Abstract
Objectives: This study aimed to explore the effect and mechanism of Yunkang oral liquid (YK) on polycystic ovary syndrome (PCOS). Methods: PCOS model rats were prepared by injecting exogenous androgen dehydroepiandrosterone, and YK was administered simultaneously for 28 days during modeling. The morphology of ovaries and uterus was observed using H&E staining, and serum levels of testosterone (T), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) were determined by radioimmunoassay. Additionally, serum lipids (TG, HDL-c), blood glucose (GLU), and aminotransferase (AST, ALT) levels were detected. The expression of androgen receptor (AR) protein was determined by Western blotting. Results: YK treatment resulted in reduced serum levels of T, LH and FSH, ameliorated ovarian polycystic-like pathological changes and uterine morphology in PCOS rats, and decreased serum TG, GLU, AST and ALT levels, elevated serum HDL-c levels, and improved abnormalities of glycolipid metabolism accompanying PCOS. Moreover, YK decreased the expression of ovarian AR in PCOS rats. Conclusions: This study indicates that YK may protect the ovaries by inhibiting the expression of AR, which could be a potential treatment for PCOS.
Introduction
Polycystic ovarian syndrome (PCOS) is a female endocrine disorder characterized by high levels of androgens, which can hinder the development and maturation of follicles, resulting in polycystic ovarian changes [Citation1]. The incidence rate of PCOS in women of reproductive age is approximately 6%–20%, which is the most common endocrine-metabolic disorder affecting a vast population worldwide [Citation2,Citation3]. Patients with PCOS typically present with menstrual disorders, infertility, and often accompanied by obesity, abnormalities in glycolipid metabolism, and hepatic impairment [Citation4]. The PCOS symptoms can persist throughout woman’s lifetime, seriously affecting physical and mental health, and the treatment of PCOS needs to be taken seriously. However, there is still a lack of specific therapeutic drugs and methods for PCOS, especially the adverse reactions caused by modern medicine, and long-term treatment is often not acceptable to patients [Citation5].
Traditional Chinese medicine (TCM) is one of the oldest health care medical systems in China and other Asian countries and has been used for centuries to treat a wide range of human diseases because of their therapeutic efficacy, minimal side effects, and widespread application [Citation6]. TCM is particularly effective in treating gynecological diseases and is widely used in clinical practice [Citation7,Citation8]. It should be noted that in recent years, the treatment of PCOS with TCM has achieved certain efficacy, and its advantages of multi-targets, multi-links, multi-pathways, low side effects, and high cure rate have been recognized by the medical profession and patients [Citation9]. Yunkang oral liquid (YK) is included in the Pharmacopeia of the People’s Republic of China for the treatment of obstetrics and gynecology diseases of the traditional Chinese medicine, clinically commonly used for abortion and menopausal syndrome [Citation10]. Prior research indicates that YK increases estrogen and progesterone levels, enhances endocrine regulation within the gonadal axis, upregulates the expression of estrogen and progesterone receptors in uterine decidual tissue, and increases the expression of vascular endothelial growth factor protein in this tissue. This enhances endometrial receptivity and reduces the embryo loss rate in mice with recurrent spontaneous abortion [Citation11]. Additionally, YK modulates the balance of Th1/Th2 immune factors, promoting decidual development and facilitating blastocyst implantation in mice with bacterial lipopolysaccharide-induced abortions [Citation12]. Nevertheless, to date, the role and mechanism of YK in PCOS have not been confirmed.
The pathogenesis of PCOS is primarily caused by dysfunction of the HPO axis, which hinders follicular development and leads to ovulation disorders, ultimately resulting in infertility [Citation13]. Additionally, the androgen receptor (AR) can be involved in follicular development and ovulation through the regulation of androgen secretion, which is closely linked to the occurrence of PCOS [Citation14]. It is currently unclear whether YK can promote follicular development and treat PCOS by inhibiting AR expression. In this study, the PCOS model was prepared by injecting the exogenous androgen dehydroepiandrosterone, and the therapeutic effects of YK on PCOS were observed by detecting AR expression, sex hormone levels, glycolipid metabolism, and liver enzyme functions, thus providing a scientific basis for the novel treatment of PCOS.
