Abstract
Objective
To evaluate FTO concentrations in follicular fluid (FF) of women with ovarian endometriosis (OE) and controls women without OE undergoing in vitro fertilization-embryo transfer (IVF-ET).
Methods
FTO concentrations in FF were measured in 74 patients (37 in the control group and 37 in the OE group) by ELISA. We measured the expression of FTO in GCs of 40 patients (19 in the control group and 21 in the OE group) by RT-qPCR. The level of m6A in GCs was measured in 20 patients (10 in the control group and 10 in the OE group) by colorimetry.
Results
Compared with the control group, FTO concentrations in FF (6.92 ± 0.44 vs. 5.67 ± 0.40 ng/ml) (p <.05) and FTO mRNA level in GCs of OE group were decreased significantly (p <.05), and the level of m6A was increased (0.21 ± 0.01 vs. 0.17 ± 0.03 ng) (p >.05).
Conclusions
The FTO concentrations in FF of infertility women with OE are decreased, which may be related to the impaired oocyte quality in endometriosis patients.
Introduction
Endometriosis is a commonly gynecologic disease characterized by presence of ectopic active endometrial tissue [Citation1]. The disease is not only medical, but also sociological, pshychological and economic problem [Citation2–4]. It has been estimated about 10% in women of reproductive age and as high as 30–50% in infertile women [Citation5]. Ovarian endometriosis (OE) occurs when ectopic endometrial tissue appears in the ovary. Although it is benign, chronic recurrent bleeding and inflammation in ectopic lesions can lead to ovarian tissue destruction [Citation6].
In vitro fertilization-embryo transfer (IVF-ET)/intracytoplasmic sperm injection (ICSI) has become an important treatment for endometriosis related infertility. However, the clinical pregnancy rate of IVF/ICSI-ET is compromised by endometriosis [Citation7]. Some studies have reported that endometriosis impairs oocyte quality and quantity, thereby reducing fertilization and implantation rates [Citation8], and leading to a decrease in cumulative live birth rate[Citation7,Citation9]; Also, it may cause ovarian fibrosis and aging[Citation10,Citation11]. However, the mechanism has not been clearly reported.
N6-methyladenosine (m6A) is the most abundant RNA modification in eukaryotes and is involved in the regulation of germ cell and early embryonic development [Citation12–14]. Fat mass and obesity-associated gene (FTO) is one of the m6A demethylases [Citation15]. Recent studies have shown that FTO can participate in ovarian aging, premature ovarian insufficiency (POI) [Citation16], polycystic ovary syndrome (PCOS) and other reproductive related diseases by regulating m6A modification [Citation17]. In addition, FTO polymorphism influences fat mass in individuals [Citation18] and body mass index (BMI) might influence IVF outcome [Citation19]. Previous research has shown an interesting link between BMI and endometriosis that endometriosis is inversely associated with BMI, and infertile obese women were at lower risk for development of endometriosis [Citation20]. Therefore, the association between FTO and endometriosis may affect IVF results of patients with endometriosis. However, no association between FTO and endometriosis with infertility has been reported.
Follicular fluid (FF) is the microenvironment for oocyte growth and development. The changes of various components and contents in FF are closely related to the developmental potential of oocytes [Citation21]. Granulosa cells (GCs), which secrete steroid hormones and growth factors, are essential for the process of follicle maturation. GCs regulate oocyte development and maturation by autocrine, paracrine and gap junction [Citation22,Citation23]. The aim of this study was to compare the expression level of FTO gene in FF of infertile women with and without OE after ovarian stimulation.
Methods
Participants
A total of 87 infertile patients who underwent IVF/ICSI-ET between May 2021 and April 2022 in the Reproductive Medicine Center of the First Hospital of Lanzhou University were enrolled. All patients provided written informed consent before participating in this study. The study was approved by the Reproductive Medicine Ethics Committee of the First Hospital of Lanzhou University (LDYYLL2022-436).
