Abstract
Obejective
To compare the effectiveness of endometrial receptivity and pregnancy outcomes of four common immunomodulatory therapies for patients with thin endometrium.
Method
This systematic review and network meta-analysis using a literature search up to January 2024, to identify relevant trials comparing endometrial receptivity and pregnancy outcomes of human chorionic gonadotropin (hCG), platelet-rich plasma (PRP), infusion of granulocyte colony-stimulating factor (IG-CSF), and peripheral blood mononuclear cell (PBMC) for patients with thin endometrium. We used surface under the cumulative ranking (SUCRA) to ranked four common immunomodulatory therapies on endometrium thickness, implantation rate (IR), clinical pregnancy rate (CPR), and live birth rate (LBR). RoB2 and ROBINS-I were used to assess the certainty of evidence.
Results
The pooled results of 22 studies showed that hCG (mean difference [MD]: 3.05, 95% confidence interval [CI]: 1.46–4.64) and PRP (MD: 0.98, 95% CI: 0.20–1.76) significantly increase endometrium thickness. The hCG was the best among the IG-CSF (MD = −2.56, 95% CI = −4.30 to −0.82), PBMC (MD = −2.75, 95% CI = −5.49 to −0.01), and PRP (MD = −2.07, 95% CI = −3.84 to −0.30) in increasing endometrium thickness. However, IG-CSF and PRP significantly improved IR (IG-CSF: risk ratio (RR; IG-CSF: RR = 1.33, 95% CI = 1.06–1.67; PRP: RR = 1.63, 95% CI = 1.19–2.23), and LBR (IG-CSF: RR = 1.53, 95% CI = 1.16–2.02; PRP: RR = 1.59, 95% CI = 1.08–2.36).
Conclusions
Available evidence reveals that hCG and subcutaneous or intrauterine CSF (SG-CSF) may be the best treatment options for current thin endometrium patients. However, future high-quality and large-scale studies are necessary to validate our findings.
Introduction
In vitro fertilization (IVF) and embryo transfer are the most effective assisted reproductive technologies (ART) for treating infertility [Citation1]. Successful embryo implantation depends on perfect synchrony between embryonic development and good endometrial receptivity [Citation2]. The ability of the endometrium to allow normal implantation is known as receptivity, which is the specific period when ‘the trophectoderm of the blastocyst can attach to the endometrial epithelium and subsequently invade the endometrial stroma and vasculature’ [Citation1], known as ‘the implantation window’ opens 4–5 d after ovulation. The success rate of pregnancies was shown to be primarily influenced by endometrial factors (approximately 60%) and embryonic factors (about 40%) [Citation3]. Therefore, increasing endometrial receptivity has become a critical concern of reproduction research [Citation4].
The major indicator of endometrial receptivity is endometrial thickness, and a successful pregnancy greatly depends on an adequate endometrial thickness [Citation5]. Many reasons, such as inflammation and iatrogenic factors, can lead to thinning of the endometrium, which is called thin endometrium and considered an ongoing challenge to female infertility and conception failure [Citation6]. Thin endometrium occurs in 1.5%–9.1% of cases despite attempts at various treatments to maintain endometrial thickness [Citation7–9]. Although the condition is uncommon, clinicians continue to work to find ways to treat thin endometrium and improve pregnancy outcomes for infertile patients [Citation10], such as extending estradiol administration, using low-dose aspirin, administering vitamin E, L-arginine, and sildenafil citrate in intravaginal treatment [Citation11,Citation12]. However, despit the use of these treatments, a small percentage of patients remain unresponsive. Therefore, better treatment strategies are needed [Citation13,Citation14]. Accumulating evidence suggests that active involvement of local immune cells at the implantation site impairs endometrium receptivity [Citation15]. Therefore, various immunomodulatory therapies have been investigated, including intrauterine human chorionic gonadotropin (hCG) [Citation16,Citation17] subcutaneous (SG) or intrauterine (IG) infusion of granulocyte colony-stimulating factor (CSF)) [Citation4,Citation18], peripheral blood mononuclear cells (PBMCs) [Citation19], and autologous platelet-rich plasma (PRP) [Citation20,Citation21]. However, which of these immunomodulatory therapies work best remains controversial. Therefore, this network meta-analysis aims to provide an evidence base for clinical application by comparing the effectiveness of four common immunomodulatory therapies to patients with thin endometrium.
Material and methods
Study design
We conducted this study followed Cochrane Handbook [Citation22] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for network meta-analysis (PRISMA-NMA) [Citation23]. Ethics approval was not required, since data was not collected directly from patients.
Selection criteria
Studies were selected followed criteria as follows:
Studies targeted on participants clinically diagnosed with thin endometrium (<7 mm);
Intervention groups received immunomodulatory monotherapy or combination therapy;
Control groups received any other anti-thin endometrium treatment;
Study endpoints included post-treatment endometrial thickness or any pregnancy outcomes (IR, CPR, and LBR).
