TFSC and LIPUS.
Methods
POF rats model and granulosa cell model were successfully induced by tripterygium glycosides and cyclophosphamide, respectively. After that, model rats and cells received TFSC plus LIPUS administration. Then ovarian histomorphology, senescence, estrus cycle, and serum sex hormone levels were detected in rats. Ovarian tissue and granulosa cells autophagy and apoptosis levels were also assessed.
Results
Disturbed sex hormone levels, atrophied and senescent ovaries, and abnormal estrous cycle were found in POF rats. Meanwhile, cell autophagy was inhibited and cell apoptosis was activated in POF ovarian tissue and granulosa cells. However, TFSC combined with LIPUS improved these changes, and this combination treatment exhibited synergistic effects. The abnormal expression of the cell apoptosis-, autophagy-, and PI3K/AKT/mTOR signaling pathway-related proteins were also improved by combination treatment.
Conclusion
The study found that the combination of TFSC and LIPUS can alleviate POF by modulating cell autophagy and apoptosis. The findings may provide a viable scientific basis for POF treatment.
Introduction
Premature ovarian failure (POF) is a syndrome of persistent amenorrhea and sexual organ atrophy in women before the age of 40, accompanied by decreased level of estradiol (E2) and increased level of follicle-stimulating hormone (FSH) [Citation1]. Except for the side impact on the quality of women’s life, POF seriously endangers physical and mental health and even leads to female infertility [Citation2]. Effective treatment for the damaged ovarian tissue structure and reduced reserve function of POF is insufficient [Citation3,Citation4]. Conventional treatments mostly use replacement therapy, but many adverse effects have emerged. Therefore, its urgent to explore effective therapeutic strategies to alleviate POF. POF is essentially to accelerate follicular atresia, and granulosa cells play an important role in follicular development and maturation [Citation5]. Abnormal apoptosis and autophagy of granulosa cells are considered to be the key pathological mechanisms of POF [Citation6–8]. Wang proved that activating granulosa cells autophagy and reducing apoptosis of damaged cells could effectively alleviate POF [Citation9]. Liu et al. also found the activation of the PI3K/AKT/mTOR-regulated autophagy delays POF in mice [Citation10]. Therefore, targeting granulosa cells autophagy and apoptosis is expected to alleviate POF.
The semen cuscuta (SC) is an important herbal medicine in Chinese and is commonly used for the treatment of gynecological endocrine disorders with proven efficacy [Citation11,Citation12]. The total flavonoids from SC (TFSC) is the main bioactive component [Citation13]. Flavonoids have been shown to possess various biological activities, such as anti-apoptosis [Citation14], anti-inflammation [Citation15], and antioxidation [Citation16]. In addition, flavonoids can also regulate the secretion of sex and steroid hormones in ovarian granulosa cells and inhibit POF disease progression [Citation17]. Some pharmacological studies have found that SC and TFSC have beneficial effects in improving reproductive endocrine function [Citation18], anti-postmenopausal osteoporosis [Citation12], and antioxidative activities [Citation19]. And yet, it is not clear whether TFSC can mitigate POF disease progression, as well as its role in regulating cell autophagy and apoptosis. In addition, low-intensity pulsed ultrasound (LIPUS) is a noninvasive treatment that uses pulses as the mode of emission to effectively avoid infection or tissue damage [Citation20]. Studies have shown that LIPUS can improve ovarian function and is beneficial for POF repairs [Citation21]. At the same time, LIPUS in combination with drugs has been reported to improve drug targeting and reduce adverse effects [Citation22]. However, the role of LIPUS in combination with TFSC in POF is lacking in studies.
Therefore, this study was conducted to observe the effect of LIPUS in combination with TFSC on ovarian function, as well as the granulosa cells’ autophagy and apoptosis status in the POF rats, with the hope of providing a scientific basis for the further application of LIPUS and TFSC in clinical practice.
Materials and methods
TFSC preparation
TFSC was prepared according to the methods already reported [Citation23]. The SC was crushed and sieved, defatted with petroleum ether, added 75% ethanol and extracted twice by heating. The extract was diluted to 0.1 g/mL, then eluted with gradient ethanol and the eluate was collected. The total flavonoids were obtained by concentration, drying, crushing and sieving.
Experimental animals and ethics
Fifty female SD rats (weighing 170-180 g) were purchased from Shanghai SLAC Laboratory Animal Co.,Ltd, experimental animal license No.: SCXK-2017-0005. All experimental animals were approved by the Animal Experimentation Ethics Committee of Zhejiang Eyong Pharmaceutical Research and Development Center. (Approval NO.: SYXK-2021-0033).
Animal grouping and POF rats model establishment
The SD rats were fed for one week for acclimatization, and the estrus cycle was monitored by vaginal smear. 50 SD rats with normal estrus cycles were randomly divided into control group, model group, LIPUS group, TFSC group, LIPUS + TFSC group, 10 rats in each group [Citation24]. The control group was given equal amount of saline and the rest of the rats were gavaged with 50 mg/kg tripterygium glycosides (TG) (Yuanye bio-technology, B20709) every morning for 15 d to establish POF rats model [Citation25]. Prolonged and irregular estrous cycle in rats is considered as successful establishment of the POF model. In the TFSC groups rats, 140 mg/kg TFSC was gavaged every afternoon for 15 d [Citation23]. The LIPUS group was given LIPUS treatment for 15 d according to the previous method [Citation26]. Meanwhile, the LIPUS + TFSC group rats were given 140 mg/kg TFSC gavage plus LIPUS treatment.