Inclusion criteria: OE group patients were ≤38 years of age, diagnosed with endometriosis, and there were still ‘ground glass’ lesions on the ovary detected by transvaginal ultrasound during controlled ovarian stimulation. The diagnostic criteria for endometriosis are confirmed as endometriosis by laparoscopy or laparotomy or aspiration combined with pathological examination. The control group consisted of age-matched patients with regular menstrual cycle (21–35 days), anti-mullerian hormone (AMH) >1.1 (ng/ml), previous diagnosis of endometriosis excluded by laparoscopy or laparotomy, and fallopian tube factor was the indication of IVF/ICSI-ET. Exclusion criteria: patients with PCOS, thyroid or adrenal dysfunction, diabetes, loss of one ovary, chromosomal abnormalities, and severe impairment of liver and kidney function.
ART procedure
The ovarian stimulation protocol was determined based on the ovarian reserve and the conventional protocols employed by the center. Cases included in this study mainly adopted follicular phase long protocol, luteal phase long protocol and antagonist protocol. According to the patient’s age, antral follicle count (AFC), AMH, and body mass index (BMI), 150–300 U/d of recombinant human follicle stimulating hormone and/or urinary follicle stimulating hormone were given. Adjust the gonadotropin dosage in time according to the growth of follicles. When at least two follicles were 18 mm in diameter, human chorionic gonadotrophin (HCG) was administered to induce ovulation. Oocyte retrieval was performed by transvaginal ultrasound-guided 36–38h after HCG injection. IVF/ICSI fertilization was performed according to the semen condition, and embryo culture was performed routinely. One to two cleavage stage embryos on day 3 or one blastocyst on day 5 was transferred. Cleavage stage embryos and blastocysts were scored according to the Scott classification and Gardner classification, respectively.
FF and GCs collection
On the day of oocyte retrieval, follicles with a diameter of ≥ 12 mm on the ovary were punctured and aspirated through vaginal ultrasound guidance [Citation24]. FF without visible blood was collected and sent to the laboratory for processing within 1 h. (1) the FF was centrifuged at 2000 rpm for 15 min. Collect the supernatant and freeze it at −80 °C for later use; (2) drop 1 ml hyaluronidase (Solarbio, China) into the cell precipitate, incubate for 15 min at 37 °C, centrifuge at 2000 rpm for 15 min, and discard the supernatant; (3) add 1 ml of red blood cell lysis buffer into the precipitate, stand for 10 min, then centrifuge for 10 min at 2000 rpm, and discard the supernatant; (4) add 1 ml of lymphocytes separation medium (Ficoll) (Solarbio, P8900, China) to the precipitate, and GCs were isolated from the middle layer after centrifugation at 1600 rpm for 10 min; (5) GCs precipitation was washed with 1 ml phosphate buffer, centrifuged at 2000 rpm for 10 min, and the supernatant was discarded to obtain GCs for RNA extraction.
Determination of FTO and other m6A modification related proteins in FF
The concentrations of FTO, ALKBH5, METTL3, METTL14, WTAP, KIAA1429 and YTHDF2 in FF were measured using Human FTO/ALKBH5/METTL3/METTL14/WTAP/KIAA1429/YTHDF2 ELISA Kit (Jianglai, Shanghai, China) according to the manufacturer’s instructions.
PCR technology
Total RNA was extracted from GCs and used for RT-qPCR when the A260/280 ratio was 1.8–2.0. The RNA was reverse transcribed into cDNA following the instructions of the FastKing gDNA Dispelling RT SuperMix kit (TIANGEN, Beijing, China). The cDNA was amplified using QuantStudioTM5 real-time PCR instrument (Thermo Fisher, Singapore) according to the instructions of SuperReal PreMix Plus (SYBR Green) (TIANGEN, Beijing, China). Primer sequences were used as follows: Human GAPDH forward primer 5′-GGAGCGAGATCCCTCCAAAAT-3′, GAPDH reverse primer 5′-GGCTGTTGTCATACTTCTCATGG-3′; Human FTO forward primer 5′-TGGGTTCATCCTACAACGG, FTO reverse primer 5′-CCTCTTCAGGGCCTTCAC-3′; Human METTL3 forward primer 5′- CTGCTGATTTCTCAACCT-3′, METTL3 reverse primer 5′- CTGGCTATTTACTTGATTCATT-3′; Human KIAA1429 forward primer 5′- GAATACTTGATGGTCTGGTGCTA-3′, KIAA1429 reverse primer 5′- CTTGGCTGTGGTCTTGGA-3′. The relative expression of gene was analyzed by 2-△△CT method.
