ABSTRACT
Biphonation, deterministic chaos, sidebands and subharmonics are four non-linear phenomena (NLP) that have been identified as common additions in the phonations of animals. NLP have been hypothesised to communicate urgency, caller identification, fitness and arousal/valence states for a variety of species but have yet to be studied in detail for bottlenose dolphins. For this study, the signature whistles of nine bottlenose dolphins residing at the US Navy Marine Mammal Program (MMP) were opportunistically recorded during routine periods of separation from conspecifics. NLP were found to be common additions onto the spectral structure of signature whistles, occurring in 53% of recorded whistles (340/642). Sidebands were the most common NLP type produced. Although less frequently emitted, biphonations were characterised by a significantly longer persistence than the other NLP types. Age had a negative correlation with overall NLP presence, and more specifically, sideband presence. Individual differences in NLP use existed between dolphins; however, all dolphins were recorded producing a minimum of two NLP types. We describe NLP prevalence in dolphin whistles in order to provide a useful baseline for continued research to further identify changes in NLP across behavioural and/or health conditions.
Introduction
In typical mammalian vocalisation, air is pushed from the lungs into the vocal tract and through a pair of oscillating vocal folds that then vibrate in synchrony (Fitch et al. Citation2002). Multiple studies have shown that due to the non-linear nature of the vocal system and of the vocal folds themselves, a variety of non-linear phenomena (NLP) were produced when the oscillating folds were not in synchrony and/or the system was pushed past its limits (Wilden et al. Citation1998; Fitch et al. Citation2002; Riede et al. Citation2004; Cazau et al. Citation2016). NLP have since been described as additions to the spectral structure of a call’s fundamental frequency and harmonic structure (Herzel et al. Citation1995). These additions have been found in the vocalisations of a wide variety of vertebrates, including amphibians, birds and non-human mammals (Riede et al. Citation2004; Suthers et al. Citation2006; Zollinger et al. Citation2008). The prevalence and communicative purpose of NLP in animal calls remains unclear but may be related to behavioural context and/or the arousal/valence status of the caller. Previous studies have suggested that the presence of NLP was used to convey urgency (e.g. response to a threat), individual recognition, body size, age and fitness (Fitch et al. Citation2002; Mann et al. Citation2006; Townsend and Manser Citation2011; Cazau et al. Citation2016). While the following categories may not incorporate all of the possible NLP types, for the purposes of the present study, we examined the presence of four common categories of non-linear phenomena (i.e. biphonations, deterministic chaos, sidebands and subharmonics) in the signature whistles of nine adult bottlenose dolphins (Tursiops truncatus).
Dolphins have been recorded producing many different call types (for a review of call repertoires, see Herman and Tavolga Citation1980; Popper Citation1980; Herzing Citation1996, Citation2000; Boisseau Citation2005; Jones et al. Citation2019). The three sound categories most commonly produced are clicks used for echolocation (i.e. SONAR used for hunting and navigation) (Kellogg et al. Citation1953; Au Citation1993; Au and Banks Citation1998), burst pulses (i.e. rapid bursts of packets of clicks commonly used in social contexts, with an inter-click interval (ICI) of typically less than 0.004 ± 0.001) (Tyack and Clark Citation2000; Blomqvist and Amundin Citation2004; Luís et al. Citation2016), and frequency and amplitude modulated whistles (i.e. tonal, narrowband, frequency and amplitude modulated sounds) (Kellogg et al. Citation1953; Dreher Citation1961; Lilly and Miller Citation1961; Caldwell and Caldwell Citation1965; Caldwell et al. Citation1990). Dolphins drive their sound production by pressurising two nasal cavities that sit below the blowhole (Mead Citation1975). Between breaths, the dolphin larynx is tightly closed, isolating the lungs from the paired nasal cavities above. Whistles are produced by pushing the pressurised air from the nasal cavities past a pair of phonic lips, one pair in the left and one pair in the right nasal cavity (Ridgway et al. Citation1980, Citation2015; Huggenberger et al. Citation2009; Madsen et al. Citation2013). Past experiments have found that dolphins mostly used the left pair of phonic lips for whistle production, and the right pair of phonic lips (i.e. the larger of the two), for click production (Ridgway et al. Citation1980, Citation2009; Cranford et al. Citation2011; Madsen et al. Citation2013). Although dolphins have vocal folds (i.e. the phonic lips) rather than vocal ‘cords’ like humans and other terrestrial mammals do, and because the larynx is closed during sound production, the term ‘vocalisation’ and ‘whistle’ is a misnomer for dolphin communication (Reidenberg and Laitman Citation1988; Madsen et al. Citation2011). However, for consistency with the literature, we hereafter refer to and use the term vocalisation to describe any sound produced by an animal’s vocal production apparatus regardless of the presence of vocal cord structure.
Signature whistles in dolphins, first described by Caldwell and Caldwell in 1965, were defined as the whistle contours produced most often by an animal when separated from groupmates. Stereotyped and individually distinctive, the frequency-time contour of signature whistles typically occurred between 1.0 kHz and 30.0 kHz and was produced in bouts with 1.0–10.0 seconds between each whistle (Janik et al. Citation2012; Janik and Sayigh Citation2013). Although they have been well studied, descriptions of the presence and characteristics of NLP on signature whistles had yet to be explored. We hypothesised that NLP produced with the signature whistles of bottlenose dolphins will be a common occurrence, as suggested by previous NLP analysis of other marine mammal repertoires.
Biphonations were documented in the calls of marine mammals (Filatova et al. Citation2009; Jones et al. Citation2019), primates (Riede et al. Citation2004), canids (Volodina et al. Citation2006; Schneider and Anderson Citation2011; Frey et al. Citation2016), deer (Reby et al. Citation2016) and in birds (Aubin et al. Citation2000) to date. Two definitions of biphonations have been presented in the literature. Biphonations were typically categorised as either 1) the production of two fundamental frequencies, independently yet simultaneously produced from a single sound source (i.e. two vocal folds) and harmonically unrelated to each other (Wilden et al. Citation1998; Fitch et al. Citation2002; Kriesell et al. Citation2014; Papale et al. Citation2015) or 2) under the ‘two voiced’ phenomenon for vocal structures with two sound sources (i.e. the two pairs of phonic lips for cetaceans and in the syrinx in birds), where both pairs of vocal folds are independently and simultaneously producing calls (Lilly and Miller Citation1961; Wilden et al. Citation1998; Zollinger et al. Citation2008; Madsen et al. Citation2011; Schneider and Anderson Citation2011; Jones et al. Citation2019). We recognise that because two simultaneous sounds could be coming from two different sound structures in the dolphin’s vocal tract, some may not consider this a NLP. For the purposes of this study, we have defined biphonations as two call types (e.g. two frequency modulated whistles or a frequency modulated whistle with an overlapped burst pulse or click train with all or part of the vocalisations overlapped in time) produced simultaneously by one individual (Jones et al. Citation2019).
