Abstract
Objective
Estrogen receptor-related receptor γ (ERRγ), is implicated in cancer cell proliferation and metastasis. The function of ERRγ in tumor angiogenesis, however, is to be revealed. This study was designed to elaborate the regulatory effect of ERRγ on angiogenesis in endometrial cancer (EC).
Methods
Immunohistochemistry (IHC) was adopted to determine the protein expression of ERRγ, VEGFA, CD31 and hypoxia-inducible factor-1 (HIF-1) in tumor tissues. HEC-1A cells stably expressing ERRγ were established bytransfection, and then an endothelial cell tube formation assay was performed. CCK-8 assay was employed for cell viability, and wound healing assay for cell migration ability. Besides, western blot, ELISA and qRT-PCR were used to examine the VEGFA expression. After hypoxia treatment of ERRγ overexpressing HEC-1A cells, the ERRγ expression and VEGFA expression were determined by western blot. Finally, EC xenografts in nude mice were constructed by subcutaneous injection of ERRγ stably expressing HEC-1A cells and control HEC-1A cells.
Results
IHC results revealed a negative correlation between the expression of ERRγ and VEGFA in EC tissues. ERRγ overexpression significantly decreased the level of HIF-1 in tumor tissue of nude mice. ERRγ overexpression down-regulated inhibited angiogenesis capability and inhibited the proliferation and migration of HEC-1A cells. Furthermore, ERRγ expression was suppressed under the condition of hypoxia while restoration of ERRγ partially inhibited hypoxia-induced VEGFA expression in HEC-1A cells.
Conclusions
ERRγ is an angiogenesis suppressor and involved in hypoxia-induced VEGFA expression in EC. Hence, ERRγ might be a promising antiangiogenic target for human EC.
Introduction
Endometrial cancer (EC), one of the most common female genital tract malignancies, accounts for 20–30% of all gynecological malignancies. The incidence of EC is increasing worldwide. The growing morbidity of EC makes it necessary to search for effective molecular markers and preventive measures for EC diagnosis and prognosis. As we all know, prolonged or unopposed estrogen exposure is the most important risk factor for EC [Citation1]. In this process, estrogen and estrogen receptors (ERs) exert crucial functions [Citation2].
Angiogenesis, a process of new vasculature formation, supports the sustained growth of tumors including EC. It can not only provide nutrients and oxygen for tumor cells but also is a passage by which cells can remove waste. Vascular endothelial growth factor A (VEGFA) is the most significant pro-angiogenic factor in tumor [Citation3,Citation4], and the transcription of VEGFA can be activated by hypoxia [Citation5]. There is extensive evidence that oxygen availability plays a crucial role in regulating tumor angiogenesis. For instance, a hypoxic intratumoral environment can promote the blood vessel formation by inducing the protein level of VEGFA [Citation5]. Under hypoxic environments, the hypoxia-inducible factor-1 (HIF-1) α is translocated into the nucleus where it heterodimerizes with HIF-1β and binds to hypoxia-responsive elements of various genes, including VEGFA.
Estrogen receptor-related receptor γ (ERRγ) belongs to the orphan nuclear receptor family whose ligand has not been discovered yet [Citation6]. ERRγ is always constitutively activated without endogenous ligand-binding and contain high levels of sequence identity to the estrogen receptors [Citation7]. As a transcriptional factor, ERRγ can directly bind to the promoter sequence and modulate the expression of its target genes. Also, ERRγ s able to regulate a number of genes related to cancer metabolism, energy homeostasis, cell proliferation and metastasis [Citation3]. In addition, ERRγ also promotes angiogenesis in skeletal muscle and retina by elevating the expression of VEGFA [Citation8,Citation9]. Conversely, ERRγ suppresses the expression of VEGFA in placenta tissues of preeclampsia patients [Citation10]. As far as we know, there was no evidence that ERRγ exerted functions in tumor angiogenesis. Some studies have claimed that ERs play essential role in the process of EC vessel formation [Citation2], and ERs and ERRs can identify similar promoter regions and share many target genes [Citation6]. Therefore, we suppose that ERRγ may also function in the angiogenesis of EC.
In the present study, the expression of ERRγ was inversely correlated with VEGFA in EC; and upregulation of ERRγ significantly suppressed angiogenesis by reducing the expression and secretion of VEGFA in vitro and in vivo. In addition, ERRγ also implicated in hypoxia-induced VEGFA expression. All these findings indicated that the emerging functions of ERRγ in EC angiogenesis by virtue of VEGFA regulation.