M6A determination
EpiQuik m6A RNA Methylation Quantification Kit (Epigentek, Wuhan, China) was used to quantify m6A methylation levels in GCs. RNA purity requirements: A260/280 > 1.9, A260/230 > 1.7.
Clinical observation indexes
The data of fertilization rate, cleavage rate, top quality embryo rate (cleavage stage embryo), available embryo rate, implantation rate, clinical pregnancy rate during IVF were collected.
Statistical analysis
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis and data mapping. Data were expressed as mean ± standard deviation. If the data were normally distributed, Student’s t-test was used for analysis. If the data were not normally distributed, the Mann-Whitney test was used. p < 0.05 was considered statistically significant.
Results
A total of 87 cases were included, of which FF from 74 cases (37 in control group and 37 in OE group) were used for ELISA, GCs from 40 cases (19 in control group and 21 in OE group) were used for RT-qPCR, and GCs from 20 cases (10 in control group and 10 in OE group) were used for the determination of m6A.
Characteristics of the OE and control patients whose samples were used for ELISA
lists the clinical characteristics of the 74 patients who underwent ELISA analysis. Compared with the control group, the levels of AMH and AFC in the OE group were significantly lower (p < .01, p < .0001), and the basal FSH were significantly higher (p < .05). There was no significant difference in other parameters between the two groups ().
Table 1. Characteristics of the OE and control patients whose samples were used for ELISA Analyses.
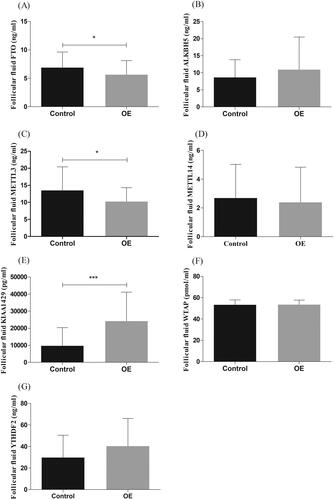
FTO and METTL3 expressions were decreased while the expression of KIAA1429 increased in FF of OE patients
Compared with the control group, the concentration of FTO and METTL3 in FF of OE group was significantly lower (p < .05; ), KIAA1429 concentration was significantly increased (p < .0001; ). There was no significant difference in other m6A modification related proteins between the two groups ().
Figure 1. The concentrations of protein in follicular fluid (FF) of control group (control) and ovarian endometriosis group (OE). The concentrations of FTO and METTL3 in FF of women with OE (n = 37) were lower than that of the control group (n = 37, *p <.05, (A,C). The concentrations of KIAA1429 in FF of women with OE (n = 37) were higher than that of the control group (n = 37, ***p <.0001, E).
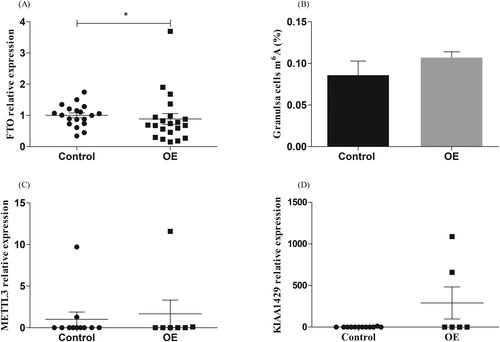
Expression of the FTO, METTL3 and KIAA1429 gene in GCs of OE group and control group
We detected the transcription level of FTO, METTL3 and KIAA1429 in GCs and found that the level of FTO in GCs of OE patients was significantly lower than that of the control group (p <. 05; ). This was consistent with the expression trend of FTO in FF. We further measured the m6A level of GCs RNA, the results showed that the m6A in OE group tended to increase (p >. 05; ).