Deterministic chaos, hereafter referred to as chaos, has been described as broad-band segments of non-random noise centred around a portion of the fundamental frequency (Riede et al. Citation2004). Chaos was found to be a result of desynchronised and irregularly vibrating vocal folds (Fitch et al. Citation2002; Riede et al. Citation2004; Tyson et al. Citation2007; Schneider and Anderson Citation2011). These chaotic segments, while visually similar to turbulent noise on a spectrogram, retained some periodic energy associated with the fundamental frequency of the vocalisation, unlike turbulent noise which had distributed energy across a broadband of frequencies (Fitch et al. Citation2002; Tyson et al. Citation2007; Cazau et al. Citation2016). Subharmonics were produced when one oscillator vibrated at twice, sometimes three times, the frequency of the other, which visually resulted in integer harmonics seen above and below the fundamental frequency and in between the fundamental’s typical interval harmonic structure (Fitch et al. Citation2002; Mann et al. Citation2006; Schneider and Anderson Citation2011). Subharmonics and chaos have both been hypothesised to help avoid habituation to a call type, as the ‘harsh’ or ‘rough’ sound quality could be attention getting for the listener (Blumstein et al. Citation2010; Townsend and Manser Citation2011). Townsend and Manser (Citation2011) conducted experimental playbacks of meerkat (Suricata suricatta) alarm calls with and without NLP and found that meerkat foraging decreased when alarm calls with subharmonics were played, compared to alarm calls without them. Stoeger et al. (Citation2011) reported that 92.0% of roar calls from infant African elephants (Loxodonta africana) contained NLP, with chaos having been the most prevalent. This study concluded that chaos production was highest during high urgency, high arousal settings, and suggested that the addition of NLP was useful for gaining the attention of the intended listener (in that case the elephant’s mother). Similarly, fledglings from different crane species increased their rates of NLP as they got older, in order to keep the attention of their parents before they had to leave the nest (Goncharova et al. Citation2015).
Sidebands, described by Frommolt (Citation1999) as an amplitude or frequency deviation from a portion of the fundamental frequency, appeared on the spectrogram as visible bands above and below the fundamental frequency. Sidebands, when viewed on the spectrogram, were tightly coupled to the fundamental contour, where the difference between the upper sideband and the fundamental was equal to the difference between the fundamental and the lower sideband (Herzel and Reuter Citation1996; Frommolt Citation1999). Riede et al. (Citation2000, Citation2004) defined sidebands as a type of biphonation associated with ‘cyclic amplitude fluctuations in the timeseries waveform’. Similarly, Goncharova et al. (Citation2015) and Zollinger et al. (Citation2008) defined sidebands as an interaction between the fundamental frequency and a much smaller, independent second fundamental frequency, which resulted in additional contour bands above and below the original fundamental. For this study, we classified sidebands as additional frequency bands that visually appeared above and below, yet tightly coupled to, the fundamental frequency, and as a separate NLP category independent from biphonation. Frequency jumps, another common NLP observed in animal calls, were defined as sudden shifts in the call’s contour to a higher or lower frequency (Riede et al. Citation2004). While we see breaks in the fundamental frequency contour in the dolphin whistles analysed here, we were unsure if a break in the whistle contour was the result of a frequency jump from the vocal apparatus or from an amplitude modulation where the contour did not stand out against the background noise. To avoid difficulty in quantifying this, frequency jumps were not analysed for this study.
Acoustic indicators for changes in arousal and/or valence have been utilised in passive welfare monitoring for animals in managed care (Manteuffel et al. Citation2004; Briefer and Le Comber Citation2012). In pigs and cattle, high-pitched sounds were typically associated with high arousal and negative valence (i.e. stress- or fear-induced scenarios), while low-pitched sounds were associated with social contact (Manteuffel et al. Citation2004). This type of acoustic analysis allowed for the identification of call characteristics (including NLP) that alerted farmers to times of high stress amongst live stock (Manteuffel et al. Citation2004; Sadeghi et al. Citation2015). Alternatively, NLP was considered as a positive welfare biomarker in a study on red wolf (Canis rufus) vocalisations that found that biphonations and sidebands in calls were most commonly produced when wolves were playing or socially interacting with conspecifics (Schneider and Anderson Citation2011). In this case, the authors proposed a connection between high arousal and positive valence (i.e. excitement) and the presence of NLP (Schneider and Anderson Citation2011).
It has also been hypothesised that for social animals, the ability to produce biphonations may provide more opportunity for added call features to improve individual and/or group recognition (Volodina et al. Citation2006; Filatova et al. Citation2009; Papale et al. Citation2015, Citation2020). For example, emperor (Aptenodytes forsteri) and king (Aptenodytes patagonicus) penguins that lived and reared their chicks in large, nest-free colonies were found to use biphonations for mate and parental identification (Aubin et al. Citation2000). Lilly and Miller (Citation1961) first reported the simultaneous emission of both clicks and whistles from dolphins, and Caldwell and Caldwell (Citation1967) reported the dual emission of burst pulses and whistles (i.e. what the authors referred to as ‘squawks’). Clicks and burst pulses could cover a portion of the whistle contour or the entire signal (Lilly and Miller Citation1961; Caldwell and Caldwell Citation1967; Root-Gutteridge et al. Citation2018; Jones et al. Citation2019). Soon after birth, calves produced calls termed ‘whistle-squawks’, a biphonic call characterised by a chaotic whistle-type vocalisation that was not as narrowband as a typical fully developed whistle contour (Caldwell and Caldwell Citation1967; Reiss Citation1988; Killebrew et al. Citation2001; Jones et al. Citation2019; Eskelinen and Jones Citation2021). The majority of calf whistles were characterised by the presence of NLP during the whistle development process, for at least 30 days post-partum (Eskelinen and Jones Citation2021). It was unclear if the NLP in dolphin calf whistles were ‘intentional’ additions that had an adaptive function, as described in other species, or were simply a by-product of an immature and underdeveloped vocal production system (Killebrew et al. Citation2001).
Information about the body size and physical fitness of a caller may also be inferred through the presence of NLP. Fitch et al. (Citation2002) proposed that subharmonics and chaos lowered the perceived frequency of a call and suggested this communicated the caller’s body size and physical fitness. In one study, 41.0% of humpback whale (Megaptera novaeangliae) songs, typically associated with mating behaviour, were found to contain chaos (Cazau et al. Citation2016). Cazau et al. (Citation2016) suggested that chaos production correlated with a higher subglottal pressure (i.e. stronger airflow from larger lungs) and that larger air cavities induced more prominent NLP. Both findings related to the idea of chaos signalling a larger body size and/or stronger physical fitness, which could be utilised by females during mate selection (Cazau et al. Citation2016; Root-Gutteridge et al. Citation2018). Alternatively, analysis of chaos in the climax portion in the pant-hoot calls of primates showed that the presence of chaos was correlated with times of systemic infection and disease and that climaxes with little to no instance of chaos were evidence of better physical fitness (Riede et al. Citation2004). These conflicting results emphasise the possibility that the production of NLP may be different depending on species and motivation.
A number of non-human mammals were found to differ their NLP production depending on age. In species that had vocal apparatus that matured as the animal grew, an underdeveloped vocal structure was hypothesised to be the cause of an increased NLP presence in young animals (Killebrew et al. Citation2001; Root-Gutteridge et al. Citation2018). Both North Atlantic right whale (Eubalaena glacialis) calves and infant African elephants had higher amounts of chaos in their calls than adults (Stoeger et al. Citation2011; Root-Gutteridge et al. Citation2018). Alternatively, an exploratory study by Riley et al. (Citation2016) found an older dog to have a ‘rougher’ and ‘poorer’ voice quality than its younger canine housemate. Marx et al. (Citation2021) also reported a higher occurrence of NLP in the whines of older dogs. An increase in chaos was found for older North Atlantic right whales, which suggested a curvilinear relationship between NLP presence and age (Root-Gutteridge et al. Citation2018).