Studies with any comparative designs (i.e. randomized controlled trials [RCTs], prospective cohort studies [PC], or retrospective cohort studies [RPC]);
The exclusion criteria were
Animal/cell researches were excluded;
Studies not published in an international peer-reviewed journal;
Excluded studies with faulty randomized design or study methodology.
Literature retrieval
We retrieved studies published before January, 2024 by searching PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), Embase, and Cochrane Library. Search strategies were summarized in Supplementary Table 1. We used the following terms and other relevant key words to develop search strategy, including ‘thin endometrium,’ ‘human chorionic gonadotropin,’ ‘granulocyte colony-stimulating factor,’ ‘peripheral blood mononuclear cells,’ and ‘ platelet-rich plasma.’ We manually examined references of eligible articles and meta-analyses to identify additional studies. The literature retrieval and selection process was performed independently by two investigators. Any discrepancy was solved by discussion.
Data extraction
Two authors independently selected eligible studies by screening titles, abstracts and full texts. Then, one author (Lifei Li) extracted data, and another (Zhijian Kou) examined the data. The following information was obtained from eligible studies: (1) the first author’s name, published year, country, and study design; (2) sample size, average age, body mass index (BMI), infertility duration, number of embryos transfer, endometrium thickness before treatment, and details of interventions; (3) outcomes of interest; (4) details of risk of bias. For outcomes reported in median and standardized error or interquartile range (IQR), we used recognized formulas to calculate the required data [Citation24].
Outcome measures
Post-treatment endometrial thickness was measured as the maximum distance between the 2 interfaces of the endometrial-myometrial junction, in the midsagittal plane of the uterus by B ultrasound radiography. Pregnancy outcomes included IR, CPR, and LBR. IR was determined by the number of live embryos, CPR was considered with the presence of a gestational sac containing a yolk sac at transvaginal ultrasonography, and LBR was classified as those cycles resulting in the delivery of a live infant after 24 weeks gestation.
Evidence structure
An evidence map for each outcome was drawn to display evidence pattern. Direct comparison between two immunomodulatory therapies was represented by a solid line, and the thickness of line was weighted by the number of direct comparisons. Solid circle represented an immunomodulatory therapy, and size of the circle was weighted by the number of accumulated participants.
Risk of bias assessment
Two authors (Lifei Li and Fei Zhao) used the revised Cochrane risk-of-bias tool (RoB2) [Citation25] to evaluate the risk of bias of RCTs and the Cochrane risk of bias in non-randomized studies of interventions (ROBINS-I) tool [Citation26] to evaluate the risk of bias of PC and RPC studies. In the RoB2 tool, five domains were involved: (a) randomization, (b) incomplete data, (c) deviation from expected intervention, (d) selected reported results, and (e) outcome measurement bias, and each entry was divided into high risk, low risk, and other concerns. In the ROBINS-I tool, seven domains were involved, including confounding, selection of participants, classification of intervention, deviations from intended interventions, missing data, outcomes measurements, and selection of the reported results. Individual domain was classified as ‘low,’ ‘moderate,’ ‘serious,’ or ‘critical.’ Results of risk of bias assessment were graphically presented using ‘robvis’ package [Citation27].
Statistical analysis
Mean difference (MD) and risk ratio (RR), with 95% confidence interval (CI), were used to report pooled results. First, we conducted transitivity assessment. Second, we used the design-by-treatment interaction method [Citation28] and the node-splitting method [Citation29] to examine global and local inconsistencies. Third, random network meta-analysis was conducted to assess the relevant efficacy of various immunomodulatory therapies. Fourth, we examined loop inconsistency to evaluate the validity of pooled results. Fifth, we ranked four common immunomodulatory therapies based on the surface under the cumulative ranking (SUCRA) [Citation30]. Finally, we checked publication bias with Begg’s [Citation31] and Egger’s [Citation32] tests. The present network meta-analysis was done with STATA version 14.0 (StataCorp LP, College Station, TX).
Results
Literature selection
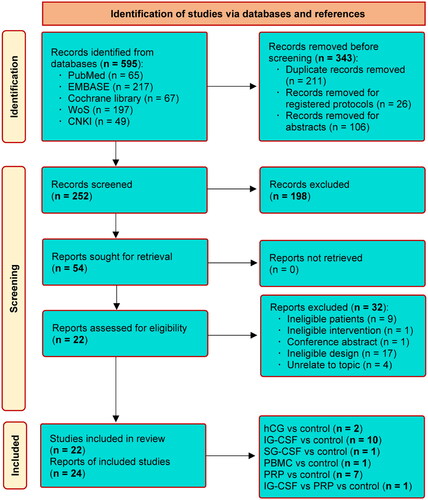
Initial search yielded 782 records. After removed 214 duplicate records, and 546 ineligible studies, 22 studies were rated for the final evaluation. Finally, 22 studies [Citation1,Citation4,Citation10,Citation13,Citation14,Citation16–21,Citation33–43] were all eligible for inclusion. The screening process and the reasons for exlucidng studies are depicted in .