Figure 2. Expression of FTO in granulosa cells (GCs) of women with ovarian endometriosis (OE). (A) FTO levels were significantly lower in women with OE (n = 21) compared with women in control group (n = 19, *p <.05). (B) The content of m6A in GCs of women with OE (n = 10) was higher than that of the control group (n = 10, p >.05).
ART cycle parameters for two groups
The comparisons of ART cycle parameters between the two groups are summarized in . Compared with the control group, the gonadotropin dose in OE group was significantly higher (p < .01), and the number of oocytes retrieved (p < .01), the mature oocytes (p < .01), the cleavage rate (p < .05), and the top-quality embryos rate (p < .05) were significantly lower ().
Table 2. ART cycle Characteristics in OE and control patients.
Discussion
To our knowledge, this is the first study to report the level of FTO in FF of OE patients with infertility. The results showed that the expression of FTO in FF and GCs of OE patients was lower than that of the controls.
The reason for the low expression of FTO in OE patients has not been clarified in this study. Previous studies on ectopic endometrial stromal cells and normal endometrial stromal cells have found that the expression of FTO in the former is lower than that in the latter at both the transcription and translation level [Citation25]. In addition, endometrial tissues on the ovary can not only affect the blood supply and neuroregulation of the ovarian cortex through mechanical compression, but also secrete a variety of cytokines, which lead to premature follicle development and accelerated atresia by activating specific signal pathways in the follicle [Citation26]. Therefore, the internal environment of follicles can be affected by OE. Healthy follicular microenvironment is essential for the normal development and maturation of oocytes [Citation27,Citation28]. If the composition of FF changed [Citation27,Citation29,Citation30], the normal morphology of the spindle may be changed, and chromosomes cannot be distributed normally, thus affecting the quality of oocytes [Citation21].
M6A modification regulates mRNA metabolism and plays an important role in gamete maturation, zygotic gene activation and early embryonic development [Citation12,Citation13]. As a demethylase, FTO can erase m6A modification on target RNA. When FTO is knocked down, the m6A modification of cyclin D1 mRNA is up-regulated, leading to accelerated degradation of cyclin D1[Citation31]. Cyclin D1 is a key regulator of G1/S transformation in the process of cell cycle, which can promote cell proliferation [Citation32]. Therefore, its degradation can lead to cell cycle arrest. Similarly, another study on single cell RNA sequencing of oocytes from patients with endometriosis found that molecules that hinder cell cycle progression (such as WEE1, the key regulator of G2/M cell cycle checkpoint) in oocytes from endometriosis patients are up-regulated [Citation33]. The regulation of D1 mRNA by FTO through m6A has been verified in a variety of cells [Citation31]. Therefore, whether such a regulation pattern also exists in GCs of OE patients is the next direction of our research. In this study, we found a decrease in FTO expression in FF and GCs of OE patients, as well as a decrease in cleavage rate and top-quality embryo rate in these patients. No difference in the transcription levels of METTL3 and KIAA1429 was found in the two groups of GCs. However, due to the limited sample size, future studies with a larger sample size are necessary. Combined with the high incidence of POI in endometriosis patients [Citation7] and the research findings that low FTO may be associated with ovarian aging [Citation16,Citation34], we hypothesized that the lower FTO in this study might be related to the decreased oocyte quality, cleavage rate and top-quality embryo rate.
In the future, we’ll further explore the specific mechanism of the effect of FTO on ovarian reserve and oocyte quality.
Conclusions
Lower levels of FTO in FF of OE infertility patients are possibly associated with the impaired oocytes potential. This provides a new perspective for understanding the epigenetic influence on the pathogenesis of endometriosis related infertility.
Authors’ contributions
Yanmei Li, Naihui Wang and Xuehong Zhang: study design; Yanmei Li, Naihui Wang, Fangfang Li and Jiajing He: data collection; Yanmei Li, Naihui Wang, Jiajing He and Fangfang Li: statiscal analysis; Yanmei Li, Naihui Wang, Yuanxue Jing and Xuehong Zhang: data interpretation; Yanmei Li and Naihui Wang: manuscript preparation. All authors read and approved the final version of the manuscript.