A few other studies to date have explored NLP presence in marine mammal calls. A study on manatee (Trichechus manatus spp.) vocalisations reported that 72.2% of manatee calls from Florida and 36.2% of manatee calls from Belize contained at least one NLP (Mann et al. Citation2006). Another study found that 92.4% of killer whale (Orcinus orca) and 65.7% of North Atlantic right whale vocalisations displayed at least one non-linear feature (Tyson et al. Citation2007). Although the literature on NLP in animal communication is growing, there has yet to be a detailed analysis of their presence in adult dolphin whistle emissions. In fact, oftentimes whistles with periods of lower signal-to-noise ratio, ‘unclear’ contours or overlapping calls were omitted from subsequent analyses. We hypothesised that NLP will be common additions to the spectral structure of signature whistles. Further, we examined what types of NLP were most common and explored differences in NLP presence for individual, sex and age.
Methods
Dolphins in this study are part of the U.S. Navy Marine Mammal Program (MMP) in San Diego Bay, CA. The MMP is AAALAC-accredited and follows the national standards of the United States Public Health Service Policy on the Humane Care and Use of Laboratory Animals and the Animal Welfare Act. The animal care and use programme at the MMP is routinely reviewed by an Institutional Animal Care and Use Committee (IACUC) and the Navy Bureau of Medicine and Surgery (BUMED). BUMED agreed with the approval of NIWC Pacific IACUC protocol #143–2021 and assigned NRD #1264 to this project. Dolphins in the MMP go out swimming and working in open water every day and then return home where they reside in groups of natural seawater pens in San Diego Bay.
As part of their routine mobility training, they also spend time in above ground pools (7 metre diameter, 1.5 metre depth). The nine dolphins (six males, three females; ) included in this study were opportunistically recorded during periods of isolation while free swimming in these pools between September 2018 and February 2021. The recordings were collected as part of a larger project which established and maintained a catalogue of vocalisations of each focal dolphin. The signature whistles for each focal dolphin have been identified and confirmed by multiple observers (minimum 3). Isolated recordings ensured that the dolphin was the producer of all of the recorded whistles and allowed analysts to confidently identify the signature whistles (along with other whistle types) for each animal. All recordings were made using one of the following devices: a self-calibrating single-channel Soundtrap (Ocean Instruments) with a bandwidth of 20 Hz to 150 kHz and a 192 kHz sampling rate or a self-calibrating four-channel Soundtrap with a 20 Hz to 90 kHz bandwidth and a 192 kHz sampling rate with a High-Tech Inc. high frequency hydrophone (2 Hz to 125 kHz frequency response).
Table 1. Dolphin demographics. An asterisk denotes that the birth year was estimated. Age range for span of recordings, from Sept. 2018 to Feb. 2021.
In order to reduce potential confounds of NLP association with health status, we identified recording dates in which the focal dolphin was confirmed ‘healthy’ based on previously defined health parameters developed with the MMP’s veterinary team (N = 79 dates).
Recordings were initially analysed in Raven Pro 1.6 using a custom, user-defined window preset for whistle identification analyses: Hann window, window size 1200, hop size 600, 2048 DFT, 50% overlap, 0.0–50.0 kilohertz (kHz) (y-axis), 7.0 seconds (s) time window (×-axis). Trained analysts identified and selected all of the whistles throughout the recordings and exported each whistle selection as an individual.wav file (16-bit, 288 kHz sample rate). A maximum of 25 signature whistles per recording day (00:00:00–23:59:59) were included for these analyses. If a dolphin emitted more than 25 signature whistles during a single day’s recording, 25 exemplars were randomly selected using a random number generator. As part of the larger study, acoustic parameters of each whistle were extracted by tracing the contour in PAMGuard 1.15.15 CORE open-source software (Gillespie et al. Citation2008) using the ROCCA plugin (Oswald et al. Citation2007, Citation2013). Signature whistle contours were traced on a Microsoft Surface Book 2 using a fine tipped stylus pen. Parameters of the contour (e.g. minimum and maximum frequency, start and end frequency, duration) were extracted for each whistle.
The signature whistles emitted by the dolphins (N = 642) were then loaded back into Raven 1.6 and zoomed in for a more detailed analysis (user-defined custom window preset for NLP analyses: Hann window, window size 2100, hop size 1050, 50% overlap, 4096 DFT, 0.0–25.0 kHz (y-axis), 2.0 s time window (×-axis)). The whistle was visually analysed for the presence (i.e. did an NLP occur) and persistence (i.e. duration of each occurrence, in seconds) of each NLP. With the selection marquee tool in Raven 1.6, each NLP was identified, tightly boxed and categorised in order to quantify the presence or absence of each NLP type across each whistle (as in Root-Gutteridge et al. Citation2018). Examples of NLP types are depicted in , and corresponding definitions of each NLP are reported in . Raven Pro 1.6 provides a measurement table for each selection box. Duration of the NLP type was measured in seconds and described as persistence (i.e. the total time an NLP type occurred over the course of one whistle). A persistence adjusted value was also calculated (i.e. the total NLP type’s persistence (s) divided by the total whistle duration (s)), which resulted in a proportion (i.e. a value between 0.0 and 1.0) of the whistle. Total NLP persistence measured the sum duration of all of the NLP occurrences produced over the course of a whistle with any periods of overlapping NLP subtracted, as more than one NLP type was commonly produced with a single whistle.
Figure 1. Spectrogram (frequency in kHz on the y-axis (0.0–30.0 kHz), time in seconds on the x-axis (1.5 s time window)) depictions of the four NLP types with corresponding waveforms (located above the spectrogram with relative amplitude on the y-axis and time in seconds on the x-axis) presented in this study. (a) Biphonation (whistle and burst pulses), (b) deterministic chaos, (c) sidebands and (d) subharmonics.
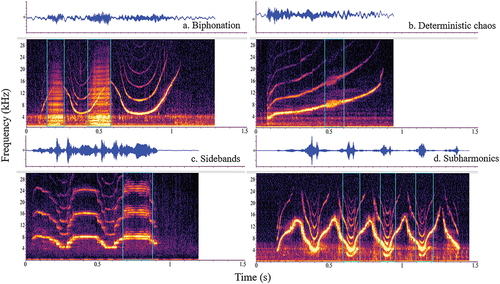
Table 2. Definitions of terms.
An inter-observer test of 10% of the total whistles used in the present study was assessed between two analysts to ensure consistent classification of NLP. Of the 181 instances of NLP that were identified over 64 whistles, an inter-observer agreement of location, persistence and categorisation of NLP was found in 83.98% of instances. All statistical analyses were conducted in IBM SPSS v.24. When dolphin age was included as a variable in the test, the mode dolphin age over the 3-year recording time frame was used.
Results
Across the 642 whistles included in this analysis, 53.0% (N = 340) had at least one type of NLP, compared to 47.0% (N = 302) that did not contain any (). When broken down by individual dolphin, a mean (M) proportion of 0.47 (± Standard Error (SE) = 0.06) of signature whistles contained at least one NLP type (range: 0.24 to 0.88) while 0.53 (± SE = 0.06) of signature whistles did not contain NLP (range: 0.12 to 0.76) (). These results confirmed that NLP were common features of healthy dolphin signature whistles.
Figure 2. To the left of the vertical grey line, the proportion of whistles with NLP (purple bar) and without NLP (pink bar) for the 642 whistles analysed is depicted. To the right of the vertical grey line depicts the breakdown of the NLP types observed across the 642 total whistles.
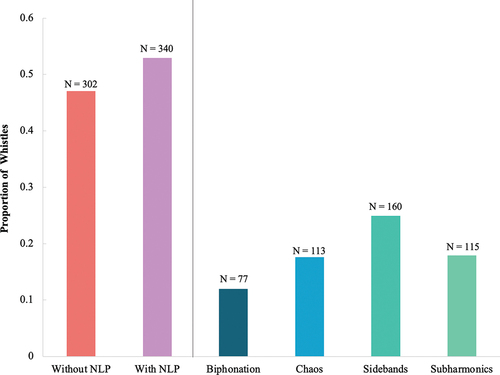
Table 3. Individual dolphin’s proportion of whistles without NLP, with NLP and NLP types.