Study characteristics
The baseline characteristics of included studies are presented in . The publication time was between 2014 and 2022. 63.6% of included studies [Citation1,Citation4,Citation10,Citation13,Citation14,Citation16,Citation17,Citation19,Citation33,Citation34,Citation39–41,Citation43] were performed in China, four studies [Citation21,Citation36,Citation37,Citation42] in Iran, two studies [Citation20,Citation35] in Russia, and other two studies in Poland [Citation38] and India [Citation18]. Eight studies [Citation1,Citation16,Citation21,Citation34,Citation36,Citation40,Citation42,Citation43] were RCTs, and other 14 studies [Citation4,Citation10,Citation13,Citation14,Citation17–20,Citation33,Citation35,Citation37–39,Citation41] were PC and RPC studies. A total of 2883 participants were accumulated, with five therapeutic modalities (i.e. hCG, IG-CSF, SG-CSF, PBMC, and PRP). The evidence structure of each outcome is shown in Supplementary Figure 1.
Table 1. Basic characteristics of included studies (n = 22).
Risk of bias of eligible studies
Supplementary Figure 2 shows the results of risk of bias assessment. Overall, all RCTs were rated as low risk or some concerns in overall methodological quality, while almost all PC and RPC studies were rated as a moderate risk in overall methodological quality except for one study, which was rated as a serious risk.
Transitivity assessment
We performed transitivity assessment according to publication year, sample size, average age and BMI of participants, number of embryos transferred, and endometrial thickness before treatment. As summarized in Supplementary Table 2, transitivity assessment confirmed that six key characteristics were evenly distributed between different comparisons (p > .05 for all).
Global and local consistency examination
There is no statistical significance shown in the global consistency test: endometrium thickness after treatment (p = .975), IR (p = .229), CPR (p = .289), and LBR (p = .268) (see Supplementary Figure 3). Comparisons of all outcomes also did not show local inconsistency (see Supplementary Table 3).
Meta-analysis of endometrium thickness
Twenty studies [Citation1,Citation4,Citation10,Citation14,Citation16,Citation17,Citation19–21,Citation33–43] reported endometrium thickness after treatment. As shown in and Citation3(a), compared with control, hCG (MD = 3.05, 95% CI = 1.46–4.64) and PRP (MD = 0.98, 95% CI = 0.20–1.76) significantly increased post-intervention endometrium thickness. hCG was better than IG-CSF (MD = −2.56, 95% CI = −4.30 to −0.82), PBMC (MD = −2.75, 95% CI = −5.49 to −0.01), and PRP (MD = −2.07, 95% CI = −3.84 to −0.30) in increasing endometrium thickness. However, except for the five comparisons mentioned earlier, differences between other comparisons did not get statistical significance.
Figure 2. Relative therapeutic effectiveness of different immunomodulatory therapies in terms of post-intervention endometrium thickness (a), IR (b), CPR (c), and LBR (d). IR: implantation rate; CPR: clinical pregnancy rate; LBR: live birth rate; hCG: human chorionic gonadotropin; IG-CSF: intrauterine infusion of granulocyte colony-stimulating factor; SG-GSF: subcutaneous intrauterine; PBMC: peripheral blood mononuclear cell; PRP: platelet-rich plasma.
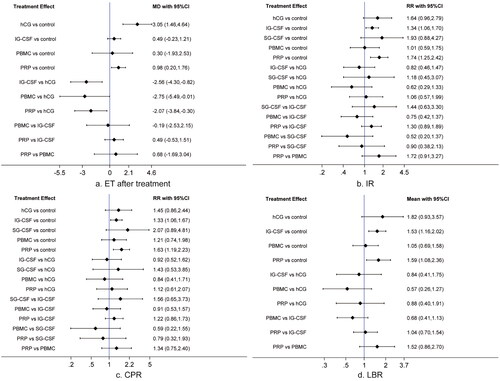
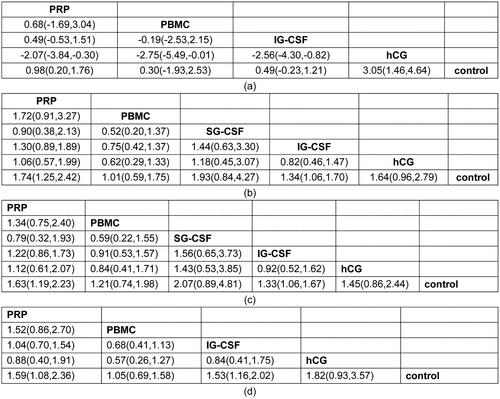
Figure 3. League tables of network meta-analysis results (a), IR (b), CPR (c), and LBR (d). IR: implantation rate; CPR: clinical pregnancy rate; LBR: live birth rate; hCG: human chorionic gonadotropin; IG-CSF: intrauterine infusion of granulocyte colony-stimulating factor; SG-GSF: subcutaneous intrauterine; PBMC: peripheral blood mononuclear cell; PRP: platelet-rich plasma.
