Abstract
The aim of this study was to investigate the effects of positive anti-thyroid peroxidase (TPO) antibodies on fertility, embryo quality, and pregnancy outcomes in women with normal thyroid function. A cross-sectional study of 1223 infertile women who received assisted reproductive technology (ART) treatment for the first time was conducted at our hospital from January 2019 to March 2022. Overall, 263 infertile women were included, comprising 263 cycles and 1813 embryos, and were divided into a positive group and a control group based on TPO antibody levels. The positive group was further divided into two subgroups according to the median antibody titer, and the therapeutic indices and pregnancy outcomes for each group were compared. The results showed that the AMH level in the positive group was significantly lower than that in the control group (2.37 (1.26-3.63) ng/ml vs. 3.54 (1.74-5.41) ng/ml, p < 0.001). The high-quality embryo rate (40.04% vs. 45.49%, p = 0.034) and live birth rate (23.26% vs. 36.16, p = 0.035) of the positive group were lower than those of the control group; the miscarriage rate was higher than that of the control group (37.50% vs. 17.95%, p = 0.035). The live birth rate in the low-titer group was significantly higher than that in the high-titer group (32.56% vs. 13.95%, p = 0.041). Studies have shown that positive anti-thyroid peroxidase antibodies are associated with a decreased ovarian reserve and decreased embryo quality. High titers of anti-thyroid peroxidase antibodies can reduce the live birth rate.
Introduction
Anti-thyroid antibodies (ATAs), mainly anti-thyroid peroxidase antibodies (TPOAb) and anti-thyroglobulin antibodies (TGAb), can be detected in the serum of 9–15% of women of childbearing age [Citation1] and in 14–27% of women with premature ovarian failure [Citation2]. The presence of ATAs is medically characterized as thyroid autoimmunity (TAI). Numerous studies have demonstrated that the presence of ATAs may negatively affect ovarian reserve [Citation3,Citation4], but the literature evaluating the relationship between ATAs and ovarian reserve has reported conflicting results. Some scholars have indicated that the presence of thyroid autoantibodies can lead to excessive or low ovarian reserve [Citation5]. However, a recent large cross-sectional study did not find differences in ovarian reserve between ATA-positive and ATA-negative groups [Citation6]. According to the findings, the presence of ATAs may have an impact on the success of IVF treatment cycles [Citation7]. It is currently unknown how ATAs affect the ability to conceive in infertile women with healthy thyroid function. Some studies have indicated that when these women use assisted reproductive technology and successfully become pregnant, the presence of ATAs can cause infertility and even early termination of embryos [Citation8,Citation9]. However, Muller, Negro, et al. evaluated the rate of abortion among women who had IVF/ICSI pregnancies and concluded that there was no distinction between the rates of abortion among ATA-positive and ATA-negative women [Citation10,Citation11]. In contrast, in a meta-analysis of 12 studies, Busnelli et al. statistically analyzed the association between thyroid autoimmunity and IVF/ICSI clinical outcomes and concluded that the presence of antithyroid antibodies increased the risk of early miscarriage and decreased the live birth rate [Citation12]. However, the above studies did not classify thyroid autoimmune antibodies, and the effects of TPOAb and TGAb on pregnancy may interact. In addition, none of the above literature showed how the titers of thyroid autoantibodies would affect pregnancy outcomes.
The goal of this cross-sectional study was to determine whether TPOAb positivity alone affects the fertility reserve of euthyroid women (AMH) as well as the relationship between TPOAb positivity and indicators related to IVF/ICSI-assisted reproductive outcomes. The number of oocytes retrieved, embryo quality, clinical pregnancy rate, miscarriage rate, and live birth rate were assessed. In addition, a subgroup analysis was performed based on the median TPOAb titers to study the effect of TPOAb titers on IVF/ICSI pregnancy outcomes.