NLP presence was further broken down by NLP type (). Each of the focal dolphins was recorded producing at least two different types of NLP. A Chi-square found a significant difference between the use of the four NLP types (Chi-square test: X2(3, 472) = 29.54, P < 0.01) for all occurrences of NLP. Sidebands were the most commonly emitted NLP (24.9% of all whistles recorded) followed by subharmonics (17.9%) and deterministic chaos (17.6%). Biphonations occurred in 12.0% of whistles. There were 77 total biphonations. Of those, 68 biphonations were whistles with overlapping clicks (88.3%), 6 were whistles with overlapping burst pulses (7.8%) and 5 were whistles with a second overlapping but unrelated whistle contour (6.5%). There was an additional 4.2% of whistles that contained a potential NLP that could not be clearly classified. While these may be known phenomena, they could not be placed into one of the NLP categories defined in this manuscript by the analysts.
A general linear mixed model with individual dolphin as a random factor, NLP type as a fixed factor, and persistence as the dependent variable was assessed. A linear mixed model with individual dolphin as a random factor found a significant relationship between the NLP type and its persistence (i.e. the total time in seconds an NLP type occurred over the duration of one whistle) (Linear Mixed Model (LMM): F(1,9.19) = 38.327, P < 0.01). While biphonations were only present with 12.0% of all whistles, on average they persisted longer than all three other NLP types (; M = 0.51 s ± SE = 0.03) (Bonferroni post-hoc univariate comparisons: chaos: M = 0.17 s ± SE = 0.01, P < 0.01; sidebands: M = 0.13 s ± 0.01, P < 0.01; and subharmonics: M = 0.08 s ± SE = 0.01, P < 0.01). Pearson’s correlation did not find a significant relationship between duration of an individual’s signature whistle and the whistle’s total NLP persistence (Pearson’s correlation: r(7) = > −0.036, P = 0.358). NLP persistence for the individual dolphins are reported in .
Figure 3. The mean persistence (in seconds) of NLP types when present. Persistence measures the duration (s) of an NLP. Error bars depict ± 1 standard error. Significance of biphonation persistence is denoted with an *.
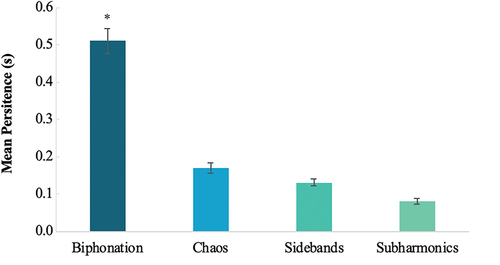
Table 4. Mean ± standard error of the persistence for each NLP type (columns) broken down by each individual dolphin (represented by each row). No standard error value represents a single whistle with that NLP type.
The persistence adjusted (i.e. the total NLP type’s persistence (s) divided by the total whistle duration (s)) gave a proportion of the whistle’s duration covered by an NLP type, between 0.00 and 1.00. A linear mixed model further supported the above results as NLP persistence adjusted was also significantly different based on the NLP type when dolphin was considered as a random factor (GLMM: F(1, 9.04) = 25.957, P < 0.01). Biphonation persistence adjusted was significantly higher than all three other NLP types (M = 0.56 s ± SE = 0.04) (Bonferroni post-hoc univariate comparisons: chaos: M = 0.17 s ± SE = 0.01, P < 0.01; sidebands: M = 0.13 s ± 0.01, P < 0.01; and subharmonics: M = 0.06 s ± SE = 0.01, P < 0.01). There were no differences between the average persistence adjusted of the other NLP types.
The proportion of whistles with and without NLP between the six male and the three female focal dolphins was compared to identify any possible differences in NLP occurrence based on sex. Male dolphin whistles made up 56.5% of the 642 whistles analysed in the study (N = 363), and female dolphins made up 43.5% of whistles (N = 279). In general, 56.0% of the total whistles produced by male dolphins and 49.0% of female dolphin whistles contained NLP. The proportion of whistles that contained NLP did not significantly differ based on the sex of the dolphin producing the whistle (Chi-square test: X2(1, 642) = 3.52, P = 0.061). The mean proportion of whistles with NLP was 0.48 (± SE = 0.10) for males, ranging between 0.24 and 0.88, and 0.45 (± SE = 0.07) for females, ranging from 0.35 to 0.57. While we acknowledge the limitations of a small sample size, these exploratory findings suggested that sex does not significantly impact the likelihood of NLP produced with a whistle.
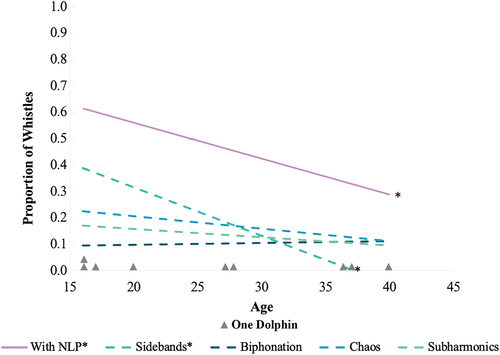
The nine dolphins in the study ranged in age from 15 to 40 years old over the course of the almost 3-year recording period (). The mode age for each animal (i.e. the age (in years) in which the dolphin had the greatest number of recording dates; ) was plotted against their proportion of whistles that contained NLP and was broken down by each NLP type (). There was a negative relationship between age and proportion of whistles with NLP (Pearson’s coefficient: r(7) = > −0.686). Correlations between age and biphonation (Pearson’s coefficient: r(7) = 0.081), age and chaos (Pearson’s coefficient: r(7) = > −0.201), and between age and subharmonics (Pearson’s coefficient: r(7) = > −0.229) were not strong. However, the proportion of whistles with sidebands was negatively correlated with age (Pearson’s coefficient: r(7) = > −0.822). We acknowledge that the sample size of unique ages is small and therefore encourage the readers to use caution when drawing broader conclusions from these findings.
Figure 4. Mode age (x-axis) of the nine focal dolphins (one dolphin represented by one grey triangle) plotted against the proportions of whistles (y-axis) with NLP (solid purple line), and for each NLP type (dashed green/blue lines). Proportion of sidebands and age were also found to have a negative correlation. Biphonation, chaos and subharmonics were not found to have a strong correlation with age. Correlation is denoted with a *. Sample sizes by age: 16 years old (yo) = 2 dolphins, 198 whistles, 17 yo = 1 dolphin, 134 whistles, 20 yo = 1 dolphin, 25 whistles, 27 yo = 1 dolphin, 9 whistles, 28 yo = 1 dolphin, 29 whistles, 37 yo = 1 dolphin, 41 whistles, 38 yo = 1 dolphin, 15 whistles, 40 yo = 1 dolphin, 22 whistles.
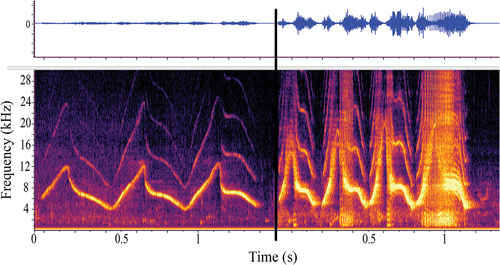
All nine dolphins produced their signature whistles both with and without NLP. Whistles with and without NLP from the same dolphin often occurred in the same recording period. depicts an example of dolphin 9’s signature whistle without NLP (left) compared to a signature whistle emission that contained sidebands, clicks and burst pulse biphonations (right). These two whistles came from the same recording within 9 minutes of each other. For this particular example, between the 9 minutes of the two whistles, a second dolphin was being put into an adjacent pool that is within hearing range of the focal dolphin. This illustrates the range of NLP production a dolphin’s signature whistle emission may contain over a brief period of time (i.e. 9 minutes apart in this example) and may suggest a possible social function to NLP emission.