Meta-analysis of pregnancy outcomes
Twenty studies [Citation1,Citation4,Citation10,Citation13,Citation14,Citation16,Citation17,Citation19,Citation20,Citation33–43] reported IR after treatment. Results showed that, as shown in and Citation3(b), IG-CSF (RR = 1.34, 95% CI = 1.06–1.70) and PRP (RR = 1.74, 95% CI = 1.25–2.42) significantly improved IR as compared with control. However, except for the two comparisons mentioned earlier, there were no significant differences for other comparisons.
All studies [Citation1,Citation4,Citation10,Citation13,Citation14,Citation16–21,Citation33–43] reported CPR after treatment. Results indicated that, as shown in and Citation3(c), the administration of IG-CSF (RR = 1.33, 95% CI = 1.06–1.67) and PRP (RR = 1.63, 95% CI = 1.19–2.23) significantly improved CPR when compared to control, while differences of other comparisons did not reach statistical significance.
LBR was available in 14 studies [Citation1,Citation4,Citation13,Citation14,Citation17,Citation19,Citation20,Citation35,Citation36,Citation38–41,Citation43]. As shown in and Citation3(d), compared with control, only IG-CSF (RR = 1.53, 95% CI = 1.16–2.02) and PRP (RR = 1.59, 95% CI = 1.08–2.36) led to a significant higher LBR. However, no significant differences were detected for other comparisons.
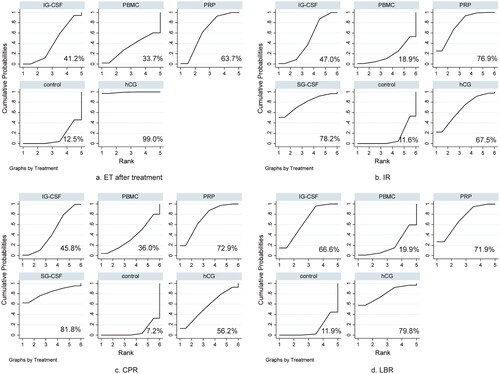
The ranking probability of SUCRA
We drew to depict probability rankings for each therapeutic modality. Regarding the endometrium thickness after treatment, hCG ranked first (99.0%), followed by PRP (63.7%), IG-CSF (41.2%), and PBMC (33.7%). For IR, SG-CSF obtained the highest probability, followed by PRP (76.9%), hCG (67.5%), IG-CSF (47.0%), and PBMC (18.9%). As far as the CPR was concerned, SG-CSF was the most effective therapeutic modality (81.8%), followed by PRP (72.9%), hCG (56.2%), IG-CSF (45.8%), and PBMC (36.0%). Finally, regarding the LBR, the most effective therapeutic modality was hCG (79.8%), followed by PRP (71.9%), IG-CSF (66.6%), and PBMC (19.9%).
Figure 4. SUCRA results of different immunomodulatory therapies in terms of post-intervention endometrium thickness (a), IR (b), CPR (c), and LBR (d). IR, implantation rate; CPR, clinical pregnancy rate; LBR, live birth rate; hCG, human chorionic gonadotropin; IG-CSF, intrauterine infusion of granulocyte colony-stimulating factor; SG-GSF, subcutaneous intrauterine; PBMC, peripheral blood mononuclear cell; PRP, platelet-rich plasma.
Heterogeneity assessment
I2 value ranged from 0.0% to 98.9% for the endometrium thickness after treatment, I2 value ranged from 0.0% to 59.7% for IR, I2 value ranged from 0.0% to 43.4% for CPR, and I2 value ranged from 0.0% to 8.3% for LBR. Details of heterogeneity examination are depicted in Supplementary Figure 4.
Loop inconsistency
As depicted in Supplementary Figure 5, the lower limits of all 95% CIs were zero, suggesting no inconsistency across all available closed loops for these results.
Publication bias
Results of Begg’s and Egger’s tests showed a risk of publication bias in endometrium thickness after treatment (Begg: p = .007, Egger: p = .00), IR (Begg: p = .042, Egger: p = .001), CPR (Begg: p = .008, Egger: p = .002), and LBR (Begg: p = .392, Egger: p = .029).
Discussion
Main findings
This network meta-analysis included 22 eligible studies with 2883 participants. Results revealed that hCG and PRP significantly increased the endometrium thickness, and hCG was superior to IG-CSF, PBMC, and PRP in terms of endometrium thickness. IG-CSF and PRP led to significantly higher IR, CPR, and LBR than the control group. SUCRA results revealed that hCG was probably the optimal modality in increasing endometrium thickness and LBR, SG-CSF might be the most effective option in improving IR and CPR, followed by PRP and hCG.