Materials and methods
Research subjects
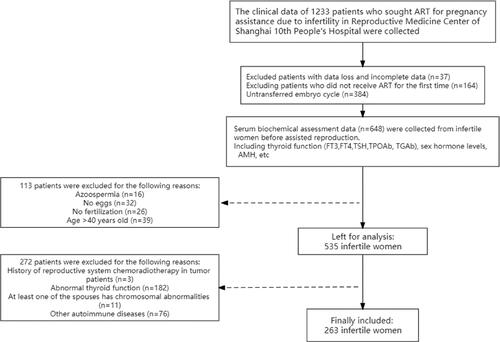
All data were obtained from Shanghai Tenth People’s Hospital, a public university-affiliated hospital in Shanghai, China. The present study analyzed data from a cross-sectional study of infertile women (January 2019 to March 2022), which was prospectively collected based on the medical history and clinical data of infertile women undergoing ART. Measurements of thyroid stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4), TPOAb (normal range 40 IU/mL), and TGAb (normal range < 110 IU/mL) were included. In addition, serum biochemical assessments, including basal sex hormone levels, anti-Mullerian hormone (AMH), infertility history, and demographic data, were recorded on Days 2 or 3 of the menstrual cycle before assisted reproduction. During the study period, 263 of the 1233 women from the Reproductive Medicine Center of Shanghai Tenth People’s Hospital were included in the study, and 1813 embryos were scored for quality.
The inclusion criteria were as follows: ① use of a standardized controlled ovulation stimulation (COS) process and successful acquisition of oocytes with a diameter of more than 16 mm; ② fresh embryo transfer or frozen-thawed embryo transfer; ③ normal thyroid test indicators (TSH: 0.38-4.34 mIU/L, FT3: 2.8-6.39 pmol/L, FT4: 10.5-24.4 pmol/L) and TGAb range; ④ no other autoimmune disease (such as antiphospholipid antibody syndrome) symptoms, rheumatoid arthritis, etc.; and ⑤ no contraindication for the use of estrogen or progesterone. The exclusion criteria were as follows: ① not meeting any of the above criteria; ② abnormal parental karyotype analysis; ③ missing treatment data or follow-up data; and ④ history of thyroid-related drug use (e.g. levothyroxine) during follow-up.
illustrates the study selection process with a flowchart.
Groupings
Patients were divided into 2 groups according to their TPOAb level: the TPOAb-positive group (TPOAb > 40 IU/mL) and the TPOAb-negative group. Then, they were divided into a high-titer group (TPOAb ≥ 297.40 IU/mL) and a low-titer group (TPOAb < 297.40 IU/mL) according to the median antibody titers of the TPOAb-positive group.
Assisted reproductive technology procedures
All patients underwent a standardized COS procedure, egg retrieval, and fertilization, followed by fresh embryo transfer or frozen-thawed embryo transfer. After the application of ovulation induction drugs, the development of follicles was detected by vaginal ultrasound, and when two or more dominant follicles grew to 17–18 mm, recombinant HCG 250 mg (eiser, Germany) was injected to trigger oocyte maturation. After 34–36 h, the eggs were retrieved by transvaginal ultrasound. On the day of egg retrieval, the partner’s sperm was collected, and according to the quality of the sperm, insemination was carried out by conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI)
Fresh embryo transfer
After successful fertilization, embryo culture was continued for another 3–5 days before 1-2 cleavage embryos or blastocysts were transferred
Frozen-thawed embryo transfer
If a woman was affected by various factors or the patient’s status did not allow for fresh embryo transfer, routine monitoring of follicular development was recommended. On the 3rd or 5th day after ovulation, according to the type of frozen embryo, cleavage embryos or blastocysts were transferred
Observation indicators
Serum HCG levels were measured 12 days after embryo transfer to confirm pregnancy. β-HCG levels greater than 10 U/L indicated a biochemical pregnancy. Transvaginal ultrasonography was performed 3 weeks after embryo transfer, and it was found that the gestational sac and original cardiac tube beat were signs of clinical pregnancy. A miscarriage was defined as the loss of a clinical pregnancy during the gestation period. A live birth was defined as a complete pregnancy with successful delivery.