Figure 5. An example of dolphin 9’s signature whistle, depicted as a waveform (top panel with relative energy over time) and spectrogram on the bottom panel (time (s) on the x-axis and frequency in kilohertz (kHz) on the y-axis), with NLP (right) and without NLP (left). These two whistles came from the same recording within 9 minutes of each other.
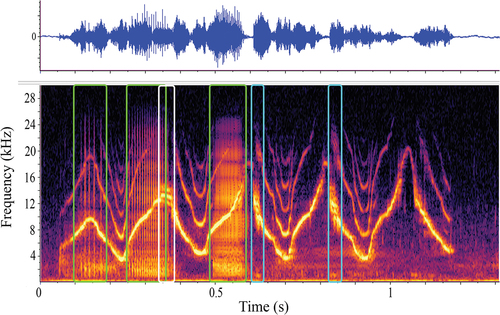
Many whistles contained more than one NLP type, and sometimes even multiple occurrences of the same NLP type, over the course of a single whistle. In , we provide an example of dolphin 2’s signature whistle that had three different types of NLP: biphonation (both clicks and burst pulses) (green boxes), sidebands (white box) and deterministic chaos (blue boxes). This whistle example demonstrates that there can be multiple NLP occurring over the course of one whistle emissions, and that those NLP sometimes overlap.
Figure 6. An example of dolphin 2’s signature whistle contour depicted as a waveform (top panel with relative energy over time) and spectrogram on the bottom panel (time (s) on the x-axis and frequency in kilohertz on the y-axis). The boxed sections highlight the multiple types of NLP emitted over the course of the whistle; clicks and burst pulse (green boxes) sidebands (white box) and deterministic chaos (blue boxes).
Discussion
Signature whistles with NLP produced by bottlenose dolphins were found to be a common occurrence, as 53.0% of the 642 whistles contained at least one NLP. All nine of the focal dolphins were recorded producing at least two different NLP types. Although they showed individual differences in NLP occurrence, NLP was still fairly common for each dolphin (proportion of whistles with NLP range: 0.24–0.88; M = 0.47 ± SE = 0.06). Individual variation in NLP production was also found in the calls of humpback whales (Cazau et al. Citation2016), rhesus macaques (Macaca mulatta) (Fitch et al. Citation2002), Asian elephants (Elephas maximus) (Beeck et al. Citation2021; Fuchs et al. Citation2021) and common chimpanzees (Pan troglodytes) (Riede et al. Citation2004). Whether these differences are a by-product of an individual’s unique vocal apparatus, individual preference, or contain communicative function are questions for future study.
Sidebands were the most observed NLP type and occurred with 24.9% of all signature whistles (). Gerhardt (Citation1998) and Frommolt (Citation1999) concluded that sidebands were a result of amplitude fluctuations in the carrier (i.e. fundamental) frequency. An example of this was seen in Riede et al. (Citation2004), as they identified sidebands as the majority NLP type present in the climax portion of the pant hoot call for chimpanzees, which was the loudest and highest frequency portion of the call. Jones et al. (Citation2022) found that the relative amplitude modulations along the frequency-time contour of a dolphin’s signature whistle were not stereotyped, so it may be that there is a relationship between the location of sidebands and the points of peak amplitude or the points that contain the highest rate of amplitude modulation along the whistle contour. Unlike the pattern found in the chimpanzee pant hoots (Riede et al. Citation2004), the relative amplitude was found to be higher in the lower frequencies across the dolphin signature whistles (Jones et al. Citation2022) so it is of interest if sidebands also commonly occur in the lower frequency aspects of signature whistles or not.
We classified three types of biphonation: a whistle with an overlapping click train (N = 68), a whistle with an overlapping burst pulse (N = 6) and two overlapping but independent frequency-modulated whistles (N = 5). Both clicks and burst pulses have been found to be highly directional compared to whistles that were more omni-directional (Branstetter et al. Citation2012). One hypothesis for the use of these biphonations is that the clicks and/or burst pulses may help direct and amplify the energy of the more omni-directional whistle towards the intended receiver. While biphonation was the least observed NLP in the dataset, it had a significantly longer duration than other NLP types when it was present. In these recordings, the dolphins were alone in above ground pools, and commonly experienced dolphins in adjacent pools that could be heard by one another. Biphonations may also be more attention getting than whistles on their own or could simply be a result of a dolphin multi-tasking (e.g. navigating and communicating simultaneously). Further investigation into the social purpose of biphonation in dolphin repertoires is needed. The production of a second fundamental frequency contour was the least observed biphonation in this dataset (N = 5). For killer whales, these types of biphonic calls were proposed to be matrilineal pod identifiers (Filatova et al. Citation2009). Similarly, Papale et al. (Citation2015) discussed this type of biphonation being used as a signature whistle by a wild dolphin. However, none of the dolphins in this study consistently used simultaneous whistle contours as their signature whistle. It could be that wild marine mammals can utilise these types of biphonations as additional individual or group identifiers when needing to differentiate from larger pods.
While sex, in our limited population, did not influence the presence of NLP, a negative correlation was found between the presence of NLP and the dolphin’s age. We acknowledge the limitations of our current sample size, with only nine dolphins contributing to six different ages, so we interpret these results cautiously, and encourage future projects to look into how NLP changes with age longitudinally within individual dolphins over time. While this study only included adult bottlenose dolphins, previous research does suggest that NLP use is very high in bottlenose dolphin calves, which would fit with the negative correlation seen here. It is then probable that the maturation and improved control of the vocal production apparatus could lead to the decrease in NLP production. Interestingly, a curvilinear trend has been reported for humans and for North Atlantic right whales (Mende et al. Citation1990; Stathopoulos et al. Citation2011; Root-Gutteridge et al. Citation2018) in that NLP was high in the young, decreased in the adult population, and then re-emerged in the vocalisations of the elder population. It is possible that with a larger dataset of geriatric dolphins we could also see this trend, but in adult dolphins, we report a negative correlation for overall NLP presence and sidebands.
It is possible that changes in the arousal level and/or valence (i.e. positive or negative) state of the animal may result in the occurrence of NLP. As in the example in , the increase in NLP seemed to correlate to the time frame that another dolphin and the animal care staff arrived at an adjacent pool. This could have been an arousal/valence response to the presence of the dolphin trainers, food, a conspecific and/or a result of extra effort to communicate with the dolphin through the laminate plastic pool walls. Caldwell and Caldwell (Citation1979) proposed the idea that heightened emotional states may lead to a loss of muscular control in the nasal system, as they saw in post-infantile dolphin calves in stressful situations. Reiss (Citation1988) noted dolphin calves produced whistle-squawks more often in ‘emotional states’, such as when separated from their mother or when they bumped into the enclosure’s wall. Briefer and Le Comber (Citation2012) stated that for mammalian phonation, the muscular tension and shape of the vocal apparatus can be affected by the emotional state of the caller. Future research on whistle use during different behavioural, social, health and stress contexts should consider whether NLP changes occur with changes in arousal level and valence.