Interpretations of findings
Previous studies revealed immunomodulatory therapy benefited ART patients [Citation44–46]. Xie et al. [Citation44] reported that compared with the control group, IR could be greatly enhanced by IG-CSF, and Liu et al. [Citation45] showed that IG-CSF could significantly increase IR, but SG-CSF was most effective for CPR. Zhao et al. [Citation46] found that a positive clinical effect may be found in pregnancy outcomes after the administration of G-CSF, and a SG injection may be the best way to administer the G-CSF. G-CSF is a glycoprotein that belongs to the growth factor family. The results of a meta-analysis published in 2021 showed that G-CSF, as a common endogenous hematopoietic growth factor, can improve clinical pregnancy rates (CPRs) in patients with RIF, which supports our findings [Citation47]. G-CSF secretion and receptor expression are present in many reproduction-related cells (endometrial epithelial cells, trophoblast cells, and placental cells, etc.) [Citation48]. The positive effects of G-CSF on immune environment and trophoblast proliferation may mediate the improvement of CPR and implantation rate (IR). In vitro and in vivo experiments have shown that G-CSFR expression by CD4 + and CD8 + T cells induced by G-CSF can promote the production of interleukin-10 (IL-10) and transforming growth factor-β(TGF-β) regulatory T cells (Tregs). While inhibiting Th1 differentiation, they polarize toward Th2 differentiation, thereby reducing Th1/Th2 and Th17/Treg ratios and promoting embryo implantation [Citation49–51]). Meanwhile, G-CSF can regulate the increase of endometrium and affected the growth of early endometriosis [Citation52], and can also improve endometrial stem cells, activate bone marrow stem cells, and promote the development of endometrial cells [Citation13]. However, there remains controversy regarding the ideal route of G-CSF administration, and the reasons for the different effects of the routes have not yet been fully clarified. So, performing more high-quality studies to clarify these issues is imperative. In addition, studies have demonstrated that endometrial thickness is very important for successful embryo implantation. Based on the effects of SG-CSF on IR and CPR, it is reasonable to speculate that SG-CSF may be also best regimen for increasing endometrium thickness and LBR. However, currently available studies have not evaluated the role of SG-CSF in both two outcomes; therefore, this open question should be further investigated.
The current network meta-analysis also found that hCG may be the best therapeutic option for increasing endometrium thickness and improving LBR. As a primary embryonic signal, hCG is crucial to initiating and maintaining a pregnancy [Citation53]. To be specific, several endometrial paracrine parameters, which correlate to endometrial differentiation, angiogenesis, implantation, and tissue remodeling, can be modulated by IG perfusion of hCG during the secretory phase [Citation54], thus promoting endometrial repairs and thickening [Citation55]. Previous network meta-analyses and meta-analysis results have also shown that hCG infusion significantly improves CPRs in patients with recurrent implantation failure (RIF) [Citation56] and significantly increases live birth rates (LBRs) [Citation57]. However, the majority of embryos transferred into a woman’s uterine cavity through IVF are eight cell stage or longer, and such embryos are unable to communicate with the mother before implantation, which is compensated for by hCG infusion before embryo transfer. Some studies have also shown that hCG infusion can inhibit the expression of insulin-like growth factor binding protein-1 (IGFBP-1) and macrophage colony-stimulating factor (M-CSF) by activating LIF, VEGF, MMP-9, and other factors to further improving endometrial, receptivity [Citation58]. However, it has also been suggested that the duration of pre-implantation hCG infusion needs to be controlled to prevent prolonged exposure of the endometrium to HCG, resulting in loss of sensitivity to the blastocyst secretion of hCG [Citation59,Citation60]. Additionally, hCG works on the endometrium and endometrial receptors (such as hCG/luteinizing hormone [hCG/LH] receptors and IL-1 receptor) to increase local angiogenesis at the embryo implantation site on the endometrium, promote embryo implantation, and reduce early embryo loss, thereby increasing LBR [Citation55]. Nevertheless, owing to the lack of eligible studies, we cannot analyze the effectiveness of SG-CFS on the endometrium thickness and LBR, so this study does not answer whether hCG is also better than SG-CSF, additional high-quality studies are required to verify the benefits of SG-CSF on these two outcomes.
Heterogeneity explanation
Significant statistical heterogeneity was detected for the comparison of hCG vs. control (98.9%), IG-CSF vs. control (78.7%), and PRP vs. control (91.3%) in endometrium thickness. Moreover, we also detected statistical heterogeneity in the comparison of IG-CSF vs. control (59.7%) in the meta-analysis of IR. We are not surprised at these results due to the variations in the study design, years of infertility, and the endometrium thickness before treatment across studies included in each comparison. For example, the RCT by Wang [Citation16] reported a significant increase in the endometrium thickness of hCG (MD = 5.80, 95% CI = 4.77–6.82), but the retrospective cohort (RPC) by Zhang et al. [Citation17] reported that hCG did not significantly increase the endometrium thickness (MD = 0.38, 95% CI = −0.01–0.77). In addition, we also recognized that most eligible studies included extremely limited sample sizes except for four studies which included more than 100 patients in each group. Studies with small sample sizes will yield more biased results than those with adequate sample sizes, inevitably introducing heterogeneity to the pooled results. In addition, as a primary outcome of interest, the measurement of endometrial thickness will be affected by operator and technology. Nevertheless, we cannot perform subgroup analysis to definitively explore which factors may be the major contributor to the significant statistical heterogeneity due to the limited number of eligible studies. So, our findings should be interpreted with caution and future studies should be performed to address these questions.