Materials and methods
Animals
Sixty female SD rats of 3 weeks old were provided by Shanghai Slake Experimental Animal Co., Ltd. with the license number of SCXK (Shanghai) 2017-0005. The feeding room shall be well ventilated, with constant temperature (22–25 °C) and humidity (65 ± 5%), and the lighting condition shall be 12 h light/12 h dark.
Reagents
Yunkang oral liquid, provided by Huiyinbi Group Zhejiang Qiqi Pharmaceutical Co., Ltd., batch number 18051211. Diane-35 (ethinylestradiol cycloproterone tablets) produced by Bayer Healthcare Co., Ltd., batch number 363 A. Dehydroepiandrosterone (DHEA) produced by Xi’an Gaoyuan Biotechnology Co., Ltd., batch number P30-Q-181002. Testosterone (T), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) radioimmunoassay kit, batch number 20190320, provided by Beijing Huaying Biotechnology Research Institute. Alanine aminotransferase (ALT) kit (batch number 180104101), aspartate aminotransferase (AST) kit (batch number 17031702), triglyceride (TG) kit (batch number 180205101), high-density lipoprotein cholesterol (HDL-c) kit (batch number 180703107), and glucose (GLU) kit (batch number 180614101) were all purchased from Ningbo Meikang Biotechnology Co., Ltd. Androgen receptor (AR) antibody (batch number. HZ02245689), Shanghai Huzhen Industrial Co., Ltd. GAPDH antibody (batch number 8998n72), Affinity Company, USA. Goat anti rabbit (H + L) HRP (batch number. J805472710), Hangzhou Dawen Biological Co., Ltd. BCA protein concentration determination kit (batch number P0012), Shanghai Biyuntian Biotechnology Co., Ltd.
Experimental design
The rats were randomly divided into six groups based on body weight: normal control group (NG), model control group (MG), Diane-35 group (9 mg/kg), YK low-dose group (YKL, 6 ml/kg), YK medium-dose group (YKM, 9 ml/kg), and YK high-dose group (YKH, 13.5 ml/kg), with 10 rats in each group. With the exception of the NG group, all other groups of rats were injected with DHEA (60 mg/kg) subcutaneously every morning from the age of 23 days [Citation15], once a day for 28 days, while rats in NG group received corresponding normal saline injections.
The clinical dosage of YK is 60 ml per day, which is converted to a rat dosage of 6 ml/kg. The three YK dose groups were administered orally at doses of 6 ml/kg, 9 ml/kg, and 13.5 ml/kg, respectively. The Diane-35 group was administered with a dose of 9 mg/kg. Rats in each treatment group were administered with the corresponding dose of test drug by gavage in the afternoon every day from the age of 23 days, once a day for four consecutive weeks. The NG and MG groups were given corresponding volumes of purified water. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals in the Zhejiang University of Technology, Hangzhou, China, and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1996).
General signs
During the administration, rats were weighed regularly, and the weight value was recorded to calculate the weight gain rate. After the last administration, the body length of rats (the distance from the tip of the nose to the anus) was measured and Lee’s index was calculated [Citation16]. Lee’s index = weight (g) ^ (1/3) x 10/length (cm).
Behavioral measurement
At the fourth week of administration, the rat’s grip strength was assessed by grasping the tail root of the rat, and when the rat forcefully grasped the elastic tension plate, applied force in a timely manner before pulling until the rat abandoned the tension plate. The instrument then read and recorded the grip strength value. Subsequently, the rat in a quiet state was placed in a holder, with its tail (approximately 2 cm from the tip of the tail) positioned in the thermal radiation area of the instrument. Upon the rat’s tail rapidly retracting from the thermal radiation zone, the instrument automatically read and recorded the pain threshold value. Furthermore, the rat’s back temperature was measured by positioning the infrared sensor’s test hole directly over the arch of the rat’s back. Once the instrument had reached a stable reading, the back temperature value was recorded.
Organ index
After 4 weeks of administration, the anesthetized rats dissected and removed the ovaries and uterus, washed them with normal saline, weighed the wet weight of organs, and calculated the organ index [Citation17,Citation18]. Organ index (%) = organ moisture × 100/body weight.