Vaginal smear
Vaginal smears were used to detect the estrus cycle in each group of rats. A small amount of vaginal secretion was taken on a slide, fixed in ethanol for 10 min and then stained with methylene blue (Nanjing jiancheng, 20210712). The cell proportions were observed under light microscopy to determine the estrus cycle.
Enzyme linked immunosorbent assay (ELISA)
ELISA was used to examine the levels of E2, progesterone prog (P), anti-Müllerian hormone (AMH), luteinizing hormone (LH), and FSH in the serum of the rats. The wells were zeroed with blank wells and the absorbance value of each well was measured at 450 nm within 15 min after the reaction was terminated.
Hematoxylin-eosin (HE) staining
Histopathological changes of rat ovaries were observed by HE staining (Servicebio, G1003). After paraffin sections of ovarian tissue were dewaxed, the sections were stained with hematoxylin staining solution for 3-5 min, returned to blue and rinsed in running water. After dehydration in gradient alcohol, eosin staining solution was added; the sections were sealed by dehydration and examined microscopically.
Senescence associated β-galactosidase (SA-β-Gal) and γH2AX staining
Rat ovarian senescence was detected by SA-β-Gal staining and immunohistochemical (IHC) staining of γH2AX. For SA-β-Gal staining, ovarian tissue sections were prepared after staining fixative (Beyotime, C0602) was added. Then, the staining working solution (Beyotime, C0602) was added and incubated at 37 °C. For immunohistochemical staining of γH2AX, the fixed ovarian tissue section was incubated antibody against γH2AXO (Abcam, ab81299) and secondary antibody, colored by DAB solution and Hematoxylin (Runnerbio, Bry-0001-01). Ovarian tissue staining was observed under the microscope.
Quantitative real-time PCR (qRT-PCR) assay
qRT-PCR was used to detect the expression of autophagy-related gene 5 (ATG5), Beclin 1, p62 and LC3 B mRNA in rats’ ovarian tissues. Ovarian tissues were completely lysed by trizol (Sangon Biotech, B511311) for extracting total RNA. The reverse transcription and qRT-PCR reactions were carried out according to the instructions. qRT-PCR primer sequences used are listed in .
Table 1. Primer sequences of PCR.
Extraction and culture of rat ovarian granulosa cells
The euthanized rats were disinfected by immersion in iodine volt, and the ovaries were dissected bilaterally in sterile saline. The ovarian surface envelope and surrounding adipose tissue were removed and the follicles were punctured in DMEM/F12 medium (Hyclone, SH30023.01) to obtain granulosa cell suspensions. The cells were incubated at 37 °C in a 5% CO2 incubator and passaged when the cell attachment rate reached 80%. The morphology of ovarian granulosa cells was observed microscopically.
Immunofluorescence staining
Ovarian granulosa cells were placed on a glass slide to grow on the slide. Then 4% paraformaldehyde was used to fix. Add 1–2 mL of 0.5% Triton X-100 (Sangon Biotech, A110694-0100) and incubate with antibody against follicle-stimulating hormone receptor (FSHR) (Invitrogen, PA5-50963), followed by DAPI staining of cell nuclei. Immunofluorescence was observed under an inverted fluorescent microscope (Motic, AE2000).
POF cell model establishment and cell grouping
Normal ovarian granulosa cells were used as a control group. The POF cell model was established by incubation with 60 μg/mL cyclophosphamide (CTX) (Yuanye bio-technology, S30563) for 24 h as in the previous method [Citation27]. The POF granulosa cells were randomly divided into four groups: model group, LIPUS group, TFSC group, LIPUS + TFSC group. The intervention conditions of TFSC were 5 μg/mL for 12 h [Citation13]. Meanwhile, LIPUS and LIPUS + TFSC groups were treated with LIPUS for 30 min. The LIPUS frequency was 0.25 MHz and the duty cycle was 20% [Citation28].
Transmission electron microscopy assay
TEM assay (Hitachi, H7650) was used to visualize the ultrastructure of ovarian granulosa cells. Ovarian granulosa cells are pre-treat and placed on a sample holder. A suitable field of view is selected and adjusted to the clearest state when photographed.
Flow cytometry assay
Ovarian granulosa cells were inoculated in 6-well plates with 1.2 × 106 cells per well. Cells were collected after 24 h of treatment as described above and the cell concentration was adjusted to 1 × 106/mL. Then 5 μL Annexin V-FITC (Elabscience, E-CK-A211) and 10 μL PI (Elabscience, E-CK-A211) were added and mixed thoroughly. Apoptosis rates were detected by flow cytometry (BD, C6).
Western blot assay
First, ovarian tissue and granulosa cell proteins were extracted and quantified using the BCA kit (Solarbio, pc0020). SDS-PAGE electrophoresis and membrane transfer were used to obtain the target bands. The membrane was closed and placed in a dilution of primary antibody. The secondary antibody were added after the reaction of the antigen and antibody. The ECL luminescence reagent were mixed 1:1, and exposed to the chemiluminescence meter. Antibody information was presented in .