Limitations of the current network meta-analysis
Undeniably, there are some limitations in this network meta-analysis. First, this study did not register formal protocol, but we strictly followed the PRISMA-NMA statement. Second, details of randomization, assignment hiding method, and blind method were not reported in some eligible RCTs, which may introduce bias in selection and measurement. Third, over half of the studies in this systematic review are from China, so our findings inevitably have a geographical bias. Fourth, the meta-analysis eliminated conference abstracts and studies that were not in English and Chinese, with a relatively small number of studies included and only two on hCG, one on PBMC, and one on SG-CSF. Fifth, although we found that the dose of the same drug varied in different studies, we did not classify the included studies into subgroups to conduct further analysis due to the limited number of eligible studies and insufficient sample size. Sixth, safety assessments for different drugs were not ensured since the adverse events in tested treatments were reported in very few studies. Seventh, we combined studies with different designs to estimate the comparative efficacy of different immunomodulatory therapies. Therefore, our findings will inevitably be biased because non-RCTs are more susceptible to confounding factors than RCTs. Finally, we need to clearly know that SUCRA cannot show whether the difference between treatments is clinically meaningful, and ranking of treatments can distract attention from the evidence and issues underlying individual studies.
Conclusions
In summary, among four kinds of immunomodulatory therapies for the treatment of patients with thin endometrium, SG-CSF, and hCG may increase endometrium thickness and improve IR rate through the regulation of IG immune environment, which is the optimal immunotherapy based on current evidence. Given the potential limitations, our findings need to be treated with caution in clinical practice.
Patient consent
Not required.
Author contributions
Substantially contributed to conception or design: Lifei Li and Yan Wang. Contributed to data acquisition, analysis, or interpretation: Lifei Li, Zhijian Kou, and Fei Zhao. Drafted the manuscript for important content: Lifei Li. Critically revised the manuscript for important intellectual content: Xuehong Zhang. Gave final approval: All authors.
Supplemental Material
Download Zip (7.7 MB)Acknowledgments
We would like to deeply appreciate all authors who performed all eligible studies which have been included in the present network meta-analysis.
Availability of data and materials
All data generated or analyzed during this study are included in this published article/as Supplementary Information Files.
Disclosure statement
None.
Additional information
Funding
References
- Mao X, Zhang J, Cai R, et al. Therapeutic role of granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with persistent thin endometrium: a prospective and randomized study. Int J Gynaecol Obstetric. 2020;150(2):194–199.
- Traub ML, Van Arsdale A, Pal L, et al. Endometrial thickness, Caucasian ethnicity, and age predict clinical pregnancy following fresh blastocyst embryo transfer: a retrospective cohort. Reprod Biol Endocrinol. 2009;7(1):33. doi: 10.1186/1477-7827-7-33.
- Al Chami A, Saridogan E. Endometrial polyps and subfertility. J Obstet Gynaecol India. 2017;67(1):9–14. doi: 10.1007/s13224-016-0929-4.
- Jiang L, Xu X, Cao Z, et al. Comparison of frozen embryo transfer outcomes between uterine infusion of granulocyte colony-stimulating factor and growth hormone application in patients with thin endometrium: a retrospective study. Front Endocrinol (Lausanne). 2021;12:725202. doi: 10.3389/fendo.2021.725202.
- Salamonsen LA, Evans J, Nguyen HP, et al. The microenvironment of human implantation: determinant of reproductive success. American J Rep Immunol. 2016;75(3):218–225. Mardoi: 10.1111/aji.12450.
- Lebovitz O, Orvieto R. Treating patients with "thin" endometrium - an ongoing challenge. Gynecol Endocrinol. 2014;30(6):409–414. doi: 10.3109/09513590.2014.906571.
- Kasius A, Smit JG, Torrance HL, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(4):530–541. Jul-Aug doi: 10.1093/humupd/dmu011.
- Wu Y, Gao X, Lu X, et al. Endometrial thickness affects the outcome of in vitro fertilization and embryo transfer in normal responders after GnRH antagonist administration. Reprod Biol Endocrinol. 2014;12(1):96. doi: 10.1186/1477-7827-12-96.
- Aydin T, Kara M, Nurettin T. Relationship between endometrial thickness and in vitro Fertilization-Intracytoplasmic sperm injection outcome. Int J Fertil Steril. 2013;7(1):29–34.
- Lian R, Wang X, Lin R, et al. Evaluation of granulocyte colony-stimulating factor on the treatment of thin endometrium during frozen-thawed embryo transfer cycles: a retrospective cohort study. Gynecol Endocrinol. 2020;36(4):370–374. doi: 10.1080/09513590.2019.1658187.
- Gargett CE, Healy DL. Generating receptive endometrium in Asherman’s syndrome. J Hum Reprod Sci. 2011;4(1):49–52. doi: 10.4103/0974-1208.82361.
- Senturk LM, Erel CT. Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol. 2008;20(3):221–228. doi: 10.1097/GCO.0b013e328302143c.