It has been well established that signature whistles convey information about individual identity through the frequency-time contour (Caldwell and Caldwell Citation1965; Janik et al. Citation2006). Each of our dolphins produced their signature whistles both with and without NLP. This suggests that, at least in this focal group, NLP was not a stereotyped feature of signature whistles that increased its caller distinctiveness from other’s whistles. NLP presence may encode different information over a signature whistle emission outside of identity. For a species that often inhabits occluded waters, and whose main source of communication is acoustic, enhancing that communication through NLP raises interesting hypotheses about the evolution of these NLP types and the systems they come from. Some proposed hypotheses for the function of NLP have been communication of urgency, body size, physical fitness, individual identification and arousal/valence states. We acknowledge that the whistles recorded for this study were not taken in their usual setting, as dolphins are typically in social pods with conspecifics. Therefore, NLP presence and rates could be different in their highly social settings where group cohesion and management are more pertinent.
Historically, whistles with NLP features have not been included in call type description and analysis, as it was typical to focus on basic call description parameters of ‘clean’ whistles (e.g. non-overlapping, good signal-to-noise ratio, parameters appear clear when viewed via spectrogram) (Briefer and Le Comber Citation2012; Cazau et al. Citation2016). However, this study has shown that dolphin whistles with NLP are common occurrences, and therefore future analysis should avoid disregarding calls with these features. These findings highlight the importance of doing research both in the wild and in managed care to get a more complete understanding of animal behaviour and communication. When recording the vocalisations of wild dolphin populations, it was typically unclear if overlapping calls (e.g. two whistles, a burst pulse and a whistle, etc.) came from one or multiple dolphins vocalising at the same time. By recording dolphins in a calm but isolated setting, we were able to confidently identify the rates of NLP in adult dolphins, a task that has been elusive for wild dolphin studies.
Conclusion
NLP were found to be common with the signature whistles of adult bottlenose dolphins. Sidebands were observed the most out of the four NLP types. Biphonation, while the least observed NLP, had the longest persistence when present on a whistle. All dolphins in the study produced at least two NLP types and showed individual variation in both the NLP types emitted and the persistence of NLP over their signature whistle emissions. There was no difference between the proportion of signature whistles that contained NLP between males and females, but NLP in general, and more specifically sideband presence, decreased with age.
The present study provides a baseline for NLP production with signature whistles for bottlenose dolphins. With the knowledge that NLP are common occurrences in the vocal repertoire of dolphins, we can further our understanding of dolphin communication by including these factors in subsequent analysis. With two pairs of phonic lips capable of producing a variety of call types, there is a lot to explore. A better understanding of the use of NLP across behavioural contexts, social interactions, arousal levels and health statuses may allow us to use NLP as a non-invasive biomarker when monitoring for changes in these contexts.
Ethical statement
The US Navy Marine Mammal Program (MMP) is AAALAC-accredited and follows the national standards of the United States Public Health Service Policy on the Humane Care and Use of Laboratory Animals and the Animal Welfare Act. The animal care and use programme at the MMP is routinely reviewed by an Institutional Animal Care and Use Committee (IACUC) and the Navy Bureau of Medicine and Surgery (BUMED). BUMED agreed with the approval of NIWC Pacific IACUC protocol #143–2021 and assigned NRD #1264 to this project.
Acknowledgements
The authors are very grateful to the U.S. Navy Marine Mammal Program for allowing us to opportunistically record the dolphins in its care. Thanks to Mark Baird and Risa Daniels for all of their time and effort in data collection and project discussions. Thank you to all of the animal care and veterinary staff who take wonderful care of these animals. Specifically, Jaime Bratis, Megan Dunn, Amanda Naderer, Sarah Hammar and their amazing crews for their constant support and enthusiasm for the project. Thank you to Dr Abby McClain for her assistance identifying healthy dolphins from the Navy’s health database. Thank you to the veterinary records team for maintaining and helping us to access an incredible health database. We would also like to thank the Office of Naval Research (ONR) for their continuous support for this research through ONR Grant #N00014-18-1-2643 and ONR Grant #N00014-21-1-2414. This is National Marine Mammal Foundation Contribution #340 to peer reviewed scientific literature.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Au WWL. 1993. Characteristics of dolphin sonar signals. In: The sonar of dolphins. 1st ed. New York (NY): Springer; p. 115–139. doi:10.1007/978-1-4612-4356-4_7.
- Au WWL, Banks K. 1998. The acoustics of the snapping shrimp Synalpheus parneomeris in Kaneohe Bay. J Acoust Soc Am. 103(1):41–47. doi:10.1121/1.423234.
- Aubin T, Jouventin P, Hildebrand C. 2000. Penguins use the two-voice system to recognize eachother. Proc Biol Sci. 267(1448):1081–1087. doi:10.1098/rspb.2000.1112.
- Beeck VC, Heilmann G, Kerscher M, Stoeger AS. 2021. A novel theory of Asian elephant high-frequency squeak production. BMC Biol. 19(1):121–137. doi:10.1186/s12915-021-01026-z.
- Blomqvist C, Amundin M. 2004. High-Frequency burst-pulse sounds in agonistic/aggressive interactions in bottlenose dolphins, Tursiops truncatus. In: Thomas J, Moss C, Vater M, editors. Echolocation of bats and dolphins. Chicago (IL): The University of Chicago Press; p. 425–431.
- Blumstein DT, Davitian R, Kaye PD. 2010. Do film soundtracks contain nonlinear analogues to influence emotion? Biol Lett. 6(6):751–754. doi:10.1098/rsbl.2010.0333.
- Boisseau O. 2005. Quantifying the acoustic repertoire of a population: the vocalizations of free-ranging bottlenose dolphins in Fiordland, New Zealand. J Acoust Soc Am. 117(4):2318–2329. doi:10.1121/1.1861692.
- Branstetter BK, Moore PW, Finneran JJ, Tormey MN, Aihara H. 2012. Directional properties of bottlenose dolphin (Tursiops truncatus) clicks, burst-pulse, and whistle sounds. J Acoust Soc Am. 131(2):1613–1621. doi:10.1121/1.3676694.
- Briefer EF, Le Comber S. 2012. Vocal expression of emotions in mammals: mechanisms of production and evidence. J Zool. 288(1):1–20. doi:10.1111/j.1469-7998.2012.00920.x.
- Caldwell MC, Caldwell DK. 1965. Individualized whistle contours in bottle-nosed dolphins (Tursiops truncatus). Nat. 207:434–435.
- Caldwell MC, Caldwell DK. 1967. Intraspecific transfer of information via the pulsed sound in captive odontocete cetaceans. In: Busnel R, editor. Animal Sonar Systems: Biology and Bionics. Jouy-en-Josas (France): Laboratoire de Physiologie Acoustic; p. 879–936.
- Caldwell MC, Caldwell DK. 1979. The whistle of the Atlantic bottlenosed dolphin (Tursiops truncatus) - Ontogeny. In: Winn H, Olla B, editors. Behavior of Marine Animals. Vol. 3: Cetaceans. St. Augustine (FL): Springer; p. 369–401. doi:10.1007/978-1-4684-2985-5_11.
- Caldwell MC, Caldwell DK, Tyack PL. 1990. Review of the signature-whistle hypothesis for the Atlantic bottlenose dolphin. In: Letherwood S, Reeves R, editors. The bottlenose dolphin. San Diego (CA): Academic Press; p. 199–234.
- Cazau D, Adam O, Aubin T, Laitman JT, Reidenberg JS. 2016. A study of vocal nonlinearities in humpback whale songs: from production mechanisms to acoustic analysis. Sci Rep. 6:31660–31672. doi:10.1038/srep31660.