Laboratory testing
All blood samples were analyzed in the examination department of the hospital. TSH, FT3, and FT4 levels were measured with an automated chemical radiation immune analyzer (Immulite 1000, Siemens, Germany), and TPOAb and TGAb levels were measured with a magnetic particle radiation chemical analyzer (Autolumo A2000, Antubio, China). The levels of sex hormones such as E2, FSH, LH, and AMH were measured with an electrochemical radiant immune analyzer (Cobas e601, Roche Diagnostics, Germany).
Statistical processing
SPSS 26.0 statistical software was used to analyze the patient data. Quantitative data with a normal distribution are represented by the mean (standard deviation [SD]), and quantitative data that did not conform to a normal distribution are represented by the median (interquartile range) [M (P25, P75)]. Enumeration data are expressed as rates (%). Continuous variables were compared using the t test or Mann–Whitney U test, and categorical variables were compared using the chi-square test. Logistic regression analysis was performed to calculate odds ratios (ORs) for 95% confidence intervals after adjusting for correlation factors and analyzing the association between TPOAb levels and pregnancy outcome (live birth). p < 0.05 was regarded as statistically significant.
Results
General information
A total of 263 patients were included in the study, of whom 177 (67.3%) were TPOAb negative and 86 (32.7%) were TPOAb positive. There was no significant difference in age, body mass index (BMI), basal sex hormone levels, or antral follicle count (AFC) between the two groups (p > 0.05). There were no significant differences in thyroid function-related indices (FT3, FT4, and TSH) or the causes of infertility. The AMH level in the TPOAb-positive group was 2.37 (1.26–3.63) ng/ml, while the level in the control group was 3.54 (1.75–4.41) ng/ml, with a statistically significant difference (p < 0.001) ().
Table 1. Comparison of baseline characteristics of TPOAb-positive and TPOAb-negative women.
Cycle treatment indicators and pregnancy outcomes
The differences in days of gonadotropin (Gn) use, egg cleavage rate, endometrial thickness on the day of transfer, number of embryos transferred, biochemical pregnancy rate, and clinical pregnancy rate were not statistically significant between the two groups (p > 0.05). The number of eggs obtained in the TPOAb-positive group (8 (5–12) vs. 10 (6–15), p = 0.017), the rate of superior embryos (40.04% vs. 45.49%, p = 0.034), and the live birth rate (23.26% vs. 37.85%, p = 0.025) were significantly lower than those in the TPOAb-negative group, and the fertilization rate (93.97% vs. 90.70%, p = 0.010) and abortion rate (37.50% vs. 18.29%, p = 0.048) were significantly higher in the TPOAb-positive group than in the control group (p < 0.05) ().
Table 2. Comparison of IVF/ICSI cycle treatment indicators and pregnancy outcomes in TPOAb-positive and TPOAb-negative women.
TPOAb titers and pregnancy outcome
Subgroup analysis was conducted according to the median antibody titers of the TPOAb-positive group, and there was no significant difference in the clinical pregnancy rate between the high-titer group and the low-titer group (p > 0.05). The abortion rate in the high-titer group (53.85%) was 2.05 times that in the low-titer group (26.32%), but the difference was not statistically significant (p > 0.05). The live birth rate of the high-titer group was significantly lower than that of the low-titer group (13.95% vs. 32.56%, p = 0.041), and the difference was statistically significant (p < 0.05) ().
Table 3. Subgroup analyses of pregnancy outcomes according to median TPOAb titers.
Correlation between univariate analysis and live birth
To investigate the factors influencing the outcome of live birth in patients with normal thyroid function and TPOAb positivity, we performed a univariate analysis of relevant variables that may affect the live birth outcome and found no significant differences between the live birth group and the non-live birth group in terms of type of transfer, BMI, number of transferred eggs, number of MII eggs, number of fertilized eggs, and number of high-quality embryos. Logistic regression analysis was performed with live birth as the dependent variable and variables that differed significantly in univariate analysis, such as the number of eggs obtained, number of egg clefts, TPOAb titer, and baseline variables related to clinical live birth, such as age, as covariates. The results showed that there was a statistically significant effect of the number of oocytes (OR = 1.145, 95% CI: 1.011 ∼ 1.298, p < 0.05) and the TPOAb titer (OR = 0.336, 95% CI: 0.115 ∼ 0.982, p < 0.05) on the live birth outcome ().