Materials and methods
Patients and samples
The EC tissues were collected from Wuhan Union Hospital affiliated to Tongji Medical College, Huazhong University of Science and Technology. All tissues were collected under the highest ethical standards with the consent of the patients. Besides, this study was approved the ethics committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No: IORG0003571). Paraffin-embedded tissue samples were obtained from well-established primary EC cases (n = 23), and immunohistochemistry (IHC) was utilized to analyze the protein levels of ERR γ and VEGFA.
Cell culture and hypoxic conditions
The human EC cell lines HEC-1A was purchased from American Type Culture Collection (ATCC). Human umbilical vascular endothelial cells (HUVECs) were obtained from Key-GEN Biotech (Nanjing, China). The HEC-1A cell line was cultured in McCoy’s 5 A (Hyclone, Logan, Utah, USA) containing 10% fetal bovine serum (FBS) and 100 U/ml penicillin/100 μg/ml streptomycin. HUVECs were cultured in RPMI1640 (Hyclone, Logan, Utah, USA) supplemented with 10% FBS. The culture condition was set as a humidified incubator containing 5% CO2 and at 37 °C. Cell hypoxia was carried out in the hypoxia chamber of 1% O2. After removal of cells from hypoxic incubator, conditioned medium (CM), cell total protein and RNA were collected or extracted immediately. ERRγ expressing cells were derived by infecting HEC-1A cells with retroviruses carrying ERRγ gene fragments, and stable cell populations were selected by puromycin (2.5 μg/mL) administration. The transgene efficacy was confirmed by qPCR and Western blot.
Western blot analysis
The cultured EC cells were washed by ice-cold PBS for three times and then lysed in RIPA buffer containing protease inhibitors. The protein concentration was determined using the BCA protein assay kit. The same amount of protein (45 μg) was added to the 10% SDS-PAGE gels and subject to the electrophoresis. Then, the protein samples were transferred onto nitrocellulose membrane, and the membrane was blocked with 5% skimmed milk for 1 h. Afterwards, the membrane was incubated with the primary antibodies against ERRγ (1:800; Abcam, Cambridge, MA, USA), VEGFA (1:400; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (1:800; CST, MA, USA) at 4 °C overnight. On the next day, the membrane was washed and then incubated again with secondary antibodies. Subsequently, the target proteins were visualized by enhanced Chemiluminescence reagents (Thermo fisher Scientific, Waltham, USA).
RNA isolation and quantitative Real-time PCR (qRT-PCR)
The Trizol reagent (Takara, Otsu, Japan) was employed to isolate the total RNA from the cultured EC cells according to the manufacturer’s protocol. Each complementary DNA was synthesized by Prime Script RT reagent kit (Takara, Otsu, Japan). Next, the cDNA(1 μl) was mixed in 10 μl reaction solution containing Premix Ex Taq (Takara, Otsu, Japan). The primers used were shown as follows: ERRγ: 5′-C CCGACAGTGACATCAAAGCC-3′(forward) and 5′-CGTGGAGAAGCCTGGAATATGC −3′(reverse), VEGFA: 5′-GCCTTGCCTTGCTGCTCTAC-3′(forward) and 5′-ATTCTGCCCT CCTCCTTCTGC-3′ (reverse), β-actin: 5′-ATCGTGCGTGACATTAAGGAGAAG-3′ (forward) and 5′-AGGAAG GAAGGCTGGAAGAGTG-3′(reverse). Real-time PCR was conducted with StepOne. The 2−ΔΔCt method was used for relative transcript abundance determination.
Immunohistochemistry (IHC)
Fresh specimens of EC patients or nude mice were fixed in 10% formaldehyde for 24 h and then embedded in paraffin. The tissue was sectioned into four micrometer-thick in size. The sections were dewaxed in xylene and rehydrated by a graded series of ethanol. Antigen was extracted by heating sections in an autoclave for 20 min in 0.01 M sodium citrate buffer. The sections were then immersed in 3% H2O2 for 15 min to block endogenous nonspecific peroxidase activity. Next, nonspecific staining was eliminated by 10% goat serum for 30 min. Then, the sections were incubated with 200 μl primary antibodies against ERRγ (1:400; abcam, Cambridge, MA, USA), VEGFA (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD31 (1:400; proteintech, Wuhan, China) and HIF-1 (1:500; proteintech, Wuhan, China) at 4 °C overnight. After washing with PBS, the sections were incubated with the biotinylated secondary antibody for 30 min. Subsequently, the sections were supplemented with diaminobenzidine substrate for visualizing, followed by counterstaining with hematoxylin. At last, the sections were dehydrated and mounted, and the IHC scoring strategy was performed.