- Cranford TW, Elsberry WR, Bonn WGV, Jeffress JA, Chaplin MS, Blackwood DJ, Carder DA, Kamolnick T, Todd MA, Ridgway SH. 2011. Observation and analysis of sonar signal generation in the bottlenose dolphin (Tursiops truncatus): evidence for two sonar sources. J Exp Mar Bio Ecol. 407(1):81–96. doi:10.1016/j.jembe.2011.07.010.
- Dreher JJ. 1961. Linguistic considerations of porpoise sounds. J Acoust Soc Am. 33(12):1799–1800. doi:10.1121/1.1908584.
- Eskelinen HC, Jones BL. 2021. Acoustic characteristics of bubblestream-associated whistles produced by Atlantic bottlenose dolphins (Tursiops truncatus) during the first thirty days of life. Aquat Mamm. 47(4):337–348. doi:10.1578/am.47.4.2021.337.
- Filatova OA, Fedutin ID, Nagaylik MM, Burdin AM, Hoyt E. 2009. Usage of monophonic and biphonic calls by free-ranging resident killer whales (Orcinus orca) in Kamchatka, Russian far East. Acta Ethol. 12:37–44. doi:10.1007/s10211-009-0056-7.
- Fitch WT, Neubauer J, Herzel H. 2002. Calls out of chaos: the adaptive significance of nonlinear phenomena in mammalian vocal production. Anim Behav. 63(3):407–418. doi:10.1006/anbe.2001.1912.
- Frey R, Volodin IA, Fritsch G, Volodina EV, Aubin T. 2016. Potential sources of high frequency and biphonic vocalization in the Dhole (Cuon alpinus). PLoS One. 11(1):e0146330. doi:10.1371/journal.pone.0146330.
- Frommolt K-H. 1999. Sidebands- facts and artefacts. Bioacoustics. 10(2–3):219–224. doi:10.1080/09524622.1999.9753432.
- Fuchs E, Beeck VC, Baotic A, Stoeger AS, Döllinger M. 2021. Acoustic structure and information content of trumpets in female Asian elephants (Elephas maximus). PLoS One. 16(11):e0260284. doi:10.1371/journal.pone.0260284.
- Gerhardt HC. 1998. Acoustic signals of animals: Recording, field measurements, analysis and description. In: Hopp S, Owren M, Evans C, editors. Animal acoustic communication: Sound Analysis and Research Methods. Berlin (Heidelberg): Springer; p. 1–25. doi:10.1007/978-3-642-76220-8_1.
- Gillespie D, Gordon J, Mchugh R, Mclaren D, Mellinger DK, Redmond P, Thode A, Trinder P, Deng XY. 2008. PAMGUARD: semiautomated, open source software for real-time acoustic detection and localization of cetaceans. In: Proceedings of the Institute of Acoustics. Vol. 30. Southampton (UK). p. 54–62.
- Goncharova MV, Klenova AV, Bragina EV. 2015. Development of cues to individuality and sex in calls of three crane species: When is it good to be recognizable? J Ethol. 33(3):165–175. doi:10.1007/s10164-015-0428-6.
- Herman LM, Tavolga WN. 1980. The communication systems of cetaceans. In: Herman L, editor. Cetacean behavior: mechanisms and functions. New York (NY): Wiley - Interscience; p. 149–197.
- Herzel H, Berry D, Titze I, Steinecke I. 1995. Nonlinear dynamics of the voice: signal analysis and biomechanical modeling. Chaos. 5(1):30–34. doi:10.1063/1.166078.
- Herzel H, Reuter R. 1996. Biphonation in voice signals. AIP Conf Proc. 375:644–657. doi:10.1063/1.51002.
- Herzing DL. 1996. Vocalizations and associated underwater behavior of free-ranging Atlantic spotted dolphins, Stenella frontalis and bottlenose dolphins, Tursiops truncatus. Aquat Mamm. 22(2):61–79. doi:10.12966/abc.02.02.2015.
- Herzing DL. 2000. Acoustics and social behavior of wild dolphins: implications for a sound society. In: Au W, Popper A, Fay R, editors. Hearing by whales and dolphins. 1sted. New York (NY): Springer; p. 225–272. doi:10.1007/978-1-4612-1150-1_5.
- Huggenberger S, Rauschmann MA, Vogl TJ, Oelschläger HHA. 2009. Functional morphology of the nasal complex in the harbor porpoise (Phocoena phocoena L.). Anat Rec. 292(6):902–920. doi:10.1002/ar.20854.
- Janik VM, King SL, Sayigh LS, Wells RS. 2012. Identifying signature whistles from recordings of groups of unrestrained bottlenose dolphins (Tursiops truncatus). Mar Mamm Sci. 29(1):109–122. doi:10.1111/j.1748-7692.2011.00549.x.
- Janik VM, Sayigh LS. 2013. Communication in bottlenose dolphins: 50 years of signature whistle research. J Comp Physiol A. 199:479–489. doi:10.1007/s00359-013-0817-7.
- Janik VM, Sayigh LS, Wells RS. 2006. Signature whistle shape conveys identity information to bottlenose dolphins. PNAS. 103(21):8293–8297. doi:10.1073/pnas.0509918103.
- Jones B, Tufano S, Daniels R, Mulsow J, Ridgway S. 2022. Non-Stereotyped amplitude modulation across signature whistle contours. Behav Processes. 194:104561. doi:10.1016/j.beproc.2021.104561.
- Jones B, Zapetis M, Samuelson MM, Ridgway S. 2019. Sounds produced by bottlenose dolphins (Tursiops): a review of the defining characteristics and acoustic criteria of the dolphin vocal repertoire. Bioacoustics. 29(4):399–440. doi:10.1080/09524622.2019.1613265.
- Kellogg WN, Kohler R, Morris HN. 1953. Porpoise sounds as sonar signals. Science. 117(3036):239–243. doi:10.1126/science.117.3036.239.
- Killebrew DA, Mercado E,sIII, Herman LM, Pack AA. 2001. Sound production of a neonate bottlenose dolphin. Aquat Mamm. 27(1):34–44.
- Kreul EJ, Hecker MHL. 1971. Descriptions of the speech of patients with cancer of the vocal folds. Part II: judgments of age and voice quality. J Acoust Soc Am. 49(4B):1283–1287. doi:10.1121/1.1912491.
- Kriesell HJ, Elwen SH, Nastasi A, Gridley T, Russo D. 2014. Identification and characteristics of signature whistles in wild bottlenose dolphins (Tursiops truncatus) from Namibia. PLoS One. 9(9):e106317. doi:10.1371/journal.pone.0106317.
- Lilly JC, Miller AM. 1961. Sounds emitted by the bottlenose dolphin: the audible emissions of captive dolphins under water or in air are remarkably complex and varied. Scince. 133(3465):1689–1693. doi:10.1126/science.133.3465.1689.
- Luís AR, Couchinho MN, dos Santos ME, Sokolowski B. 2016. A quantitative analysis of pulsed signals emitted by wild bottlenose dolphins. PLoS One. 11(7):e0157781. doi:10.1371/journal.pone.0157781.
- Madsen PT, Jensen FH, Carder D, Ridgway S. 2011. Dolphin whistles: a functional misnomer revealed by heliox breathing. Biol Lett. 8(2):211–213. doi:10.1098/rsbl.2011.0701.
- Madsen PT, Lammers M, Wisniewska D, Beedholm K. 2013. Nasal sound production in echolocating delphinids (Tursiops truncatus and pseudorca crassidens) is dynamic, but unilateral: Clicking on the right side and whistling on the left side. J Exp Biol. 216(21):4091–4102. doi:10.1242/jeb.091306.
- Mann DA, O’Shea TJ, Nowacek DP. 2006. Nonlinear dynamics in manatee vocalizations. Mar Mamm Sci. 22(3):548–555. doi:10.1111/j.1748-7692.2006.00036.x.