Table 4. Logistic regression analysis to Identify factors affecting live birth outcomes.
Discussion
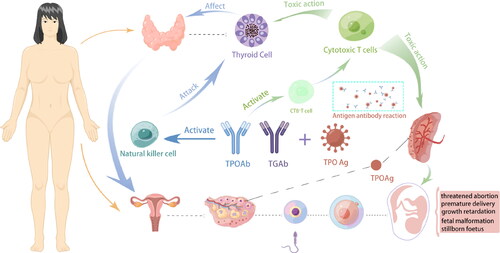
Compared with TGAb, TPOAb are more prevalent in infertile women, and most reports link them to diminished female reproductive function and poor outcomes of assisted reproductive technology. However, the specific impact mechanism of ATAs on the clinical outcomes of IVF/ICSI is not yet clear. According to relevant research articles, we have summarized several possible mechanisms, as shown in . One is that the presence of thyroid autoantibodies can cause an imbalance in the autoimmune system of women with TAI. This imbalance can lead to changes in the proportion of certain cells in the body, and the activation of natural killer (NK) cells and natural killer-like (NK-like) T cells also affects pregnancy outcomes negatively [Citation13]. Second, the presence of thyroid autoantibodies can lead to insufficient thyroid reserve, making it difficult for the thyroid to make adaptive changes during COS and early pregnancy [Citation14], and there are reports that human chorionic gonadotropin (HCG) may also be affected by TPOAb [Citation15]. Third, it has been suggested that the risk of adverse pregnancy is increased because of the older age of TAI patients [Citation16], and advanced age, not thyroid autoantibodies, has been reported to be the factor affecting pregnancy [Citation17].
There are many indicators for evaluating ovarian reserve function, including the antral follicle count, FSH level, and AMH level. In recent years, many studies worldwide have proposed that AMH can be used to effectively predict ovarian response according to multivariate regression models, and its predictive value is better than that of the FSH level and the AFC, which have become the preferred methods for assessing ovarian reserve [Citation18–20]. AMH was chosen to characterize the ovarian reserve in infertile patients in our study due to its objectivity and stability throughout the menstrual cycle. We found that the AMH level of the TPOAb-positive patients was significantly lower than that of the control patients, indicating that the TPOAb-negative patients had a higher ovarian reserve. A 2015 article focussed on the relationship between AITD and ovarian reserve (AMH) investigated the correlation between ovarian function (AMH) and TPOAb levels in 5000 women and found no relationship between TPOAb positivity and low ovarian reserve [Citation6]. However, many current reports show that TPOAb positivity is the most common autoimmune dysfunction in patients with reduced ovarian reserve [Citation8,Citation21,Citation22], and it is often found in clinical diagnosis and treatment that patients with premature ovarian failure have autoimmune thyroid disease (AITD). This is consistent with the findings of AYESHA et al. [Citation23], who also indicated that the presence of TPOAb would harm the ovarian reserve of patients. In addition, we compared the number of antral follicles between the two groups. Although there was no significant difference between the two groups, the number of antral follicles in the TPOAb-positive group was slightly lower than that in the control group. In addition, we found that, compared with the TPOAb-negative group, the TPOAb-positive group exhibited lower ovarian responsiveness, more total gonadotropin (Gn) requirements, and lower oocyte retrieval at the same stimulation time. This finding was consistent with the report of Revelli et al. [Citation24]. It is speculated that TAI patients are in a state of immune imbalance during COS, and follicular development and follicle quality may be affected, which was confirmed in a correlation study [Citation13]. In 2011, a research team reported that thyroid autoantibodies (TGAb and TPOAb) were found in follicular fluid and that their concentrations were positively correlated with serum antibody levels [Citation25]. Researchers have reported that during follicular maturation, ATAs cross the blood-follicular barrier and reach the follicular fluid and that the cytotoxic effects of ATAs have deleterious effects on egg cell quality. The research team further detected the expression of thyroid peroxidase (TPO) on the surface of granulosa cells in the follicular fluid of infertile patients, which indicates that the TPO antigen on ovarian tissue may be attacked by TPOAb, resulting in compromised egg cell quality [Citation26].