Table 2. Antibody information of Western blot.
Statistical assay
SPSS 20.0 statistical software was used for data analysis. All data are expressed as mean ± standard deviation, One-way ANOVA with Tukey’s test was performed for comparison between groups, p < .05 is considered statistically significant.
Results
LIPUS combined with TFSC restores estrus cycle and sex hormone levels in POF rats
The vaginal smear was used to evaluate the POF model, and the results showed that the rats in the control group had a normal estrus cycle. In contrast, the rats in the model group had a prolonged and irregular estrus cycle, and the rats in the LIPUS group, TFSC group, and LIPUS + TFSC group had different degrees of improvement in estrus cycles (). In addition, compared with the control group, the body weight, ovarian index, and uterine index of the model rats were reduced, while LIPUS, TFSC, and LIPUS + TFSC improved these indices (). The measurement of the serum sex hormone levels showed that compared to the control group, the levels of AMH, E2, and P decreased and the levels of LH and FSH increased in the model group, and these changes were reversed in the LIPUS, TFSC, and LIPUS + TFSC groups (Figure1E–I).
Figure 1. LIPUS combined with TFSC restores estrus cycle and sex hormone levels in POF rats. (A) Vaginal smears were performed to observe the estrus cycle in rats (magnification, 200 ×). (B–D) body weight, ovarian index and uterine index of rats were examined after the last dose, n = 10, compared with control group, ▲p < .05, ▲▲p < .01; compared with model group, ★p < .05, ★★p < .01. (E-I) ELISA was used to detect AMH, E2, P, LH and FSH in rats serum, n = 10, compared with control group, ▲p < .05, ▲▲p < .01; compared with model group, ★p < .05, ★★p < .01. POF: premature ovarian failure; ELISA: enzyme linked immunosorbent assay; AMH: anti-Müllerian hormone; E2: estradiol; P: progesterone prog; LH: luteinizing hormone; FSH: follicle-stimulating hormone.
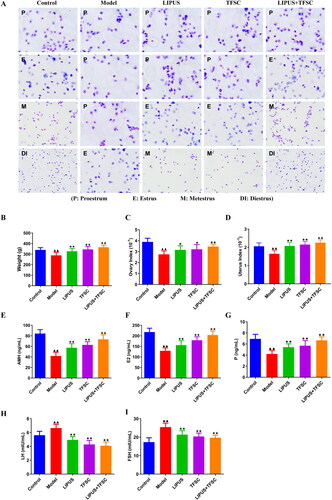
LIPUS and TFSC combination inhibit ovarian tissue structural disorders and cellular senescence in POF rats
HE staining was used to observe the histomorphology of rats’ ovaries, the results showed that follicles at all levels of the ovaries were clearly visible, while primary and secondary follicles were rare observed in the model group ovary, and atretic follicle was increased (). Compared with the model group, the numbers of primary and secondary follicles in ovarian tissues of LIPUS, TFSC, and LIPUS + TFSC rats were increased, while atretic follicles were reduced (, ). As shown in , the results of SA-β-Gal staining of ovarian senescence suggested that there were only a few blue positive cells in the ovarian tissues of the control rats but increased numbers of SA-β-Gal-positive cells in the ovarian tissues of the model rats (). However, the positive staining rate of LIPUS, TFSC, and LIPUS + TFSC groups was lower than the model group, and the decrease was most obvious in the LIPUS + TFSC group (). IHC staining of γH2AX also demonstrated similar results ().
Figure 2. LIPUS and TFSC inhibit ovarian tissue structural disorders and cellular senescence in POF rats. (A) Histopathological changes in the ovaries of rats were observed by HE staining (magnification, 40 ×, 400 μm; 200 ×, 100 μm; 400 ×, 50 μm), n=6, compared with control group, ▲P<.05, ▲▲P<.01; compared with model group, ★P<.05, ★★P<.01. (B) SA-β-Gal staining was used to detect senescence in rat ovaries (magnification, 40 ×, 400 μm; 200 ×, 100 μm), n=6, compared with control group, ▲P<0.05, ▲▲P<0.01; compared with model group, ★P<.05, ★★P<.01. (C) γH2AX staining was used to detect senescence in rat ovaries (magnification, 200 ×, 100 μm), n=6, compared with control group, ▲P<.05, P<.01; compared with model group, ★P<.05, ★★P<.01. LIPUS: low intensity pulsed ultrasound; TFSC: total flavonoids from semen cuscutae; HE: hematoxylin-eosin; SA-β-Gal: senescence associated β-galactosidase.