Two independent researchers blinded to the clinical and pathological data were responsible for scoring. ERRγ and VEGFA staining were evaluated by observing both the number of glandular epithelial cells with positive nuclear and cytoplasmic staining and the intensity of the staining in each positive nucleus and cytoplasm. The percentage of positive cells was graded as follows: 0 (0–5%); 1 (5–25%); 2 (26–50%); 3 (51–75%); 4 (75%-100%). The intensity was graded as follows: 0 (negative); 1 (weak); 2 (moderate); 3 (strong). Final scores were calculated by multiplying the two scores above, and the results were divided as follows: negative (‘–’, score 0–1), weak positive (‘+’, score 2–4), moderate positive (‘++’, score 5–8) and strong positive (‘+++’, score 9–12).
Endometrial cancer xenografts
All animal experiments were approved by the Animal Care Committee of Tongji Medical College (approval number: Y20080290). Female BALB/C nude mice aged 4–6 weeks (n = 5 per group) were injected with 5 × 106 ERRγ stable HEC-1A cells and control HEC-1A cells subcutaneously in the right flank. Volume of tumor was calculated as follows: V = length × width2/2 every four days. At 42 days after injection, mice were sacrificed, and tumors were excised, weighed and sectioned. Finally, IHC was performed to analyze the protein levels of ERR γ, VEGFA and CD31.
Enzyme-linked immunosorbent assay (ELISA)
Human VEGFA ELISA kit was purchased from Elabscience. Cell samples were seeded in plates. At 48 h of post-transfection, the medium was replaced by McCoy 5 A, and the cell samples were cultured in hypoxic chamber (1% O2) or conventional incubator (normoxic) for another 24 h. Then the supernatants were collected, and VEGFA secretion in the medium was quantified according to the manufacturer’s instructions.
CCK-8 method
Cell viability was detected using the CCK-8 kit (Beyotime, China). In short, the transfected HEC-1A cells and Ishikawa cells were seeded onto 96 well plates with 1 × 104 cells per well. After treatment with hypoxia as above, the cells were cultured for 24 h at 37 °C and 5% CO2. Then, the CCK-8 solution was added to the corresponding wells, and the incubation was continued for 2 h. Eventually, the absorbance at 450 nm was measured using a microplate reader (Thermo Fisher Scientific, Waltham, USA).
Wound healing test
Cell migration was identified by scratch test. Firstly, HEC-1A cells were seeded in 6-well plates until the fusion degree reached 95%. The adherent cells were scratched by a 10 μL tip, and a wound was formed. Next, the shed cells were washed with PBS for 3 times then cultured in a serum-free medium. Wounded cells were immediately photographed using an inverted microscope (Tokyo, Japan), and representative pictures were collected within 24 h to assess the cells’ ability to migrate by assessing the size of the scratches.
Double luciferase reporter gene assay
Wild-type (WT) pmirGLO-VEGFA with ERRγ binding site was constructed (Ribobio, China). Also, pmirGLO-VEGFA mutant (MUT) with ERRγ binding site mutation was constructed. The reporter vector was then co-transfected into HEC1A cells with ERRγ overexpression vector (ERRγ) or a negative control (Mock). After 48 h of transfection, cells were collected and luciferase assay was performed using a dual luciferase reporter assay kit (Beyotime, China). Lastly, the fluorescence intensity was measured by a F-4500 fluorescence spectrophotometer (Hitachi, Japan).
Endothelial cell tube formation assay
A 96-well plate was coated with 50 ml Matrigel (BD Science, Sparks, MD, USA) per well and kept at 37 °C for 30 min. Then, HUVECs were suspended in the CM and seeded into the pre-coated 96-well plate at a density of 30000 cells per well. The CM was collected from the supernatant of HEC-1A cells cultured under hypoxic or normoxic condition for 24 h. After incubation of cell samples at 37 °C for 6 h, the total length of the capillary-like tubes that had formed were counted and averaged in randomly selected five microscopic fields. Last but not the least, morphological changes of cell samples were observed under a microscope and photographed at 100* magnification.