In this study, we found that the presence of TPOAb had a significant effect on embryo quality in women with normal thyroid function with an ART-assisted pregnancy, which was consistent with the results of Weghofer et al. [Citation27]. TPOAb have been reported to reduce the rate of optimal embryos in women with normal thyroid function who received ICSI. In addition, we found that the fertilization rate of the TPOAb-positive group was higher than that of the control group, but the cleavage rate was comparable to that of the control group, suggesting that TPOAb played a negative role in the development of fertilized eggs into cleavage embryos. It has been reported that the rate of superior embryos in IVF cycles is significantly lower than that in ICSI cycles, possibly due to immaturity in the oocytes retrieved and fertilized in IVF cycles [Citation28]. However, some studies have reported that the proportion of high-quality embryos is comparable regardless of IVF or ICSI [Citation29], but this study did not differentiate the TAI status of patients. In addition, we found that, compared with control women, TPOAb-positive patients had a lower rate of high-quality embryos, while clinical pregnancy rates were comparable between the two groups. Therefore, the presence of TPOAb may have a greater negative impact on the presence of embryonic early developmental processes [Citation30].
The effect of TAI on IVF/ICSI outcomes has been widely reported, but the findings remain controversial. Some scholars have suggested that ATA-positive women have significantly lower clinical pregnancy rates and live birth rates than ATA-negative women [Citation22,Citation31,Citation32]; however, five studies found that ATA positivity did not affect clinical pregnancy rates and live birth rates [Citation11,Citation33–36]. Two other studies showed no difference in the miscarriage rate between ATA-positive and ATA-negative women [Citation34,Citation36], but four studies showed a significantly higher miscarriage rate in ATA-positive women than in ATA-negative women [Citation22,Citation36–39]. Especially in Thangaratinam’s study, it was found that the miscarriage rate in the ATA-positive group was more than double that of the ATA-negative group [Citation39]. In our study, we found that after excluding TGAb-positive ATA patients, TPOAb positivity alone did not affect the clinical pregnancy rate in euthyroid women, but the TPOAb-positive group had higher rates of miscarriage and fewer live births. It is speculated that the development process of the embryo after implantation may be affected by TPOAb, leading to abortion [Citation40]. When we performed a subgroup analysis, we found significant differences in live birth rates between the TPOAb high-titer and low-titer groups. In contrast to our findings, Chai J et al. [Citation35] discovered that thyroid autoantibody titers did not affect IVF/ICSI live birth outcomes. The difference in the results may be caused by the differences in the enrolled patients. Our research subjects were patients with thyroid indices within the normal reference value ranges. However, their study included patients with subclinical hypothyroidism or subclinical hyperthyroidism, which may be due to the interaction of TSH with TPOAb, resulting in no significant effect of different titers of TPOAb on IVF/ICSI outcomes.
The strength of our study is that it was conducted in a real-world setting, including different causes of infertility and different types of assisted reproductive technology (IVF/ICSI). The inclusion criteria were very strict, and we excluded women with positive TGAb, which can also cause thyroid autoimmunity, and women treated with LT4 were excluded. In addition, we determined that thyroid function, thyroid autoimmune antibodies, and basal sex hormone levels should be measured only on Day 2 or Day 3 of the natural menstrual cycle before ovarian stimulation. In other studies, this time node is often omitted. In addition, data from our medical-record control study were collected prospectively. Of course, our study has some limitations, and there were slight differences in the fertilization techniques used among the enrolled patients. The fertilization rate may be affected to some extent after oocytes undergo IVF or ICSI treatment. Moreover, considering that this is a cross-sectional study, we used the most stringent inclusion criteria, and of course, a reduction in sample size was difficult to avoid. As a result, we will conduct additional prospective studies in the future to compare the embryo quality difference of IVF/ICSI treatment in patients with normal thyroid function and TPOAb positivity as well as changes in TPOAb levels in patients after pregnancy to confirm our findings.