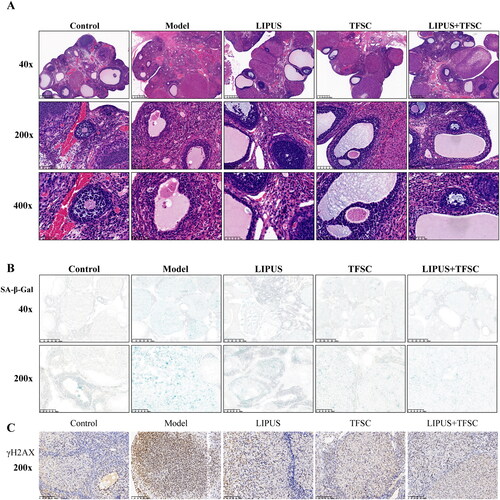
Table 3. Number of follicles in ovarian tissue (mean ± SD).
LIPUS combined with TFSC promotes autophagy and inhibits apoptosis via PI3K/AKT/mTOR pathway in ovarian tissues of POF rats
qRT-PCR was used to detect the ATG5, Beclin-1, p62, LC3 B mRNA expression in rats ovarian tissues (). Compared with the control group, ATG5, Beclin1, and LC3 B mRNA expression levels were decreased and p62 mRNA expression levels were increased in the ovarian tissues of the model group rats (). In contrast, LIPUS, TFSC, and LIPUS + TFSC antagonized the above changes (). Western blot results suggested that compared with the model group, the ovarian tissues of the LIPUS, TFSC and LIPUS + TFSC groups showed higher expression levels of Beclin-1, LC3 II/I, Bcl-2 proteins and lower protein expression levels of p62, Bax, p-PI3K/pI3K, p-AKT/AKT, p-mTOR/mTOR () ().
Figure 3. LIPUS combined with TFSC promotes autophagy and inhibits apoptosis in ovarian tissues. (A-D) qRT-PCR was used to detect the ATG5, Beclin-1, p62, LC3 II/I mRNA expression in rats ovarian tissues, n = 3, compared with control group, ▲p < .05, ▲▲p < .01; compared with model group, ★p < .05, ★★p < .01. (E–K) the Beclin 1, LC3 II/I, p62, Bax, Bcl-2 protein levels in rats ovarian tissues were detected by Western blot, n = 3, compared with control group, ▲p < .05, ▲▲p < .01; compared with model group, ★p < .05, ★★p <.01. qRT-PCR: Quantitative real-time PCR; ATG5: autophagy-related gene 5; p62: sequestosome I; LC3: microtubule associated protein light chain 3; Bax: BCL2-Associated X; Bcl-2: B cell lymphoma 2.
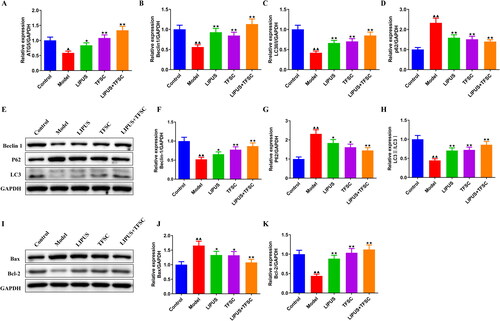
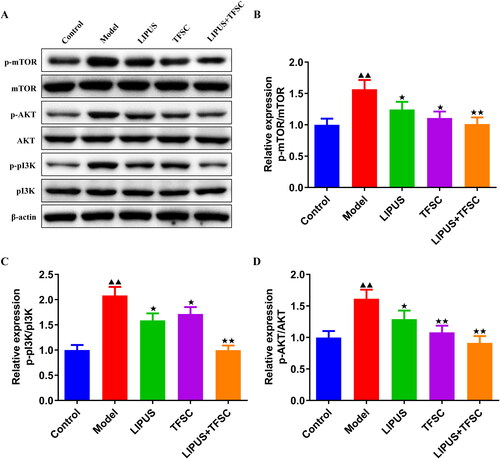
Figure 4. LIPUS combined with TFSC inhibit the PI3K/AKT/mTOR pathway in POF rats. (A–D) Western blot was used to detect the protein expression levels of p-PI3K/PI3K, p-AKT/AKT, p-mTOR/mTOR in rats ovarian tissues, n = 3, compared with control group, ▲p < .05, ▲▲p < .01; compared with model group, ★p < .05, ★★p < .01. PI3K: Phosphatidylinositol 3-kinase; AKT: protein kinase B; mTOR: mammalian target of rapamycin.
Extraction and identification of rats ovarian granulosa cells
The ovarian granulosa cells were initially round or oval, and after 48 h the cells were multinodular or spindle-shaped and interconnected by pseudopods (). Immunofluorescent staining was used to detect the cell marker FSHR expression in ovarian granulosa cells, and positive FSHR staining localization on the cell membrane of ovarian granulosa cells was observed obviously ().
Figure 5. Extraction and identification of rats ovarian granulosa cells. (A) Primary rats ovarian granulosa cells morphology was observed (magnification, 100×). (B) FSHR antibody expression in ovarian granulosa cells was detected by immunofluorescence (magnification, 200×). FSHR: follicle-stimulating hormone receptor.