Statistical analysis
Statistical analysis was conducted by GraphPad Prism v5.0 statistical software. All data were expressed as mean ± SEM (standard error of the mean). Difference among groups was analyzed by Student’s t-test or One-way analysis of variance (ANOVA). Pearson’s coefficient correlation was used to evaluate the association between ERRγ and VEGFA expression levels. p < .05 indicated statistically significant differences.
Results
Inverse correlation of ERRγ expression with VEGFA in EC tissues
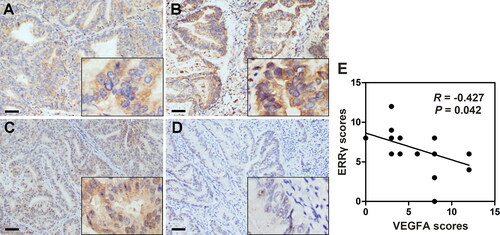
To determine the relationship between ERRγ and VEGFA in EC tissues, IHC staining of ERRγ and VEGFA was performed in 23 tissue specimens derived from EC patients. IHC scores for each patient showed negative correlation between ERRγ and VEGFA expression in paired samples (, Table.S1). Representative weak and intense ERRγ staining images were shown in (A and B), and the VEGFA expression in paired tissues were shown in (C and D). Our clinical data provided the first evidence that ERRγ expression was negatively correlated with VEGFA expression in EC tissues (correlation coefficient R = −0.427, p = .042).
Figure 1. Negative association between ERRγ and VEGFA expression in human endometrial cancer tissue specimens. A–B, Representative immunohistochemical staining for ERRγ in EC tissues with low (A) and high (B) intensity. C–D, Immunohistochemical staining for VEGFA in the same areas of ERRγ (Original magnification: 400 × for the inserts, 100 × for all others; scale bar, 50 μm). E, Histological scores of ERRγ and VEGFA in the 23 EC specimens. ERRγ, estrogen receptor-related receptor γ; VEGFA, vascular endothelial growth factor A; EC, endometrial cancer.
ERRγ reduces the expression and secretion of VEGFA in HEC-1A cells
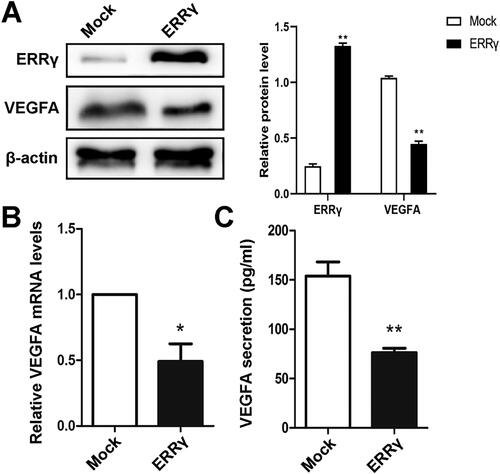
On the basis of above clinical findings, we proposed a potential relationship between ERRγ and VEGFA in EC cells. The expression of ERRγ in EC cell lines was so low that it was undetectable. Therefore, to further investigate ERRγ function and its correlation with VEGFA in EC, we established HEC-1A populations that stably expressed human ERRγ by infecting with a lentivirus containing ERRγ (ERRγ group) and a mock control virus (Mock group) (). The results of western blot and qRT-PCR showed decreased protein and mRNA levels of VEGFA after overexpression of ERRγ in HEC-1A cells (), and such results were consistent with the study by Luo et al. [Citation10]. Moreover, VEGFA secretion in conditioned medium (CM) of HEC-1A cells were also reduced as indicated by ELISA (). These findings indicated that ERRγcould inhibit the expression and secretion of VEGFA in EC cell line HEC-1A.
Figure 2. ERRγ reduces the expression and secretion of VEGFA in HEC-1A cells. A, Western blot analysis of the effect of ERRγ overexpression on protein expression of VEGFA in HEC-1A cells. HEC-1A cells were infected with a lentivirus harboring ERRγ (ERRγ) or a Mock lentivirus (Mock) for 48 h (multiplicity of infection = 2). Protein expression was measured relative to that in Mock-infected cells, with β-actin as the internal control. B, qRT-PCR analysis of the effect of ERRγ overexpression on mRNA expression of VEGFA in HEC-1A cells. C, ELISA analysis of the effect of ERRγ overexpression on secretion of VEGFA in HEC-1A cells. The concentration of VEGFA in the CMs collected from HEC-1A cells infected with a lentivirus harboring ERRγ (ERRγ) or a mock lentivirus (Mock). Protein band densities and RNA level were quantified, and β-actin serves as the internal control. Values represented the means (± SEM) of independent experiments (n = 3–4 per group). Graphs showed protein/mRNA expression relative to that in control cells (Mock). (*p < .05; **p < .01; ***p < .001). ERRγ, estrogen receptor-related receptor γ; VEGFA, vascular endothelial growth factor A; CM, conditioned medium.