Observation of pathological tissue staining
The ovaries and uterus of rats were fixed in 4% paraformaldehyde for 48 h, followed by dehydration treatment with xylene and ethanol. The tissues were then embedded in paraffin slices. The tissue sections were then deparaffinized using a gradient of ethanol and xylene. Subsequently, the sections were stained with H&E dyes. Finally, the sections were examined under micrograph for observation.
Determination of serum indicators
Serum was obtained from rats at the end of administration and the concentrations of serum T, FSH, and LH were measured by radioimmunoassay. In addition, serum levels of TG, HDL-c, GLU, ALT, and AST were determined using automated blood biochemistry analyzer.
Ovarian AR protein expression by Western blot assay
The ovarian tissue was fully ground and the appropriate amount of protein lysate was added, and after extracting the tissue proteins, the protein concentration was detected by BCA protein assay kit. After preparing the gel, the samples were loaded. Following electrophoretic membrane transfer, the membrane was closed for 2 h on a shaker. The primary antibody AR was incubated overnight at 4 °C. Subsequently, the samples were incubated with an HRP-labeled secondary antibody on shaker and finally colored with chemiluminescence. The relative expression level of the target protein was calculated by analyzing protein grayscale values using ImageJ software.
Statistical treatment
All data were expressed as mean ± standard deviation (mean ± SD). SPSS 19.0 statistical software was used to analyze the experimental data. Groups were compared by t-tests, and one-way analysis of variance. p<.05 was considered that indicates the difference is statistically significant.
Results
YK reversals normalize PCOS phenotypes
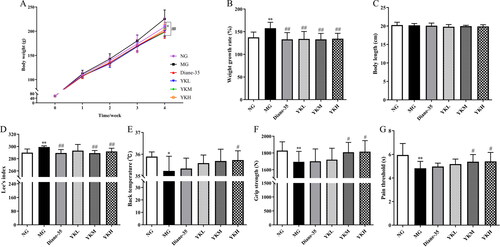
After 4 weeks of administration, the MG group rats showed significant increases in body weight, weight gain rate, and Lee’s index compared to the NG group (p < .05, .01). However, there was no significant difference in body length. Following YK intervention, the weight and weight gain rate of PCOS rats significantly decreased (p < .01). Additionally, Lee’s index of rats in the YKM and YKH groups significantly reduced (p < .01). Further examination of physical indicators revealed that the back temperature, grip strength, and pain threshold values of PCOS rats were significantly reduced (p < .05, .01). Compared with the MG group, the YKM and YKH groups of rats showed a significant increase in grip and pain threshold values (p < .05). Additionally, the back temperature of YKH group rats was obviously increased (p < .05; ).
Figure 1. Effect of YK on body weight, Lee’s index, back temperature, grip strength and pain threshold of PCOS rats. (A) Body weight. (B) Weight growth rate. (C) Body length. (D) Lee’s index. (E) Back temperature. (F) Grip strength. (G) Pain threshold. Data are shown as mean ± SD (n = 10). Compared with the NG group, *p < .05, **p < .01, compared with the MG group, #p < .05, ##p < .01.
YK improves ovarian function in PCOS rats
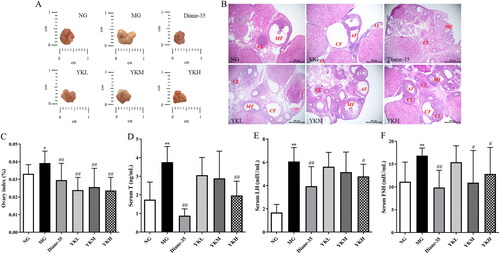
PCOS is characterized by polycystic ovary changes and disrupted secretion of sex hormones. The surface of the ovaries of the rats in the NG group was reddish in color, while H&E staining revealed that follicles and Corpus luteum of all developmental periods were visible in the ovaries, and the granulosa cells were intact in morphology and neatly arranged.