Overall, this study shows that in infertile women with normal thyroid function, simple anti-thyroid peroxidase antibody positivity is associated with decreased ovarian reserve function and decreased embryo quality and that such women have a significantly higher rate of miscarriage while on ART. In particular, the presence of high titers of TPOAb is a risk factor for live birth outcomes. Therefore, it is necessary to use TPOAb as a routine screening indicator for infertile women undergoing ART, even if the patient’s routine thyroid hormone tests are normal.
Ethics approval consent to participate
The study was carried out following the principles of the Declaration of Helsinki. Approved by the Research Ethics Committee of Shanghai 10th People’s Hospital affiliated with Tongji University (Ethics approval number: SHYS-IEC-5.0/22K163/P01). Informed consent was obtained from all individual participants included in the study.
Authors’ contribution
Kun Qiao: study design, manuscript writing, and critical discussion.
Shi-Xi Wei and Ling Wang: study design, data collection, data analysis, manuscript writing, and critical discussion. Contributed equally to this work.
Yu-Bing Liu, Qiu-Lin Fan, Yu Fan: data collection and manuscript comment.
All authors read and approved the final manuscript.
Consent for publication
All authors give their consent to publish the article.
Availability of data and material
The dataset(s) supporting the conclusions of this article is(are) included within the article (and its additional file(s)).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Stagnaro-Green A, Roman SH, Cobin RH, et al. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA. 1990;264(11):1–7. doi: 10.1001/jama.1990.03450110068029.
- Kelkar RL, Meherji PK, Kadam SS, et al. Circulating auto-antibodies against the zona pellucida and thyroid microsomal antigen in women with premature ovarian failure. J Reprod Immunol. 2005;66(1):53–67. doi: 10.1016/j.jri.2005.02.003.
- Novosad JA, Kalantaridou SN, Tong ZB, et al. Ovarian antibodies as detected by indirect immunofluorescence are unreliable in the diagnosis of autoimmune premature ovarian failure: a controlled evaluation. BMC Women’s Health. 2003;3(1):2. doi: 10.1186/1472-6874-3-2.
- Goswami R, Marwaha RK, Goswami D, et al. Prevalence of thyroid autoimmunity in sporadic idiopathic hypoparathyroidism in comparison to type 1 diabetes and premature ovarian failure. J Clin Endocrinol Metab. 2006;91(11):4256–4259. doi: 10.1210/jc.2006-1005.
- Huang N, Chen L, Lian Y, et al. Impact of thyroid autoimmunity on in vitro fertilization/intracytoplasmic sperm injection outcomes and fetal weight. Front Endocrinol (Lausanne). 2021;12:698579. doi: 10.3389/fendo.2021.698579.
- Polyzos NP, Sakkas E, Vaiarelli A, et al. Thyroid autoimmunity, hypothyroidism and ovarian reserve: a cross-sectional study of 5000 women based on age-specific AMH values. Hum Reprod. 2015;30(7):1690–1696. doi: 10.1093/humrep/dev089.
- Vissenberg R, Manders VD, Mastenbroek S, et al. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum Reprod Update. 2015;21(3):378–387. doi: 10.1093/humupd/dmv004.
- Kim CH, Ahn JW, Kang SP, et al. Effect of levothyroxine treatment on in vitro fertilization and pregnancy outcome in infertile women with subclinical hypothyroidism undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2011;95(5):1650–1654. doi: 10.1016/j.fertnstert.2010.12.004.
- van den Boogaard E, Vissenberg R, Land JA, et al. Significance of (Sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2011;17(5):605–619. doi: 10.1093/humupd/dmr024.
- Muller AF, Verhoeff A, Mantel MJ, et al. Thyroid autoimmunity and abortion: a prospective study in women undergoing in vitro fertilization. Fertil Steril. 1999;71(1):30–34. doi: 10.1016/s0015-0282(98)00394-x.