Ethical approval and consent to participate
This study was approved by the Reproductive Medicine Ethics Committee of the First Hospital of Lanzhou University.
Acknowledgments
The authors would like to thank the Reproductive Medicine Center of the First Hospital of Lanzhou University and the Key Laboratory of Reproductive Medicine and Embryology of Gansu Province for their help in the research process.
Disclosure statement
The authors report there are no competing interests to declare
Additional information
Funding
References
- Chapron C, Marcellin L, Borghese B, et al. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15(11):1–6. doi:10.1038/s41574-019-0245-z.
- Gao X, Outley J, Botteman M, et al. Economic burden of endometriosis. Fertil Steril. 2006;86(6):1561–1572. doi:10.1016/j.fertnstert.2006.06.015.
- Aerts L, Grangier L, Streuli I, et al. Psychosocial impact of endometriosis: from co-morbidity to intervention. Best Pract Res Clin Obstet Gynaecol. 2018;50:2–10. doi:10.1016/j.bpobgyn.2018.01.008.
- Garalejić E, Bojović-Jović D, Damjanović A, et al. Hamilton anxiety scale (Hama) in infertile women with endometriosis and its correlation with magnesium levels in peritoneal fluid. Psychiatr Danub. 2010;22(1):64–67.
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244–1256. doi:10.1056/NEJMra1810764.
- Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15(4):441–461. doi:10.1093/humupd/dmp007.
- Li A, Zhang J, Kuang Y, et al. Analysis of IVF/ICSI-FET outcomes in women with advanced endometriosis: influence on ovarian response and oocyte competence. Front Endocrinol (Lausanne). 2020;11:427. doi:10.3389/fendo.2020.00427.
- Corachan A, Pellicer N, Pellicer A, et al. Novel therapeutic targets to improve IVF outcomes in endometriosis patients: a review and future prospects. Hum Reprod Update. 2021;27(5):923–972. doi:10.1093/humupd/dmab014.
- Yin Y, Mao Y, Liu A, et al. Insufficient cumulus expansion and poor oocyte retrieval in Endometriosis-Related infertile women. Reprod Sci. 2021;28(5):1412–1420. doi:10.1007/s43032-020-00410-4.
- Donnez J, Donnez O, Orellana R, et al. Endometriosis and infertility. Panminerva Med. 2016;58(2):143–150.
- Ke J, Ye J, Li M, et al. The role of matrix metalloproteinases in endometriosis: a potential target. Biomolecules. 2021;11(11):1739. doi:10.3390/biom11111739.
- Hu Y, Ouyang Z, Sui X, et al. Oocyte competence is maintained by m(6)a methyltransferase KIAA1429-mediated RNA metabolism during mouse follicular development. Cell Death Differ. 2020;27(8):2468–2483. doi:10.1038/s41418-020-0516-1.
- Ivanova I, Much C, Di Giacomo M, et al. The RNA m(6)a reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and oocyte competence. Mol Cell. 2017;67(6):1059–1067.e1054. doi:10.1016/j.molcel.2017.08.003.
- Li C, Jiang Z, Hao J, et al. Role of N6-methyl-adenosine modification in mammalian embryonic development. Genet Mol Biol. 2021;44(2):e20200253. doi:10.1590/1678-4685-GMB-2020-0253.
- Jia G, Fu Y, Zhao X, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–887. doi:10.1038/nchembio.687.
- Ding C, Zou Q, Ding J, et al. Increased N6-methyladenosine causes infertility is associated with FTO expression. J Cell Physiol. 2018;233(9):7055–7066. doi:10.1002/jcp.26507.
- Castillo-Higuera T, Alarcón-Granados MC, Marin-Suarez J, et al. A comprehensive overview of common polymorphic variants in genes related to polycystic ovary syndrome. Reprod Sci. 2021;28(9):2399–2412. doi:10.1007/s43032-020-00375-4.