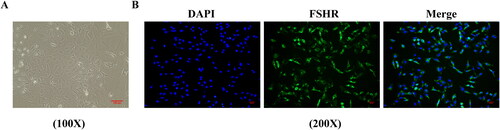
LIPUS combined with TFSC promotes ovarian granulosa cell autophagy and inhibits apoptosis via the PI3K/AKT/mTOR pathway
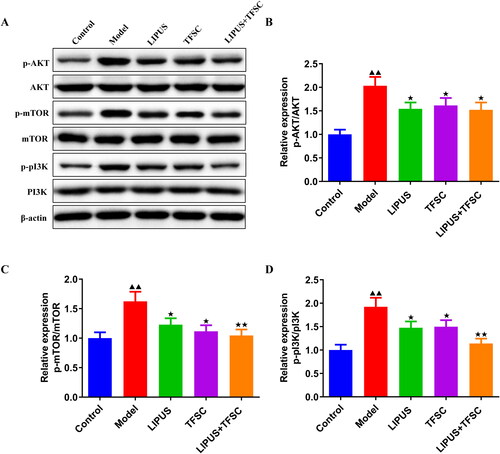
TEM assay for the ultrastructure of ovarian granulosa cells revealing a large number of autophagic vesicles in the control group while a decrease of autophagic vesicles in the model group (). Compared to the model group, the LIPUS, TFSC, and LIPUS + TFSC groups showed an increase in autophagic vesicles (). The content of autophagy proteins in ovarian granulosa cells was measured by Western blot (). We found that LIPUS combined with TFSC promoted Beclin-1 and LC3 II/I and decreased p62 expression levels in cells compared with the model group (). Ovarian granulosa cell apoptosis rate was detected by flow cytometry (). The results show that the rate of apoptosis was lower in the LIPUS, TFSC, and LIPUS + TFSC groups than in the model group (). In addition, compared with the model group, Bcl-2 protein level was elevated, Bax, Cleaced caspase 3/caspase 3, p-PI3K/pI3K, p-AKT/AKT, p-mTOR/mTOR protein levels were decreased in the LIPUS, TFSC, and LIPUS + TFSC groups (, ).
Figure 6. LIPUS combined with TFSC promotes autophagy in ovarian granulosa cells. (A) Transmission electron microscopy was used to observe changes in the ultrastructure of ovarian granulosa cells (magnification, 15,000 ×). (B–E) Western blot was used to detect the protein expression levels of p62, Beclin 1, LC3 II/I, n = 3, compared with control group, ▲p < .05, ▲▲p < .01; compared with model group, ★p < .05, ★★p < .01.
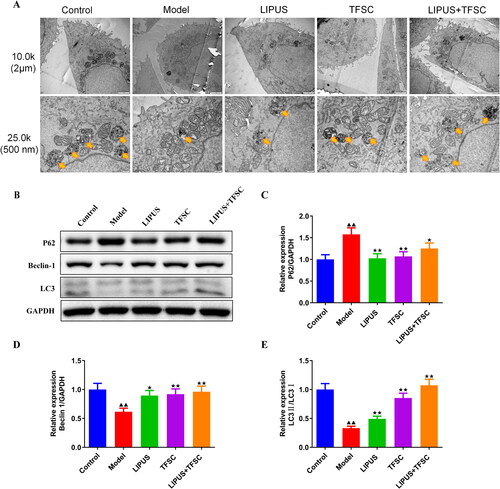
Figure 7. LIPUS combined with TFSC inhibits apoptosis in ovarian granulosa cells. (A–B) The apoptosis of each group of granulosa cells was detected by flow cytometry, n = 3, compared with control group, ▲p < .05, ▲▲p < .01; compared with model group, ★p < .05, ★★p < .01. (C–E) Western blot was used to detect the protein levels of Bcl-2 and Bax in ovarian granulosa cells, n = 3, compared with control group, ▲p < .05, ▲▲p < .01; compared with model group, ★p < .05, ★p < .01.
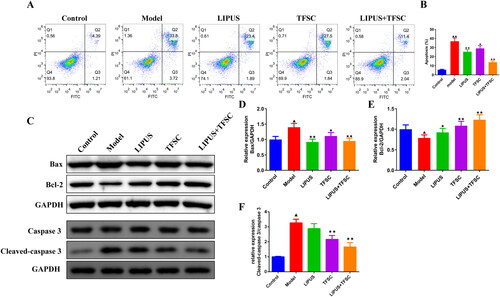
Figure 8. LIPUS combined with TFSC inhibits PI3K/AKT/mTOR pathway in ovarian granulosa cells. (A–D) The protein expression levels of p-PI3K/PI3K, p-AKT/AKT, p-mTOR/mTOR in ovarian granulosa cells were detect by Western blot, n = 3, compared with control group, ▲p < .05, ▲▲p < .01; compared with model group, ★p < .05, ★p < .01.
Discussion
In this study, to explore the protective effect of TSFC and LIPUS against POF, we therefore used TG and CTX to induce POF animal and cellular models, respectively. We found that LIPUS and TFSC effectively improved POF in rats, and the combination treatment had synergistic effects. The potential molecular mechanism is that LIPUS combined with TFSC alleviates POF by promoting ovarian granulosa cell autophagy and suppressing excessive apoptosis.