- Negro R, Formoso G, Coppola L, et al. Euthyroid women with autoimmune disease undergoing assisted reproduction technologies: the role of autoimmunity and thyroid function. J Endocrinol Invest. 2007;30(1):3–8. doi: 10.1007/BF03347388.
- Busnelli A, Paffoni A, Fedele L, et al. The impact of thyroid autoimmunity on IVF/ICSI outcome: a systematic review and meta-analysis. Hum Reprod Update. 2016;22(6):775–790. doi: 10.1093/humupd/dmw019.
- Miko E, Meggyes M, Doba K, et al. Characteristics of peripheral blood NK and NKT-like cells in euthyroid and subclinical hypothyroid women with thyroid autoimmunity experiencing reproductive failure. J Reprod Immunol. 2017;124:62–70. doi: 10.1016/j.jri.2017.09.008.
- Busnelli A, Vannucchi G, Paffoni A, et al. Levothyroxine dose adjustment in hypothyroid women achieving pregnancy through IVF. Eur J Endocrinol. 2015;173(4):417–424. doi: 10.1530/EJE-15-0151.
- Hou Y, Liu A, Li J, et al. Different thyroidal responses to human chorionic gonadotropin under different thyroid peroxidase antibody and/or thyroglobulin antibody positivity conditions during the first half of pregnancy. Thyroid. 2019;29(4):577–585. doi: 10.1089/thy.2018.0097.
- Strieder TG, Prummel MF, Tijssen JG, et al. Risk factors for and prevalence of thyroid disorders in a cross-sectional study among healthy female relatives of patients with autoimmune thyroid disease. Clin Endocrinol (Oxf). 2003;59(3):396–401. doi: 10.1046/j.1365-2265.2003.01862.x.
- Venables A, Wong W, Way M, et al. Thyroid autoimmunity and IVF/ICSI outcomes in euthyroid women: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2020;18(1):120. doi: 10.1186/s12958-020-00671-3.
- Fleming R, Seifer DB, Frattarelli JL, et al. Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reprod Biomed Online. 2015;31(4):486–496. doi: 10.1016/j.rbmo.2015.06.015.
- Bedenk J, Vrtačnik-Bokal E, Virant-Klun I. The role of anti-Müllerian hormone (AMH) in ovarian disease and infertility. J Assist Reprod Genet. 2020;37(1):89–100. doi: 10.1007/s10815-019-01622-7.
- Steiner AZ, Herring AH, Kesner JS, et al. Antimüllerian hormone as a predictor of natural fecundability in women aged 30-42 years. Obstet Gynecol. 2011;117(4):798–804. doi: 10.1097/AOG.0b013e3182116bc8.
- Jha V, Goswami D. Premature ovarian failure: an association with autoimmune diseases. J Clin Diagn Res. 2016;10(10):QC10–QC12. doi: 10.7860/JCDR/2016/22027.8671.
- Poppe K, Glinoer D, Tournaye H, et al. Assisted reproduction and thyroid autoimmunity: an unfortunate combination? J Clin Endocrinol Metab. 2003;88(9):4149–4152. doi: 10.1210/jc.2003-030268.
- Poppe K, Glinoer D, Van Steirteghem A, et al. Thyroid dysfunction and autoimmunity in infertile women. Thyroid. 2002;12(11):997–1001. doi: 10.1089/105072502320908330.
- Revelli A, Casano S, Piane LD, et al. A retrospective study on IVF outcome in euthyroid patients with anti-thyroid antibodies: effects of levothyroxine, acetyl-salicylic acid and prednisolone adjuvant treatments. Reprod Biol Endocrinol. 2009;7(1):137. doi: 10.1186/1477-7827-7-137.
- Monteleone P, Parrini D, Faviana P, et al. Female infertility related to thyroid autoimmunity: the ovarian follicle hypothesis. Am J Reprod Immunol. 2011;66(2):108–114. doi: 10.1111/j.1600-0897.2010.00961.x.