ERRγ inhibits angiogenesis in vitro and in tumor xenografts
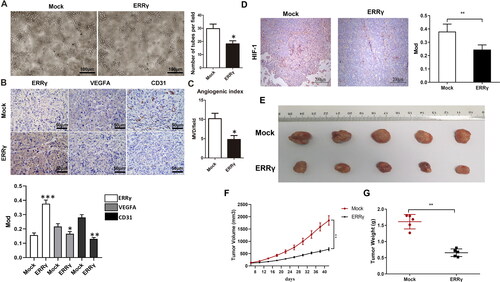
To determine whether ERRγ could alter the angiogenesis capacity of endothelial cells in EC, HUVECs were placed on the basement membrane matrix to trigger capillary-like tube formation in the CMs collected from HEC-1A cells of ERRγ and Mock groups, respectively. Tube formation was quantified by calculating the average number of tubes per field in five randomly chosen fields. As expected, CM from ERRγ group significantly inhibited tube formation and branching of HUVECs (). To confirm this effect in vivo, we constructed EC xenografts in nude mice. IHC were performed to detect the expression of ERRγ, VEGFA, CD31 and HIF-1 in transplanted tumors (). CD31 staining was conducted as a marker of tumor vessels. Specifically, the ERRγ group exhibited a lower angiogenesis index and VEGFA expression than the Mock group in the xenograft tumors (). In addition, compared with the Mock group, the expression level of HIF-1 in tumor tissue of nude mice in the ERRγ group was significantly reduced (). Furthermore, the ERRγ group presented a significant reduction in tumor volume and weight relative to the Mock group (). In a nutshell, the above outcomes demonstrated that upregulation of ERRγ in EC cells impeded angiogenesis by reducing VEGFA secretion in vivo and in vitro.
Figure 3. ERRγ inhibits angiogenesis in vitro and in tumor xenografts. A, Tube formation of HUVECs. 96-well dishes were coated with Matrigel, and HUVEC tube formation assays were performed 6 h after incubation with CMs collected from HEC-1A cells infected with a lentivirus harboring ERRγ or a mock lentivirus (Mock). Left, representative photographs were shown at 100 × magnification. Right, relative number of tubes per field were counted at 100 × magnification. B, Representative microphotographs of ERRγ, VEGFA and CD31 staining in nude mice tumor tissues (magnification: 400 ×, scale bar: 50 μm). C, for evaluation of the angiogenic index in nude mice tumor tissues, the CD31-positive blood vessels in the hotspot areas were counted at 400* magnification. Data represented the mean (± SEM) of 5 grafts in each condition. (*p < .05). D, the expression level of HIF in Mock group and ERRγ group was detected by immunohistochemistry. E–G, Representative tumor images (E), volume (F) and weight (G) of nude mice in Mock and ERRγ groups. (**p < .01). ERRγ, estrogen receptor-related receptor γ; VEGFA, vascular endothelial growth factor A; CM, conditioned medium; HUVEC, human umbilical vascular endothelial cells; HIF, hypoxia-inducible factor.
ERRγ inhibits hypoxia-induced proliferation and migration of HEC-1A cells
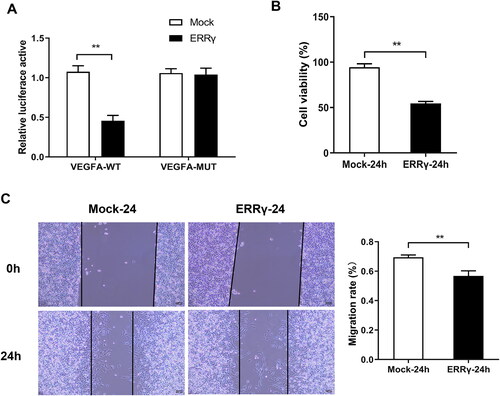
Subsequently, the effect of ERRγ overexpression on the proliferation and migration of HEC-1A cells was investigated under hypoxia conditions. Double luciferase reporter gene experiment revealed a targeted binding relationship between ERRγ and VEGFA (). Besides, results of CCK-8 assay showed that ERR overexpression significantly reduced HEC-1A cell viability compared with the Mock group (). Moreover, the results of wound healing experiment showed that the migration ability of cells in the ERRγ-24h group was significantly reduced compared with the Mock- group (). Consequently, upregulation of ERRγ in hypoxic environment could inhibit the proliferation and migration of EC cells to slow the further development of cancer.