- Xu B, Zhang Q, Hao J, et al. Two protocols to treat thin endometrium with granulocyte colony-stimulating factor during frozen embryo transfer cycles. Reprod Biomed Online. 2015;30(4):349–358. doi: 10.1016/j.rbmo.2014.12.006.
- Chang Y, Li J, Wei LN, et al. Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine (Baltimore). 2019;98(3):e14062. doi: 10.1097/MD.0000000000014062.
- Kunicki M, Łukaszuk K, Woclawek-Potocka I, et al. Evaluation of granulocyte colony-stimulating factor effects on treatment-resistant thin endometrium in women undergoing in vitro fertilization. Biomed Res Int. 2014;2014:913235–913235. doi: 10.1155/2014/913235.
- Wang L. Effect of intrauterine hCG infusion on IVF-ET outcome of thin endometrium. Chin Foreign Med Res. 2020;18(28):127–129.
- Zhang W, Huang H, Chen C. Effect of intrauterine perfusion of human chorionic gonadotropin on the clinical outcome of thin endometrial freezethaw cycle. Chin Foreign Med Res. 2021;19(34):9–12.
- Banerjee K, Singla B, Verma P. Efficacy of subcutaneous granulocyte colony stimulating factor infusion for treating thin endometrium. Clin Exp Reprod Med. 2022;49(1):70–73. doi: 10.5653/cerm.2021.04833.
- Li S, Wang J, Cheng Y, et al. Intrauterine administration of hCG-activated autologous human peripheral blood mononuclear cells (PBMC) promotes live birth rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J Reprod Immunol. 2017;119:15–22. Feb doi: 10.1016/j.jri.2016.11.006.
- Dzhincharadze L, Abubakirov A, Mishieva N. Effectiveness of intrauterine administration of autologous platelet-rich plasma to prepare ‘thin’ endometrium for the defrosted embryo transfer program. Akusherst Ginekol (Russian Federat). 2021;2:90–Z5.
- Nazari L, Salehpour S, Hoseini S, et al. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: a double-blind RCT. Int J Reprod Biomed. 2019;17(6):443–448. doi: 10.18502/ijrm.v17i6.4816.
- Higgins J, Thomas J, Chandler J. Cochrane handbook for systematic reviews of interventions version. Vol. 6. Hoboken (NJ): Cochrane Library; 2021.
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135.
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919.
- McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. doi: 10.1002/jrsm.1411.
- Tu YK. Using generalized linear mixed models to evaluate inconsistency within a network meta-analysis. Value Health. 2015;18(8):1120–1125. doi: 10.1016/j.jval.2015.10.002.
- Higgins JP, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. doi: 10.1002/jrsm.1044.
- Mbuagbaw L, Rochwerg B, Jaeschke R, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6(1):79. doi: 10.1186/s13643-017-0473-z.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629.
- Chang Y, Zhang X, Yang X. Platelet-rich plasma promoted the growth of endometrium and pregnancy rate in women with thin endometrium. J Pract Obstetric Gynecol. 2016;32(06):445–449.
- Cheng H, Liu K, Liu Y. Influence of platelet-rich plasma on the pregnancy outcome of thin endometrial patients with freeze-thaw embryo transfer. Prog Obstet Gynecol. 2020;29(06):450–452.
- Dzhincharadze L, Abubakirov A, Mishieva N. Intrauterine use of granulocyte colony-stimulating factor in patients with thin endometrium in frozen embryo transfer programs. Akusherst Ginekol (Russian Federat). 2020;2020(8):106–110.
- Eftekhar M, Neghab N, Naghshineh E, et al. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwanese J Obstetric Gynecol. 2018;57(6):810–813.
- Eftekhar M, Sayadi M, Arabjahvani F. Transvaginal perfusion of G-CSF for infertile women with thin endometrium in frozen ET program: a non-randomized clinical trial. Iranian J Reprod Med. 2014;12(10):661–666.
- Kunicki M, Łukaszuk K, Liss J, et al. Granulocyte colony stimulating factor treatment of resistant thin endometrium in women with frozen-thawed blastocyst transfer. Syst Biol Reprod Med. 2017;63(1):49–57. doi: 10.1080/19396368.2016.1251505.
- Li Y, Pan P, Chen X, et al. Granulocyte colony-stimulating factor administration for infertile women with thin endometrium in frozen embryo transfer program. Reprod Sci. 2014;21(3):381–385. doi: 10.1177/1933719113497286.
- Long H, Wang F, Wu D. Comparison of therapeutic effect of two intrauterine perfusion treatments on patients with thin endometrium. J Reprod Med. 2020;29(11):1421–1426.
- Qi G, Wang Y, Xu S. Influence of intrauterine infusion platelet-rich plasma on the pregnancy outcome of thin endometrial patients with freeze-thaw embryo transfer. Chin Remedi Clin. 2018;18(06):1023–1025.
- Sarvi F, Arabahmadi M, Alleyassin A, et al. Effect of increased endometrial thickness and implantation rate by granulocyte colony-stimulating factor on unresponsive thin endometrium in fresh in vitro fertilization cycles: a randomized clinical trial. Obstet Gynecol Int. 2017;2017:3596079–3596076. doi: 10.1155/2017/3596079.