Compared with the NG group, the ovary index and serum T, FSH and LH levels of the PCOS rats were significantly higher (p < .05, .01), and the ovaries were cystically enlarged with pale surfaces. In the ovarian tissue of MG rats, the number of follicles at different developmental stages was found to have significantly decreased, with almost no mature follicles present. The number of Corpus luteum exhibited a significant decline, while the number of atretic follicles demonstrated a notable increase. The number of cystic follicles increased, while the number of granulosa cell layers in the follicles decreased, indicating the presence of typical polycystic ovarian pathological changes. The ovary index of rats treated with YK significantly reduced (p < .01), and the ovarian morphology showed different improvements. The number of Corpus luteum and follicles at all levels in the ovaries increased significantly, cystic dilation was ameliorated, and the number of granulosa cell layers in the follicle was significantly increased, in which serum levels of T, FSH, and LH were significantly reduced in the rats of the YKH group (p < .05, .01) ().
Figure 2. Effect of YK on ovaries tissue of PCOS rats. (A) Ovaries appearance. (B) H&E staining images of the ovaries tissues. (C) Ovary index. (D) Changes in serum T level. (E) Changes in serum FSH level. (F) Changes in serum LH level. CL: Corpus luteum; MF: mature follicle; AF: atretic follicle; CF: cystic follicle. Data are shown as mean ± SD (n = 10). Compared with the NG group, *p < .05, **p < .01, compared with the MG group, #p < .05, ##p < .01.
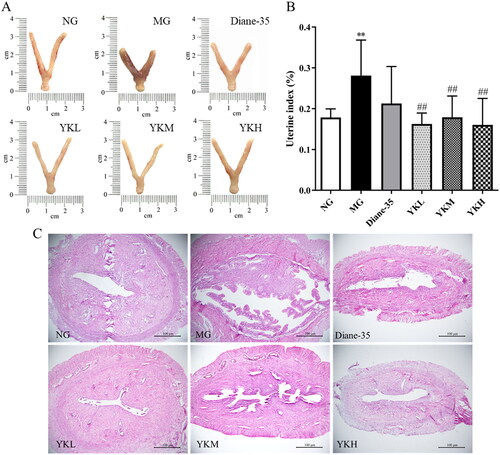
YK alleviates uterus tissue damage in PCOS rats
PCOS could cause uterine tissue to become diseased in severe cases. The uterus morphology of rats in the NG group was thin and long, and H&E staining revealed that the endometrium of rats in this group was well developed. Compared with the NG group, PCOS rats exhibited a significantly higher uterine index (p < .01), with a short and thick uterine morphology, thickening of the uterine muscle layer, abnormal development of the endometrium, and dilation and edema of the uterine mucosal glands. Compared to the MG group, the uterine index of YK treated rats was significantly reduced (p < .01), the uterine morphology was closer to that of normal rats, and all treatment groups showed improvement in uterine lesions, which were manifested by the thinning of the myometrium and the benign development of the endometrium ().
YK improves glycolipid metabolism in PCOS rats
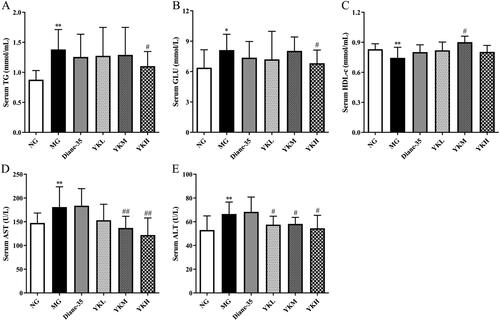
PCOS is frequently associated with irregularities in glycolipid metabolism. This study detected the levels of blood lipids, blood glucose, and transaminase in PCOS rats and found that compared with the NG group, the serum levels of TG, GLU, AST, and ALT in the MG group rats were significantly increased, while the serum levels of HDL-c were significantly reduced (p < .05, .01). Compared with the MG group, only serum ALT level was significantly reduced in the YKL group rats (p < .05). The serum HDL-c level of YKM group rats increased, while the serum AST and ALT levels showed a decrease (p < .05, .01). The serum levels of TG, GLU, AST, and ALT were significantly reduced in the YKH group rats (p < .05, .01), while there was no significant difference in the levels of serum TG, GLU, HDL-c, AST, and ALT in the Diane-35 group ().