Figure 4. ERRγ inhibits hypoxia-induced proliferation and migration of HEC-1A cells. A, Identification of the interaction ERRγ with VEGFA by double luciferase reporter gene experiment. B, CCK8 method to detect cell viability. C, the wound healing test to cell migration. (**p < .01). ERRγ, estrogen receptor-related receptor γ; VEGFA, vascular endothelial growth factor A.
Hypoxia reduces the ERRγ gene expression in EC cell lines
It has been reported that the ERRs is involved in the transcriptional response to hypoxia [Citation11], and the expression of ERRγin liver cell line HepG2 is increased by hypoxia [Citation12]. Nevertheless, the impact of hypoxia on ERRγ expression in EC has not been explored so far. To evaluate whether hypoxic condition affected the expression of ERRγ, HEC-1A cells were incubated in the hypoxia incubator for different duration. As determined by western blot, after exposure to the hypoxia, the protein level of ERRγ in the ERRγ group was decreased in a time dependent manner (). Due to the low expression of ERRγ in parental HEC-1A cells, there was still no strip of ERR γ in the nitrocellulose membrane of the mock group (). Furthermore, the mRNA level of ERRγ, determined by qRT-PCR, was reduced by half in all of the two groups (ERRγ group and Mock group) after exposure to hypoxia (). Collectively, hypoxic condition could suppress the expression of ERRγ in HEC-1A cells.
Figure 5. Reducing ERRγ expression by hypoxia induction and ERRγ upregulation can abolish hypoxia-induced expression of VEGFA. A, Western blot analysis of the effect of hypoxia on ERRγ protein expression in HEC-1A cells. ERRγ group and Mock group cells were incubated in hypoxic environment at the indicated oxygen levels for 12 h, 24 h, and those incubated in normoxic environment served as control. B, qRT-PCR analysis of the effect of hypoxia on ERRγ mRNA expression in HEC-1A cells. C, ELISA analysis of the effect of hypoxia on VEGFA secretion in HEC-1A cells. D, Western blot analysis of the effect of ERRγ overexpression on hypoxia-induced VEGFA protein expression. E, qRT-PCR analysis of the effect of ERRγ overexpression on hypoxia-induced VEGFA mRNA expression. F, ELISA analysis of the effect of ERRγ overexpression on hypoxia-induced VEGFA secretion. Protein band densities and RNA level were quantified, with β-actin as the internal control. Values represented the means (± SEM) of independent experiments (n = 3–4 per group). Graphs showed protein/mRNA expression relative to that in control cells. (*p < .05; **p < .01; ***p < .001).
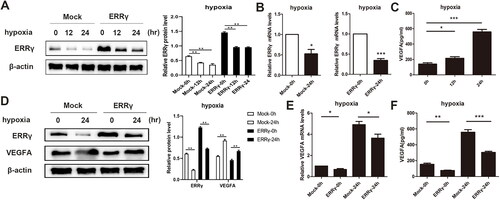
ERRγ inhibits hypoxia-induced VEGFA expression in HEC-1A cells
It is well established that hypoxia can induce tumor angiogenesis through upregulating VEGFA expression [Citation5]. What’s more, there is a research that retinal hypoxia can induce the expression of vascular endothelial growth factor through induction of ERRγ [Citation9]. Our aforementioned results indicated that ERRγ could inhibit the angiogenesis by reducing the VEGFA expression, and hypoxia condition reduced the ERRγ expression in the endometrial cell line HEC-1A. Based on these findings, we speculated that ERRγ may be involved in hypoxia-induced VEGFA expression. Consistent with the results of other research, hypoxia treatment increased the secretion of VEGFA in parental HEC-1A cells (). To further confirm whether hypoxia induced the expression of VEGFA through inhibition of ERRγ in EC, the effect of ERRγon hypoxia-induced VEGFA expression was explored by upregulation of ERRγ. Consistent with our conjecture, lentivirus-mediated upregulation of ERRγ significantly reduced the expression and secretion of VEGFA induced by hypoxia (). All above outcomes indicated that ERRγ could block hypoxia-induced VEGFA expression. Taking together, ERRγ may be involved in the hypoxia-induced angiogenesis in EC.