- Antonio J, Knafo S, Kapoor R, et al. A fat mass and obesity-associated gene polymorphism influences fat mass in exercise-trained individuals. J Int Soc Sports Nutr. 2018;15(1):40.
- Garalejic E, Arsic B, Radakovic J, et al. A preliminary evaluation of influence of body mass index on in vitro fertilization outcome in non-obese endometriosis patients. BMC Womens Health. 2017;17(1):112.
- Rowlands IJ, Hockey R, Abbott JA, et al. Body mass index and the diagnosis of endometriosis: findings from a national data linkage cohort study. Obes Res Clin Pract. 2022;16(3):235–241. doi:10.1016/j.orcp.2022.04.002.
- Da Broi MG, Malvezzi H, Paz CC, et al. Follicular fluid from infertile women with mild endometriosis may compromise the meiotic spindles of bovine metaphase II oocytes. Hum Reprod. 2014;29(2):315–323. doi:10.1093/humrep/det378.
- Da Broi MG, Giorgi VSI, Wang F, et al. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J Assist Reprod Genet. 2018;35(5):735–751. doi:10.1007/s10815-018-1143-3.
- Turathum B, Gao EM, Chian RC. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells. 2021;10(9):2292. doi:10.3390/cells10092292.
- Li Y, Liu YD, Chen SL, et al. Down-regulation of long non-coding RNA MALAT1 inhibits granulosa cell proliferation in endometriosis by up-regulating P21 via activation of the ERK/MAPK pathway. Mol Hum Reprod. 2019;25(1):17–29. doi:10.1093/molehr/gay045.
- Wang H, Liang Z, Gou Y, et al. FTO-dependent N(6)-methyladenosine regulates the progression of endometriosis via the ATG5/PKM2 axis. Cell Signal. 2022;98:110406. doi:10.1016/j.cellsig.2022.110406.
- Kitajima M, Dolmans MM, Donnez O, et al. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil Steril. 2014;101(4):1031–1037. doi:10.1016/j.fertnstert.2013.12.049.
- Abhari S, Lu J, Hipp HS, et al. A Case-Control study of follicular fluid cytokine profiles in women with diminished ovarian reserve. Reprod Sci. 2022;29(9):2515–2524. doi:10.1007/s43032-021-00757-2.
- Monniaux D. Driving folliculogenesis by the oocyte-somatic cell dialog: lessons from genetic models. Theriogenology. 2016;86(1):41–53. doi:10.1016/j.theriogenology.2016.04.017.
- Ni Z, Li Y, Song D, et al. Iron-overloaded follicular fluid increases the risk of endometriosis-related infertility by triggering granulosa cell ferroptosis and oocyte dysmaturity. Cell Death Dis. 2022;13(7):579. doi:10.1038/s41419-022-05037-8.
- Yang Z, Zhou W, Zhou C, et al. Steroid metabolome profiling of follicular fluid in normo- and hyperandrogenic women with polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2021;206:105806. doi:10.1016/j.jsbmb.2020.105806.
- Hirayama M, Wei FY, Chujo T, et al. FTO demethylates cyclin D1 mRNA and controls Cell-Cycle progression. Cell Rep. 2020;31(1):107464. doi:10.1016/j.celrep.2020.03.028.
- Wang XK, Zhang YW, Wang CM, et al. METTL16 promotes cell proliferation by up-regulating cyclin D1 expression in gastric cancer. J Cell Mol Med. 2021;25(14):6602–6617. doi:10.1111/jcmm.16664.
- Ferrero H, Corachan A, Aguilar A, et al. Single-cell RNA sequencing of oocytes from ovarian endometriosis patients reveals a differential transcriptomic profile associated with lower quality. Hum Reprod. 2019;34(7):1302–1312. doi:10.1093/humrep/dez053.
- Jiang ZX, Wang YN, Li ZY, et al. The m6A mRNA demethylase FTO in granulosa cells retards FOS-dependent ovarian aging. Cell Death Dis. 2021;12(8):744. doi:10.1038/s41419-021-04016-9.