Figure 4. Effect of YK on glycolipid metabolism of PCOS rats. (A) Changes in serum TG level. (B) Changes in serum GLU level. (C) Changes in serum HDL-c level. (D) Changes in serum AST level. (E) Changes in serum ALT level. Data are shown as mean ± SD (n = 10). Compared with the NG group, *p < .05, **p < .01, compared with the MG group, #p < .05, ##p < .01.
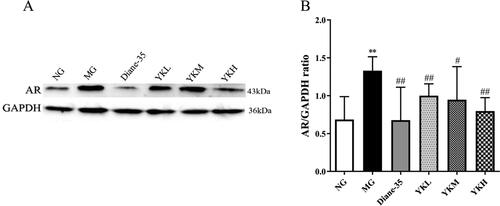
YK inhibits AR expression in PCOS rats
The AR signaling pathway is crucial in the development of androgen-induced PCOS. Western blot analysis detected elevated AR protein expression in the ovaries of PCOS rats (p < .01). However, YK intervention reduced AR protein expression in the model rats (p < .05, .01), indicating that YK may treat PCOS by inhibiting the AR signaling pathway ().
Figure 5. Effect of YK on AR protein expression in ovaries of PCOS rats. (A) The expression of AR in the ovaries was detected by Western blot. (B) Statistical analysis of AR expression by imageJ. Data are shown as mean ± SD. Compared with the NG group, *p < .05, **p < .01, compared with the MG group, #p < .05, ##p < .01.
Discussion
The DHEA-induced PCOS model is an excellent model because animals can develop many of the characteristics of human PCOS [Citation19]. DHEA is an intermediate product of synthetic testosterone biosynthesis that directly elevates androgen levels. This study found the effects of hyperandrogenism induced by 28 consecutive days of DHEA injection, which inhibited follicular maturation and hindered the development of dominant follicles. This resulted in ovulatory dysfunction and the development of polycystic ovary-like changes, which is consistent with previous studies [Citation20,Citation21].
From the perspective of TCM, kidney deficiency is the root cause and initiating factor of PCOS [Citation22] and is primarily characterized by irregular menstrual cycles, lower back and knee pain, sensitivity to cold, fatigue and weakness. This experiment measures the indicators of grasping force, pain threshold, and back temperature to quantify the TCM terminology of PCOS kidney deficiency syndrome [Citation23]. The grasping force value reflected the symptom of fatigue and weakness, the pain threshold value represented the symptom of lumbar and knee soreness, and the dorsal temperature denoted the symptom of fear of cold and limb coldness. The study results suggest that YK could enhance the grip, pain threshold, and back temperature of PCOS rats, and ameliorate the signs of kidney deficiency in PCOS.
PCOS patients exhibit alterations in the function of the HPO axis, which primarily manifest as an imbalance in hormone secretion. This imbalance is characterized by abnormal secretion of gonadotropins, with abnormally elevated LH being the most prominent feature [Citation24]. The HPO axis strictly regulates female serum sex hormone levels, which are also associated with follicular growth in the ovaries. Elevated LH levels can impact androgen secretion and synthesis in the ovaries, resulting in hyperandrogenism [Citation25]. Approximately, 80%–90% of PCOS patients can have elevated androgen levels monitored in their circulating blood [Citation26]. The increase in androgen levels accelerates the frequency of hypothalamic gonadotropin-releasing hormone pulse secretion, causing uncoordinated pituitary secretion of LH and FSH. Hyperandrogenemia is a key feature of PCOS, and this inherent ovarian hyperandrogenic state can stunt follicular development and maturation, leading to polycystic ovarian changes [Citation27]. Moreover, elevated androgen levels can lead to excessive estrogen secretion by the ovaries, increasing the risk of endometrial cancer due to prolonged exposure to high estrogen levels [Citation28]. The experiment discovered that YK can decrease the T level of PCOS rats, inhibit LH and FSH secretion, reduce the production of endogenous androgens in the ovaries, break the vicious cycle between high levels of androgens and LH, and improve the histopathological changes of target organs by inhibiting the effects of androgens on the ovaries and uterus. This suggests that YK can improve the endocrine changes of PCOS rats by regulating the HPO axis function.