- Zhang Y, Chen X, Chen S, et al. Intrauterine administration of G-CSF for promoting endometrial growth after hysteroscopic adhesiolysis: a randomized controlled trial. Hum Reprod. 2022;37(4):725–733. doi: 10.1093/humrep/deac023.
- Xie Y, Zhang T, Tian Z, et al. Efficacy of intrauterine perfusion of granulocyte colony-stimulating factor (G-CSF) for infertile women with thin endometrium: a systematic review and meta-analysis. Am J Rep Immunol. 2017;78(2). doi: 10.1111/aji.12701.
- Liu M, Yuan Y, Qiao Y, et al. The effectiveness of immunomodulatory therapies for patients with repeated implantation failure: a systematic review and network meta-analysis. Sci Rep. 2022;12(1):18434. doi: 10.1038/s41598-022-21014-9.
- Zhao J, Xu B, Xie S, et al. Whether G-CSF administration has beneficial effect on the outcome after assisted reproductive technology? A systematic review and meta-analysis. Reprod Biol Endocrinol. 2016;14(1):62. doi: 10.1186/s12958-016-0197-2.
- Hou Z, Jiang F, Yang J, et al. What is the impact of granulocyte colony-stimulating factor (G-CSF) in subcutaneous injection or intrauterine infusion and during both the fresh and frozen embryo transfer cycles on recurrent implantation failure: a systematic review and meta-analysis? Reprod Biol Endocrinol. 2021;19(1):125. doi: 10.1186/s12958-021-00810-4.
- McCracken S, Layton JE, Shorter SC, et al. Expression of granulocyte-colony stimulating factor and its receptor is regulated during the development of the human placenta. J Endocrinol. 1996;149(2):249–258. doi: 10.1677/joe.0.1490249.
- Rutella S, Pierelli L, Bonanno G, et al. Role for granulocyte colony-stimulating factor in the generation of human T regulatory type 1 cells. Blood. 2002;100(7):2562–2571. doi: 10.1182/blood-2001-12-0291.
- Rutella S, Zavala F, Danese S, et al. Granulocyte colony-stimulating factor: a novel mediator of T cell tolerance. J Immunol (Baltimore, MD: 1950). 2005;175(11):7085–7091. doi: 10.4049/jimmunol.175.11.7085.
- Sloand EM, Kim S, Maciejewski JP, et al. Pharmacologic doses of granulocyte colony-stimulating factor affect cytokine production by lymphocytes in vitro and in vivo. Blood. 2000;95(7):2269–2274. doi: 10.1182/blood.V95.7.2269.
- Jensen JR, Witz CA, Schenken RS, et al. A potential role for colony-stimulating factor 1 in the genesis of the early endometriotic lesion. Fertil Steril. 2010;93(1):251–256. Jan doi: 10.1016/j.fertnstert.2008.09.050.
- Davar R, Miraj S, Farid Mojtahedi M. Effect of adding human chorionic gonadotropin to frozen thawed embryo transfer cycles with history of thin endometrium. Int J Reprod Biomed. 2016;14(1):53–56. doi: 10.29252/ijrm.14.1.53.
- Licht P, Russu V, Lehmeyer S, et al. Molecular aspects of direct LH/hCG effects on human endometrium–lessons from intrauterine microdialysis in the human female in vivo. Reprod Biol. 2001;1(1):10–19.
- Papanikolaou EG, Kyrou D, Zervakakou G, et al. "Follicular HCG endometrium priming for IVF patients experiencing resisting thin endometrium. A proof of concept study. J Assist Reprod Genet. 2013;30(10):1341–1345. Oct doi: 10.1007/s10815-013-0076-0.
- Kong X, Tang G, Liu Y, et al. Efficacy of intrauterine infusion therapy before embryo transfer in recurrent implantation failure: a systematic review and network meta-analysis. J Reprod Immunol. 2023;156:103819. doi: 10.1016/j.jri.2023.103819.
- Xie H, Zeng H, He D, et al. Effect of intrauterine perfusion of human chorionic gonadotropin before embryo transfer after two or more implantation failures: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2019;243:133–138. Dec doi: 10.1016/j.ejogrb.2019.10.039.
- Santibañez A, García J, Pashkova O, et al. Effect of intrauterine injection of human chorionic gonadotropin before embryo transfer on clinical pregnancy rates from in vitro fertilisation cycles: a prospective study. Reprod Biol Endocrinol. 2014;12(1):9. 29doi: 10.1186/1477-7827-12-9.
- Osman A, Pundir J, Elsherbini M, et al. The effect of intrauterine HCG injection on IVF outcome: a systematic review and meta-analysis. Reprod Biomed Online. 2016;33(3):350–359. Sep doi: 10.1016/j.rbmo.2016.05.010.
- Evans J, Salamonsen LA. Too much of a good thing? Experimental evidence suggests prolonged exposure to hCG is detrimental to endometrial receptivity. Hum Reprod. 2013;28(6):1610–1619. doi: 10.1093/humrep/det055.