POF has been found to be associated with excessive apoptosis of ovarian granulosa cells [Citation29,Citation30]. Ovarian granulosa cell apoptosis caused by follicular atresia is considered to be the main pathogenesis of POF [Citation31,Citation32]. Under normal conditions, granulosa cells eliminate apoptosis through autophagy-assisted phagocytosis [Citation33]. However, POF is often accompanied by impaired autophagy [Citation34,Citation35]. Cycloferonamide was reported to cause POF by decreasing autophagic activity of ovarian granulosa cells [Citation36]. Here, we successfully established a rat and cell model of POF, by detecting autophagy and apoptosis-related biomarkers, elevated expression of ATG5, Beclin 1, LC3, Bcl-2, and decreased p62, Bax, Cleaced caspase 3/caspase 3 in ovarian tissues and granulosa cells of the model group were found. In addition, TEM and flow cytometry results also suggested that autophagy level was reduced while apoptosis was activated in POF granule cells. The above results suggest that POF correlates with the abnormal autophagy and apoptosis status.
Accordingly, activation of autophagy to reduce apoptosis becomes an effective way to alleviate POF. To investigate the action mechanism of TFSC on POF, the autophagy and apoptotic levels as well as related protein expression after TFSC and LIPUS intervention were detected. The present study also found that TFSC and LIPUS treatment restored the autophagy and apoptosis levels, which contributed to the alleviation of POF in model rats and granule cells. As an important class of active compounds from nature, flavonoids have been reported with the ability to ameliorate POF, and the mechanism of action is related to apoptosis inhibition [Citation37,Citation38]. Liu et al. also found that esculentoside A rescues granulosa cell apoptosis and folliculogenesis in POF mice to improve disease [Citation38]. The combination of SC and Fructus Lycii are able to treat spermatogenic dysfunction by inhibiting germ cell apoptosis [Citation39]. Zhang has also demonstrated that TFSC inhibits apoptosis and improves reproductive damage in rats by high-throughput transcriptomic sequencing [Citation18]. Cells senescence is an important causal factor for POF. In the present study, vaginal smear, SA-β-Gal, and γH2AX staining results also revealed that TFSC improved the estrus cycle and inhibited ovarian senescence in POF rats. In addition, LIPUS is considered an effective method to promote drug efficacy and reduce adverse effects [Citation40]. LIPUS has also been shown to promote follicular development and inhibit follicular atresia [Citation26]. Therefore, we also explored the combination effects of LIPUS and TFS, and found that these two methods exhibited synergistic effects in alleviating POF.
Increasing evidence support the critical role of the PI3K/AKT/mTOR pathway in autophagy. Cell autophagy is activated by ULK, which is controlled by mTOR, and PI3K/AKT is the upstream regulator of mTOR. In Yin et al.’s study, inducing robust autophagic flux through activating the JNK/Bcl-2 signal pathway is a major therapeutic process to improve POF in model rats and granulosa cells [Citation41]. Previous studies also report that LIPUS and TFSC can regulate the PI3K/AKT pathway to induce autophagy in multiple diseases. In glioblastoma cells, LIPUS plus voxtalisib can inhibit the PI3K/AKT/mTOR to activate autophagy, which is thought to develop more effective therapeutic approaches for glioblastoma [Citation42]. In addition, pueraria flavonoids have been reported to stimulate autophagy by inhibiting the PI3K/Akt/mTOR signaling pathway, thereby reducing intracellular lipid accumulation and inflammation levels to alleviate non-alcoholic fatty liver disease in obese mice [Citation43]. In this experiment, we examined the expression of PI3K/AKT/mTOR pathway-related proteins in ovarian tissue and granulosa cells, respectively. Consistent with the autophagy promotion in intervention groups, the results confirmed that the PI3K/AKT/mTOR pathway was activated in the model group and was significantly inhibited after the drug intervention.
In conclusion, in this study, to explore the protective effect of TSFC and LIPUS against POF, we used TG and CTX to induce POF in animal and cellular models. The study found that the combination of TFSC and LIPUS can alleviate POF by promoting autophagy and inhibiting apoptosis. Our findings may provide additional treatment options for patients with POF.
Acknowledgements
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jang S, Kim M-S, Kim P-W, et al. Korean medicine treatment for premature ovarian failure: three case reports. Explore. 2023;19(1):1–11. doi: 10.1016/j.explore.2022.08.018.
- Geng Z, Nie X, Ling L, et al. Electroacupuncture may inhibit oxidative stress of premature ovarian failure mice by regulating intestinal microbiota. Oxid Med Cell Longev. 2022;2022:4362317–4362313. doi: 10.1155/2022/4362317.
- Lin J, Li X-L, Song H, et al. A general description for Chinese medicine in treating premature ovarian failure. Chin J Integr Med. 2017;23(2):91–97. doi: 10.1007/s11655-016-2642-7.
- Shi L, Zhang Z, Deng M, et al. Biological mechanisms and applied prospects of mesenchymal stem cells in premature ovarian failure. Medicine. 2022;101(32):e30013. doi: 10.1097/MD.0000000000030013.
- Liu J, Yang Y, He Y, et al. Erxian decoction alleviates cisplatin-induced premature ovarian failure in rats by reducing oxidation levels in ovarian granulosa cells. J Ethnopharmacol. 2023;304:116046. doi: 10.1016/j.jep.2022.116046.