PCOS patients frequently present metabolic disorders alongside reproductive disorders [Citation29,Citation30]. Clinical epidemiological studies indicate that more than half of PCOS patients are overweight or obese, with a higher incidence of abnormal glycolipid metabolism and cardiovascular disease [Citation31,Citation32]. The experiment showed that YK inhibited excessive weight gain in PCOS rats, reducing Lee’s index and weight gain rate. PCOS patients can induce excessive secretion of androgens due to abnormal secretion of LH, leading to elevated levels of free fatty acids that affect glucose uptake and utilization, causing abnormalities in the glucose-lipid metabolism [Citation33]. The accumulation of ovarian androgens during this time can cause hyperlipidemia, which can worsen the occurrence of obesity and ultimately lead to the characteristic obese shape of patients with PCOS [Citation34]. The experiment results indicate that YK reduced serum GLU levels, decreased the conversion of cholesterol from high-density lipoproteins to very low-density lipoproteins, elevated serum HDL-c levels, and decreased serum TG levels in PCOS rats. Research has shown that PCOS patients often experience abnormal transaminase activity and may develop liver injury [Citation35], which could be related to elevated transaminase activity caused by disorders of glucolipid metabolism [Citation36]. The results of this study found that YK can reduce serum ALT and AST levels and ameliorated liver injury in PCOS rats. However, the positive control drug Diane-35 did not show significant effects in regulating glucolipid metabolism and improving hepatic injury, indicating that the improvement of glycolipid metabolism by YK contributes to the treatment of PCOS. It is noteworthy that Diane-35 is a commonly utilized clinical treatment for PCOS, with strong anti-androgenic activity, which can reduce the concentration of free testosterone, improve endocrine disorders, and regulate menstrual cycles [Citation37]. However, the results of this study showed that Diane-35 did not show significant effects in regulating lipid metabolism and lowering blood glucose, suggesting that YK is more effective than Diane-35 in improving PCOS with glucose and lipid metabolic complications. This may be related to the biochemical characteristics of YK’s multi-component, multi-target, and multi-action nature.
Androgens play a crucial role in female follicular development and ovulation by specifically binding to AR [Citation38]. Studies have shown that upregulation of AR activity can lead to hyperandrogenism, while overexpression of AR can increase atresia follicles and decrease mature follicles [Citation39]. AR is closely related to the development of PCOS by mediating androgen regulation [Citation40]. Reports indicate that the expression of AR in ovarian granulosa cells of PCOS patients is significantly increased [Citation41], possibly due to hyperandrogenism [Citation42,Citation43]. The present study demonstrated that YK reduced the expression of ovarian AR proteins in PCOS rats, which may be related to the fact that YK is involved in promoting follicular maturation by mediating the regulation of androgens, thereby treating PCOS. Androgens and their receptors have been shown to play a pivotal role in the pathogenesis of PCOS. Therefore, it is also a question worthy of further investigation as to whether other proteins interacting with AR may be involved in the developmental process of PCOS.
Conclusion
In summary, our study demonstrated that YK has a protective effect on the ovaries in PCOS by regulating AR and improves abnormalities of glucolipid metabolism. Thus, YK has broad potential for the treatment of PCOS and warrants further investigation.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
All the data are contained in the manuscript.
Additional information
Funding
References
- Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2(1):1. doi: 10.1038/nrdp.2016.57.
- Zhao H, Zhang J, Cheng X, et al. Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment. J Ovarian Res. 2023;16(1):9. doi: 10.1186/s13048-022-01091-0.
- Dabravolski SA, Nikiforov NG, Eid AH, et al. Mitochondrial dysfunction and chronic inflammation in polycystic ovary syndrome. Int J Mol Sci. 2021;22(8):3923. doi: 10.3390/ijms22083923.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–8. doi: 10.1210/er.2015-1104.
- Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106(3):e1071–e1083. doi: 10.1210/clinem/dgaa839.
- Liu Y, Yang S, Wang K, et al. Cellular senescence and cancer: focusing on traditional Chinese medicine and natural products. Cell Prolif. 2020;53(10):e12894. doi: 10.1111/cpr.12894.
- Chen X, Hao C, Deng W, et al. Effects of the Zishen yutai pill compared with placebo on live births among women in a fresh embryo transfer cycle: a randomized controlled trial. Obstet Gynecol. 2022;139(2):192–201. doi: 10.1097/AOG.0000000000004658.