Discussion
ERRs, consisting of ERRα, ERRβ and ERRγ, are members of the nuclear receptor superfamily. Correlations between the expression of ERRs and diverse parameters of tumor progression, especially for ERRα and ERRγ, have already been revealed [Citation3]. Previous studies proposed that, ERRs, as critical regulators of metabolic programs, affected tumor cell proliferation, migration and metastasis [Citation3,Citation13]. Additionally, overexpression of ERRα was always associated with poor prognosis while elevated ERRγ expression heralded better prognosis in tumors such as breast cancer, ovarian cancer and prostate cancer [Citation14–17]. However, the role of ERRγ in the EC progression is still unclear as there are few investigations about it. In this study, we first focused on the role of ERRγ in the angiogenesis of EC and discovered that ERRγ acted as an angiogenesis suppressor in EC.
The growth of EC is dependent on the development of neovessels. The development of neovessels is also termed angiogenesis, which can not only provide oxygen and nutrients to cells but also an avenue for cells to remove waste. VEGFA is the most critical pro-angiogenic factor in cancer. A previous research announced that VEGFA was overexpressed in 56–66% of EC tissues [Citation18]. What’s more, VEGFA is an independent predictor of a poor prognosis in the EC patients [Citation19]. However, the role of ERRγ in angiogenesis is still undetermined. Regard to the correlation between ERRγ and VEGFA expression, controversial conclusions were obtained in different tissues [Citation8–10]. Moreover, there was no evidence that ERRγ played a role in tumor angiogenesis. In the present study, we explored the role of ERRγ in the angiogenesis of EC and first validated a negative correlation between ERRγ and VEGFA expression in EC.
On basis of our above clinical findings, we proposed a potential correlation between ERRγ and VEGFA in EC. However, the ERRγ protein was hardly detected in EC cell lines. To explore the function of ERRγ in angiogenesis at the cellular level, a cell line stably expressing ERRγ was established by infecting with lentivirus in HEC-1A cells. According to the detection results, overexpression of ERRγ remarkably inhibited the expression and secretion of VEGFA. Moreover, the HEC-1A cells stably expressing ERRγ inhibited angiogenesis both in vitro and in vivo as verified by endothelial cell tube formation assay and xenografts tumors microvessel quantity comparison. These findings elucidated that ERRγcould inhibit the pro-angiogenic property of HEC-1A cells by reducing VEGFA secretion. Out results were opposite with the study in skeletal muscle and ischemic retinopathies but is consistent with that in placenta [Citation8–10].Therefore, the effect of ERRγ on VEGFA may differ among tissues. Although we first verified the role of ERRγ in EC angiogenesis, the specific molecular mechanism about how ERRγ regulated the VEGFA expression remained to be revealed in this research. As researches have reported a conserved binding site for ERRγ on the promoter of VEGFA gene [Citation8], we speculate that ERRγ may transcriptionally regulate the expression of VEGFA in EC. Such speculation deserves a further exploration.
Oxygen availability plays an important role in regulating tumor angiogenesis, and this is mediated primarily by the transcription factor HIF-1 according to the previous research [Citation5]. HIF-1 allows trans-activation of pro-angiogenic factors, among which the most notable is VEGFA. It has been reported that VEGFA is directly up-regulated by ERRα under hypoxia condition through a cooperative interaction with PGC-1a which occurs independently of the HIF in muscle [Citation20,Citation21]. Also, we hypothesized that ERRγ may be involved in hypoxia-induced VEGFA expression as ERRα and ERRγ often had opposite effect on the same aspect. To verify this hypothesis, HEC-1A cells with low and high expression of ERRγ were cultured in hypoxic condition. Based on the observation results, hypoxia remarkably decreased the protein level of ERRγ in HEC-1A cells. Furthermore, overexpression of ERRγ could partly abolish hypoxia-induced VEGFA expression. Overall, ERRγ may participate in the regulation of hypoxia-induced VEGFA expression.
In conclusion, this study have revealed an important angiogenesis-suppressive function of ERRγ in EC for the first time, and provided clinical evidences. Moreover, ERRγ has been discovered to be involved in the regulation of hypoxia-induced VEGFA expression. However, the exact molecular mechanism underlying the regulatory effect of ERRγ on VEGFA expression and hypoxia-induced VEGFA expression process requires further investigation. Even so, this study has provided a novel mechanistic insight into hypoxia-induced tumor angiogenesis, which may open new avenues for cancer therapy by targeting angiogenesis.