- Delcour C, Amazit L, Patino LC, et al. ATG7 and ATG9A loss-of-function variants trigger autophagy impairment and ovarian failure. Genet Med. 2019;21(4):930–938. doi: 10.1038/s41436-018-0287-y.
- Liu Y-X, Ke Y, Qiu P, et al. LncRNA NEAT1 inhibits apoptosis and autophagy of ovarian granulosa cells through miR-654/STC2-mediated MAPK signaling pathway. Exp Cell Res. 2023;424(1):113473. doi: 10.1016/j.yexcr.2023.113473.
- Li H, et al. The activation of reticulophagy by ER stress through the ATF4-MAP1LC3A-CCPG1 pathway in ovarian granulosa cells is linked to apoptosis and necroptosis. Int J Mol Sci. 2023;24(3):2749. doi: 10.3390/ijms24032749.
- Wang R, Wang L, Wang L, et al. FGF2 is protective towards cisplatin-induced KGN cell toxicity by promoting FTO expression and autophagy. Front Endocrinol. 2022;13:890623. doi: 10.3389/fendo.2022.890623.
- Liu XH, et al. [Effect of ginsenoside Rg_1 in delaying premature ovarian failure induced by D-gal in mice through PI3K/Akt/mTOR autophagy pathway. ]. Zhongguo Zhong Yao Za Zhi. 2020;45(24):6036–6042.
- Fan R-H, Liu C-G, Zhang Z, et al. Metabolomics analysis of semen cuscutae protection of kidney deficient model rats using ultra high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal. 2022;207:114432. doi: 10.1016/j.jpba.2021.114432.
- Yang Y, Wei Q, An R, et al. Anti-osteoporosis effect of semen cuscutae in ovariectomized mice through inhibition of bone resorption by osteoclasts. J Ethnopharmacol. 2022;285:114834. doi: 10.1016/j.jep.2021.114834.
- Gao F, Zhou C, Qiu W, et al. Total flavonoids from semen cuscutae target MMP9 and promote invasion of EVT cells via notch/AKT/MAPK signaling pathways. Sci Rep. 2018;8(1):17342. doi: 10.1038/s41598-018-35732-6.
- Yang W, Liu R, Sun Q, et al. Quercetin alleviates endoplasmic reticulum Stress-Induced apoptosis in buffalo ovarian granulosa cells. Animals. 2022;12(6):787. doi: 10.3390/ani12060787.
- Luo M, Yang Z-Q, Huang J-C, et al. Genistein protects ovarian granulosa cells from oxidative stress via cAMP-PKA signaling. Cell Biol Int. 2020;44(2):433–445. doi: 10.1002/cbin.11244.
- Wang X, Fan G, Wei F, et al. Hyperoside protects rat ovarian granulosa cells against hydrogen peroxide-induced injury by sonic hedgehog signaling pathway. Chem Biol Interact. 2019;310:108759. doi: 10.1016/j.cbi.2019.108759.
- Nie X, Sheng W, Hou D, et al. Effect of hyperin and icariin on steroid hormone secretion in rat ovarian granulosa cells. Clin Chim Acta. 2019;495:646–651. doi: 10.1016/j.cca.2018.05.004.
- Zhang B, et al. Study on mechanism of cuscutae semen flavonoids in improving reproductive damage of tripterygium glycosides tablets in rats based on high-throughput transcriptome sequencing. Zhongguo Zhong Yao Za Zhi. 2019;44(16):3478–3485.
- Wang Y, Li J, Gu J, et al. Hyperoside, a natural flavonoid compound, attenuates triptolide-induced testicular damage by activating the Keap1-Nrf2 and SIRT1-PGC1α signalling pathway. J Pharm Pharmacol. 2022;74(7):985–995. doi: 10.1093/jpp/rgac011.
- Tan Y, Guo Y, Reed-Maldonado AB, et al. Low-intensity pulsed ultrasound stimulates proliferation of stem/progenitor cells: what we need to know to translate basic science research into clinical applications. Asian J Androl. 2021;23(6):602–610. doi: 10.4103/aja.aja_25_21.
- Qin J, Chen J, Xu H, et al. Low-intensity pulsed ultrasound promotes repair of 4-Vinylcyclohexene diepoxide-induced premature ovarian insufficiency in SD rats. J Gerontol A Biol Sci Med Sci. 2022;77(2):221–227. doi: 10.1093/gerona/glab242.
- Wang XQ, Li Z-N, Wang Q-M, et al. Lipid nano-bubble combined with ultrasound for anti-keloids therapy. J Liposome Res. 2018;28(1):5–13. doi: 10.1080/08982104.2016.1239633.
- Wang YX, et al. Study of TFSC on ovarian function in rat model with premature ovarian failure. Medical Recapitulate. 2019;25(13):2695–2699.
- Wang S, et al. Acupuncture reduces apoptosis of granulosa cells in rats with premature ovarian failure via restoring the PI3K/akt signaling pathway. Int J Mol Sci. 2019;20(24):6311. doi: 10.3390/ijms20246311.
- Xu X, Tan Y, Jiang G, et al. Effects of bushen tianjing recipe in a rat model of tripterygium glycoside-induced premature ovarian failure. Chin Med. 2017;12(1):10. doi: 10.1186/s13020-017-0131-3.