- Wang X, Su P, Hao Q, et al. A Chinese classical prescription Guizhi-Fuling wan in treatment of ovarian cancer: an overview. Biomed Pharmacother. 2022;153:113401. doi: 10.1016/j.biopha.2022.113401.
- Chen H, Deng C, Meng Z, et al. Effects of TCM on polycystic ovary syndrome and its cellular endocrine mechanism. Front Endocrinol (Lausanne). 2023;14:956772. doi: 10.3389/fendo.2023.956772.
- Sun P, Tang L, Yan D, et al. Efficacy and safety of Yunkang oral liquid combined with conventional therapy for threatened miscarriage of first-trimester pregnancy a protocol for systematic review and meta-analysis. PLoS One. 2022;17(2):e0263581. doi: 10.1371/journal.pone.0263581.
- Chen B, Shi QQ, Liang KL, et al. Effect and mechanism of Yunkang oral liquid in regulating endocrine system and VEGF signaling pathway and reducing abortion rate in recurrent abortion mice. Zhongguo Zhong Yao Za Zhi. 2018;43(9):1894–1900. doi: 10.19540/j.cnki.cjcmm.2018.0064.
- Shi QQ, Yan MQ, Yu HH, et al. Effect of Yunkang oral liquid on preventing LPS-induced abortion and regulating immune tolerance in mice. Zhongguo Zhong Yao Za Zhi. 2019;44(6):1227–1232. doi: 10.19540/j.cnki.cjcmm.20181218.002.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80–97. doi: 10.1016/j.bpobgyn.2016.03.005.
- Caldwell ASL, Edwards MC, Desai R, et al. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci USA. 2017;114(16):E3334–E3343. doi: 10.1073/pnas.1616467114.
- Zhang H, Yi M, Zhang Y, et al. High-fat diets exaggerate endocrine and metabolic phenotypes in a rat model of DHEA-induced PCOS. Reproduction. 2016;151(4):431–441. doi: 10.1530/REP-15-0542.
- Xia J, Yu P, Zeng Z, et al. Lauric triglyceride ameliorates high-fat-diet-induced obesity in rats by reducing lipogenesis and increasing lipolysis and β-oxidation. J Agric Food Chem. 2021;69(32):9157–9166. doi: 10.1021/acs.jafc.0c07342.
- Qiu S, Wu C, Lin F, et al. Exercise training improved insulin sensitivity and ovarian morphology in rats with polycystic ovary syndrome. Horm Metab Res. 2009;41(12):880–885. doi: 10.1055/s-0029-1234119.
- Wu C, Lin F, Qiu S, et al. The characterization of obese polycystic ovary syndrome rat model suitable for exercise intervention. PLoS One. 2014;9(6):e99155. doi: 10.1371/journal.pone.0099155.
- Zhang Y, Yu X, Liu L, et al. Mechanisms of DHEA-induced AMH increase in the ovarian tissues of PCOS rat. Reprod Biol. 2023;23(4):100797. doi: 10.1016/j.repbio.2023.100797.
- Xie F, Zhang J, Zhai M, et al. Melatonin ameliorates ovarian dysfunction by regulating autophagy in PCOS via the PI3K-Akt pathway. Reproduction. 2021;162(1):73–82. doi: 10.1530/REP-20-0643.
- Ye R, Yan C, Zhou H, et al. Brown adipose tissue activation with ginsenoside compound K ameliorates polycystic ovary syndrome. Br J Pharmacol. 2022;179(18):4563–4574. doi: 10.1111/bph.15909.
- Qian H, Xu W, Cui L, et al. Efficacy of Bushen Huatan decoction combined with Baduanjin in the treatment of polycystic ovary syndrome with insulin resistance (IR-PCOS), kidney deficiency and phlegm dampness: study protocol for a randomized controlled trial. Trials. 2021;22(1):781. doi: 10.1186/s13063-021-05770-z.
- Dai MZ, Lyu GY, Xu YY, et al. The prevent miscarriage effects of Yun Kang oral liquid on threatened abortion rat induced by kidney deficiency and Luteum inhibition. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2019;35(1):9–12.