Supplemental Material
Download MS Word (18 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Akhmedkhanov A, Zeleniuch-Jacquotte A, Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer: review of the evidence and research perspectives. Ann NY Acad Sci. 2001;943(1):1–7. doi:10.1111/j.1749-6632.2001.tb03811.x.
- He YY, Du GQ, Cai B, et al. Estrogenic transmembrane receptor of GPR30 mediates invasion and carcinogenesis by endometrial cancer cell line RL95-2. J Cancer Res Clin Oncol. 2012;138(5):775–783. doi:10.1007/s00432-011-1133-7.
- Tam IS, Giguere V. There and back again: the journey of the estrogen-related receptors in the cancer realm. J Steroid Biochem Mol Biol. 2016;157:13–19. doi:10.1016/j.jsbmb.2015.06.009.
- Mahecha AM, Wang H. The influence of vascular endothelial growth factor-A and matrix metalloproteinase-2 and -9 in angiogenesis, metastasis, and prognosis of endometrial cancer. Onco Targets Ther. 2017;10:4617–4624. doi:10.2147/OTT.S132558.
- Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2016;2(12):758–770. doi:10.1016/j.trecan.2016.10.016.
- Giguere V, Yang N, Segui P, et al. Identification of a new class of steroid hormone receptors. Nature. 1988;331(6151):91–94. doi:10.1038/331091a0.
- Giguere V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29(6):677–696.
- Matsakas A, Yadav V, Lorca S, et al. Revascularization of ischemic skeletal muscle by estrogen-related receptor-gamma. Circ Res. 2012;110(8):1087–1096. doi:10.1161/CIRCRESAHA.112.266478.
- Do JY, Choi YK, Kook H, et al. Retinal hypoxia induces vascular endothelial growth factor through induction of estrogen-related receptor gamma. Biochem Biophys Res Commun. 2015;460(2):457–463. doi:10.1016/j.bbrc.2015.03.055.
- Luo Y, Kumar P, Chen CC, et al. Estrogen-related receptor gamma serves a role in blood pressure homeostasis during pregnancy. Mol Endocrinol. 2014;28(6):965–975. doi:10.1210/me.2014-1003.
- Ao A, Wang H, Kamarajugadda S, et al. Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc Natl Acad Sci USA. 2008;105(22):7821–7826. doi:10.1073/pnas.0711677105.
- Lee JH, Kim EJ, Kim DK, et al. Hypoxia induces PDK4 gene expression through induction of the orphan nuclear receptor ERRgamma. PLoS One. 2012;7(9):e46324. doi:10.1371/journal.pone.0046324.
- Ang J, Sheng J, Lai K, et al. Identification of estrogen receptor-related receptor gamma as a direct transcriptional target of angiogenin. PLoS One. 2013;8(8):e71487. doi:10.1371/journal.pone.0071487.
- Deblois G, Giguere V. Oestrogen-related receptors in breast cancer: control of cellular metabolism and beyond. Nat Rev Cancer. 2013;13(1):27–36. doi:10.1038/nrc3396.
- Misawa A, Inoue S. Estrogen-related receptors in breast cancer and prostate cancer. Front Endocrinol. 2015;6:83. doi:10.3389/fendo.2015.00083.
- Sun P, Wei L, Denkert C, et al. The orphan nuclear receptors, estrogen receptor-related receptors: their role as new biomarkers in gynecological cancer. Anticancer Res. 2006;26(2C):1699–1706.
- Audet-Walsh E, Yee T, McGuirk S, et al. Androgen-dependent repression of ERRgamma reprograms metabolism in prostate cancer. Cancer Res. 2017;77(2):378–389. doi:10.1158/0008-5472.CAN-16-1204.
- Hirai M, Nakagawara A, Oosaki T, et al. Expression of vascular endothelial growth factors (VEGF-A/VEGF-1 and VEGF-C/VEGF-2) in postmenopausal uterine endometrial carcinoma. Gynecol Oncol. 2001;80(2):181–188. doi:10.1006/gyno.2000.6056.
- Kamat AA, Merritt WM, Coffey D, et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res. 2007;13(24):7487–7495. doi:10.1158/1078-0432.CCR-07-1017.
- Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451(7181):1008–1012. doi:10.1038/nature06613.
- Perry MC, Dufour CR, Tam IS, et al. Estrogen-related receptor-alpha coordinates transcriptional programs essential for exercise tolerance and muscle fitness. Mol Endocrinol. 2014;28(12):2060–2071. doi:10.1210/me.2014-1281.