- Monteleone P, Faviana P, Artini PG. Thyroid peroxidase identified in human granulosa cells: another piece to the thyroid-ovary puzzle? Gynecol Endocrinol. 2017;33(7):574–576. doi: 10.1080/09513590.2017.1296424.
- Weghofer A, Himaya E, Kushnir VA, et al. The impact of thyroid function and thyroid autoimmunity on embryo quality in women with low functional ovarian reserve: a case-control study. Reprod Biol Endocrinol. 2015;13(1):43. doi: 10.1186/s12958-015-0041-0.
- Lee SH, Lee JH, Park YS, et al. Comparison of clinical outcomes between in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in IVF-ICSI split insemination cycles. Clin Exp Reprod Med. 2017;44(2):96–104. doi: 10.5653/cerm.2017.44.2.96.
- Yoeli R, Orvieto R, Ashkenazi J, et al. Comparison of embryo quality between intracytoplasmic sperm injection and in vitro fertilization in sibling oocytes. J Assist Reprod Genet. 2008;25(1):23–28. doi: 10.1007/s10815-007-9188-8.
- Andrisani A, Sabbadin C, Marin L, et al. The influence of thyroid autoimmunity on embryo quality in women undergoing assisted reproductive technology. Gynecol Endocrinol. 2018;34(9):752–755. doi: 10.1080/09513590.2018.1442427.
- Litwicka K, Arrivi C, Varricchio MT, et al. In women with thyroid autoimmunity, does low-dose prednisolone administration, compared with no adjuvant therapy, improve in vitro fertilization clinical results? J Obstet Gynaecol Res. 2015;41(5):722–728. doi: 10.1111/jog.12615.
- Kilic S, Tasdemir N, Yilmaz N, et al. The effect of anti-thyroid antibodies on endometrial volume, embryo grade and IVF outcome. Gynecol Endocrinol. 2008;24(11):649–655. doi: 10.1080/09513590802531112.
- Łukaszuk K, Kunicki M, Kulwikowska P, et al. The impact of the presence of antithyroid antibodies on pregnancy outcome following intracytoplasmatic sperm injection-ICSI and embryo transfer in women with normal thyreotropine levels. J Endocrinol Invest. 2015;38(12):1335–1343. doi: 10.1007/s40618-015-0377-5.
- Tan S, Dieterle S, Pechlavanis S, et al. Thyroid autoantibodies per se do not impair intracytoplasmic sperm injection outcome in euthyroid healthy women. Eur J Endocrinol. 2014;170(4):495–500. doi: 10.1530/EJE-13-0790.
- Chai J, Yeung WY, Lee CY, et al. Live birth rates following in vitro fertilization in women with thyroid autoimmunity and/or subclinical hypothyroidism. Clin Endocrinol (Oxf). 2014;80(1):122–127. doi: 10.1111/cen.12220.
- Mintziori G, Goulis DG, Gialamas E, et al. Association of TSH concentrations and thyroid autoimmunity with IVF outcome in women with TSH concentrations within normal adult range. Gynecol Obstet Invest. 2014;77(2):84–88. doi: 10.1159/000357193.
- Negro R, Mangieri T, Coppola L, et al. Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Hum Reprod. 2005;20(6):1529–1533. doi: 10.1093/humrep/deh843.
- Toulis KA, Goulis DG, Venetis CA, et al. Risk of spontaneous miscarriage in euthyroid women with thyroid autoimmunity undergoing IVF: a meta-analysis. Eur J Endocrinol. 2010;162(4):643–652. doi: 10.1530/EJE-09-0850.
- Thangaratinam S, Tan A, Knox E, et al. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ. 2011;342(1):d2616. doi: 10.1136/bmj.d2616.
- Lee YL, Ng HP, Lau KS, et al. Increased fetal abortion rate in autoimmune thyroid disease is related to circulating TPO autoantibodies in an autoimmune thyroiditis animal model. Fertil Steril. 2009;91(5 Suppl):2104–2109. doi: 10.1016/j.fertnstert.2008.07.1704.