- Krishnan A, Muthusami S. Hormonal alterations in PCOS and its influence on bone metabolism. J Endocrinol. 2017;232(2):R99–R113. doi: 10.1530/JOE-16-0405.
- Zeng X, Xie YJ, Liu YT, et al. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214–221. doi: 10.1016/j.cca.2019.11.003.
- Azziz R, Kintziger K, Li R, et al. Recommendations for epidemiologic and phenotypic research in polycystic ovary syndrome: an androgen excess and PCOS society resource. Hum Reprod. 2019;34(11):2254–2265. doi: 10.1093/humrep/dez185.
- Wang K, Li Y, Chen Y. Androgen excess: a hallmark of polycystic ovary syndrome. Front Endocrinol (Lausanne). 2023;14:1273542. doi: 10.3389/fendo.2023.1273542.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(5):748–758. doi: 10.1093/humupd/dmu012.
- Moran LJ, Norman RJ, Teede HJ. Metabolic risk in PCOS: phenotype and adiposity impact. Trends Endocrinol Metab. 2015;26(3):136–143. doi: 10.1016/j.tem.2014.12.003.
- Anagnostis P, Tarlatzis BC, Kauffman RP. Polycystic ovarian syndrome (PCOS): long-term metabolic consequences. Metabolism. 2018;86:33–43. doi: 10.1016/j.metabol.2017.09.016.
- Kakoly NS, Khomami MB, Joham AE, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018;24(4):455–467. doi: 10.1093/humupd/dmy007.
- Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism. 2019;92:108–120. doi: 10.1016/j.metabol.2018.11.002.
- Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132(2):321–336. doi: 10.1097/AOG.0000000000002698.
- Pan X. Metabolic characteristics of obese patients with polycystic ovarian syndrome: a meta-analysis. Gynecol Endocrinol. 2023;39(1):2239934. doi: 10.1080/09513590.2023.2239934.
- Liu C, Liu K, Zhao X, et al. The associations between alanine aminotransferase and other biochemical parameters in lean PCOS. Reprod Sci. 2023;30(2):633–641. doi: 10.1007/s43032-022-01030-w.
- Chen XX, Xu YY, Wu R, et al. Resveratrol reduces glucolipid metabolic dysfunction and learning and memory impairment in a NAFLD rat model: involvement in regulating the imbalance of Nesfatin-1 abundance and Copine 6 expression. Front Endocrinol (Lausanne). 2019;10:434. doi: 10.3389/fendo.2019.00434.
- Wu H, Ruan X, Jin J, et al. Metabolic profile of Diane-35 versus Diane-35 plus metformin in Chinese PCOS women under standardized life-style changes. Gynecol Endocrinol. 2015;31(7):548–551. doi: 10.3109/09513590.2015.1029447.
- Cheng XB, Jimenez M, Desai R, et al. Characterizing the neuroendocrine and ovarian defects of androgen receptor-knockout female mice. Am J Physiol Endocrinol Metab. 2013;305(6):E717–26.
- Walters KA, Rodriguez Paris V, Aflatounian A, et al. Androgens and ovarian function: translation from basic discovery research to clinical impact. J Endocrinol. 2019;242(2):R23–r50. doi: 10.1530/JOE-19-0096.
- Hu L, Liu Y, Dong P, et al. Protective effect of Wuzibushen recipe on follicular development via regulating androgen receptor in polycystic ovary syndrome model rats. Gynecol Endocrinol. 2023;39(1):2190808. doi: 10.1080/09513590.2023.2190808.
- Ye W, Xie T, Song Y, et al. The role of androgen and its related signals in PCOS. J Cell Mol Med. 2021;25(4):1825–1837. doi: 10.1111/jcmm.16205.
- Luo J, Ye H, Hao L, et al. SRSFS mediate the function of AR in the ovarian granulosa cells of patients with PCOS. Genes Dis. 2021;8(1):94–109. doi: 10.1016/j.gendis.2019.09.005.
- Lin LH, Baracat MC, Maciel GA, et al. Androgen receptor gene polymorphism and polycystic ovary syndrome. Int J Gynaecol Obstet. 2013;120(2):115–118. doi: 10.1016/j.ijgo.2012.08.016.