- Xu H, Xia Y, Qin J, et al. Effects of low intensity pulsed ultrasound on expression of B-cell lymphoma-2 and BCL2-Associated X in premature ovarian failure mice induced by 4-vinylcyclohexene diepoxide. Reprod Biol Endocrinol. 2021;19(1):113. doi: 10.1186/s12958-021-00799-w.
- Jiang X-L, Tai H, Kuang J-S, et al. Jian-Pi-Yi-Shen decoction inhibits mitochondria-dependent granulosa cell apoptosis in a rat model of POF. Aging. 2022;14(20):8321–8345. doi: 10.18632/aging.204320.
- Ling L, Feng X, Wei T, et al. Effects of low-intensity pulsed ultrasound (LIPUS)-pretreated human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation on primary ovarian insufficiency in rats. Stem Cell Res Ther. 2017;8(1):283. doi: 10.1186/s13287-017-0739-3.
- Zhou J. The hypothetical molecular pathways of ursolic acid to attenuate the premature ovarian failure in human. Med Hypotheses. 2021;153:110636. doi: 10.1016/j.mehy.2021.110636.
- Niu J, Yu F, Luo X, et al. Human umbilical cord mesenchymal stem cells improve premature ovarian failure through cell apoptosis of miR-100-5p/NOX4/NLRP3. Biomed Res Int. 2022;2022:3862122–3862112. doi: 10.1155/2022/3862122.
- Cai L, et al. Apoptotic mechanism of premature ovarian failure and rescue effect of traditional Chinese medicine: a review. J Tradit Chin Med. 2021;41(3):492–498.
- Qu Q, Liu L, Cui Y, et al. miR-126-3p containing exosomes derived from human umbilical cord mesenchymal stem cells promote angiogenesis and attenuate ovarian granulosa cell apoptosis in a preclinical rat model of premature ovarian failure. Stem Cell Res Ther. 2022;13(1):352. doi: 10.1186/s13287-022-03056-y.
- Yefimova MG, Lefevre C, Bashamboo A, et al. Granulosa cells provide elimination of apoptotic oocytes through unconventional autophagy-assisted phagocytosis. Hum Reprod. 2020;35(6):1346–1362. doi: 10.1093/humrep/deaa097.
- Liu L, Wang H, Xu GL, et al. Tet1 deficiency leads to premature ovarian failure. Front Cell Dev Biol. 2021;9:644135. doi: 10.3389/fcell.2021.644135.
- Lin X, Liu X, Ma Y, et al. Coherent apoptotic and autophagic activities involved in regression of chicken postovulatory follicles. Aging (Albany NY). 2018;10(4):819–832. doi: 10.18632/aging.101436.
- Liu T, Liu Y, Huang Y, et al. miR-15b induces premature ovarian failure in mice via inhibition of α-Klotho expression in ovarian granulosa cells. Free Radic Biol Med. 2019;141:383–392. doi: 10.1016/j.freeradbiomed.2019.07.010.
- Li X, Li X, Deng L. Chrysin reduces inflammation and oxidative stress and improves ovarian function in D-gal-induced premature ovarian failure. Bioengineered. 2022;13(4):8291–8301. doi: 10.1080/21655979.2021.2005991.
- Liu Z, Li F, Xue J, et al. Esculentoside a rescues granulosa cell apoptosis and folliculogenesis in mice with premature ovarian failure. Aging. 2020;12(17):16951–16962. doi: 10.18632/aging.103609.
- Guan S, Zhu Y, Wang J, et al. A combination of semen cuscutae and fructus lycii improves testicular cell proliferation and inhibits their apoptosis in rats with spermatogenic dysfunction by regulating the SCF/c-kit–PI3K–bcl-2 pathway. J Ethnopharmacol. 2020;251:112525. doi: 10.1016/j.jep.2019.112525.
- Li X, Zhong Y, Zhou W, et al. Low-intensity pulsed ultrasound (LIPUS) enhances the anti-inflammatory effects of bone marrow mesenchymal stem cells (BMSCs)-derived extracellular vesicles. Cell Mol Biol Lett. 2023;28(1):9. doi: 10.1186/s11658-023-00422-3.
- Yin N, Wu C, Qiu J, et al. Protective properties of heme oxygenase-1 expressed in umbilical cord mesenchymal stem cells help restore the ovarian function of premature ovarian failure mice through activating the JNK/bcl-2 signal pathway-regulated autophagy and upregulating the circulating of CD8 + CD28- T cells. Stem Cell Res Ther. 2020;11(1):49. doi: 10.1186/s13287-019-1537-x.
- Tutak I, Ozdil B, Uysal A. Voxtalisib and low intensity pulsed ultrasound combinatorial effect on glioblastoma multiforme cancer stem cells via PI3K/AKT/mTOR. Pathol Res Pract. 2022;239(null):154145. doi: 10.1016/j.prp.2022.154145.
- Sun C, Zhang J, Hou J, et al. Induction of autophagy via the PI3K/akt/mTOR signaling pathway by pueraria flavonoids improves non-alcoholic fatty liver disease in obese mice. Biomed Pharmacother. 2023;157(null):114005. doi: 10.1016/j.biopha.2022.114005.