- Manteuffel G, Puppe B, Schön PC. 2004. Vocalization of farm animals as a measure of welfare. Appl Anim Behav Sci. 88(1–2):163–182. doi:10.1016/j.applanim.2004.02.012.
- Marx A, Lenkei R, Fraga PP, Bakos V, Kubinyi E, Faragó T. 2021. Occurrences of non-linear phenomena and vocal harshness in dog whines as indicators of stress and ageing. Sci Rep. 11:4468–4480. doi:10.1038/s41598-021-83614-1.
- Mead JG. 1975. Anatomy of the external nasal passages and facial complex in the Delphinidae (Mammalia: Cetacea). Smithsonian Contrib to Zool. 207:1–35. doi:10.5479/si.00810282.207.
- Mende W, Herzel H, Wermke K. 1990. Bifurcations and chaos in newborn infant cries. Phys Lett A. 145(8–9):418–424. doi:10.1016/0375-9601(90)90305-8.
- Oswald JN, Rankin S, Barlow J, Lammers MO. 2007. A tool for real-time acoustic species identification of delphinid whistles. J Acoust Soc Am. 122(1):587–595. doi:10.1121/1.2743157.
- Oswald JN, Rankin S, Barlow J, Oswald M, Lammers MO. 2013. Real-Time odontocete call classification algorithm (ROCCA): software for species identification of delphinid whistles. Detection, classification and localization of marine mammals using passive acoustics 2003-2010. Paris; p. 245–266.
- Papale E, Buffa G, Filiciotto F, Maccarrone V, Mazzola S, Ceraulo M, Giacoma C, Buscaino G. 2015. Biphonic calls as signature whistles in a free-ranging bottlenose dolphin. Bioacoustics. 24(3):223–231. doi:10.1080/09524622.2015.1041158.
- Papale E, Fanizza C, Buscaino G, Ceraulo M, Cipriano G, Crugliano R, Grammauta R, Gregorietti M, Renò V, Ricci P, et al. 2020. The social role of vocal complexity in striped dolphins. Front Mar Sci. 7:1–12. doi:10.3389/fmars.2020.584301.
- Popper AN. 1980. Sound emission and detection by delphinids. In: Herman L, editor. Cetacean Behavior: Mechanisms and Functions. New York (NY): Wiley - Interscience; p. 1–44.
- Reby D, Wyman MT, Frey R, Passilongo D, Gilbert J, Locatelli Y, Charlton BD. 2016. Evidence of biphonation and source–filter interactions in the bugles of male North American wapiti (Cervus canadensis). J Exp Biol. 219(8):1224–1236. doi:10.1242/jeb.131219.
- Reidenberg JS, Laitman JT. 1988. Existence of vocal folds in the larynx of odontoceti (toothed whales). Anat Rec. 221:884–891. doi:10.1002/ar.1092210413.
- Reiss D. 1988. Observations on the development of echolocation in young bottlenose dolphins. In: Natchigall P, Moore P, editors. Animal sonar: processes and performance. Vol. 156. New York (NY): Plenum; p. 121–127.
- Ridgway SH, Carder DA, Green RF, Gaunt AS, Gaunt SLL, Evans WE. 1980. Electromyographic and pressure events in the nasolaryngeal system of dolphins during sound production. In: Busnel R, Fish J, editors. Animal Sonar Systems. Vol. 28. Boston (MA): Springer; p. 239–250.
- Ridgway S, Dibble DS, Alstyne KV, Price D. 2015. On doing two things at once: Dolphin brain and nose coordinate sonar clicks, buzzes and emotional squeals with social sounds during fish capture. J Exp Biol. 218(24):3987–3995. doi:10.1242/jeb.130559.
- Ridgway S, Keogh M, Carder D, Finneran J, Kamolnick T, Todd M, Goldblatt A. 2009. Dolphins maintain cognitive performance during 72 to 120 hours of continuous auditory vigilance. J Exp Biol. 212(10):1519–1527. doi:10.1242/jeb.027896.
- Riede T, Herzel H, Mehwald D, Seidner W, Trumler E, Böhme G, Tembrock G. 2000. Nonlinear phenomena in the natural howling of a dog–wolf mix. J Acoust Soc Am. 108(4):1435–1442. doi:10.1121/1.1289208.
- Riede T, Owren MJ, Arcadi AC. 2004. Nonlinear acoustics in pant hoots of common chimpanzees (Pan troglodytes): frequency jumps, subharmonics, biphonation, and deterministic chaos. Am J Primatol. 64(3):277–291. doi:10.1002/ajp.20078.
- Riley JL, Riley WD, Carroll LM. 2016. Frequency characteristics in animal species typically used in laryngeal research: an exploratory investigation. J Voice. 30(6):767.e17–767.e24. doi:10.1016/j.jvoice.2015.10.019.
- Robb MP, Saxman JH. 1988. Acoustic observations in young children’s non‐cry vocalizations. J Acoust Soc Am. 83(5):1876–1882. doi:10.1121/1.396523.
- Root-Gutteridge H, Cusano DA, Shiu Y, Nowacek DP, Parijs SMV, Parks SE. 2018. A lifetime of changing calls: North Atlantic right whales, Eubalaena glacialis, refine call production as they age. Anim Behav. 137:21–34. doi:10.1016/j.anbehav.2017.12.016.
- Sadeghi M, Banakar A, Khazaee M, Soleimani MR. 2015. An intelligent procedure for the detection and classification of chickens infected by clostridium perfringens based on their vocalization. Braz J Poult Sci. 17(4):537–544. doi:10.1590/1516-635x1704537-544.
- Schneider JN, Anderson RE. 2011. Tonal vocalizations in the red wolf (Canis rufus): potential functions of nonlinear sound production. J Acoust Soc Am. 130(4):2275–2284. doi:10.1121/1.3628331.
- Stathopoulos ET, Huber JE, Sussman JE. 2011. Changes in acoustic characteristics of the voice across the lifespan: measures from individuals 4-93 years of age. J Speech Lang Hear Res. 54(4):1011–1021. doi:10.1044/1092-4388(2010/10-0036).
- Stoeger AS, Charlton BD, Kratochvil H, Fitch WT. 2011. Vocal cues indicate level of arousal in infant African elephant roars. J Acoust Soc Am. 130(3):1700–1710. doi:10.1121/1.3605538.
- Suthers RA, Narins PM, Lin W-Y, Schnitzler H-U, Denzinger A, Xu C-H, Feng AS. 2006. Voices of the dead: complex nonlinear vocal signals from the larynx of an ultrasonic frog. J Exp Biol. 209(24):4984–4993. doi:10.1242/jeb.02594.
- Townsend SW, Manser MB. 2011. The function of nonlinear phenomena in meerkat alarm calls. Biol Lett. 7(1):47–49. doi:10.1098/rsbl.2010.0537.
- Tyack PL, Clark CW. 2000. Communication and acoustic behaviour of dolphins and whales. In: Au W, Popper A, Fay R, editors. Hearing by whales and dolphins. New York (NY): Springer; p. 156–224. doi:10.1007/978-1-4612-1150-1_4.
- Tyson RB, Nowacek DP, Miller PJO. 2007. Nonlinear phenomena in the vocalizations of North Atlantic right whales (Eubalaena glacialis) and killer whales (Orcinus orca). J Acoust Soc Am. 122(3):1365–1373. doi:10.1121/1.2756263.
- Volodina EV, Volodin IA, Isaeva IV, Unck C. 2006. Biphonation may function to enhance individual recognition in the Dhole, Cuon alpinus. Ethol. 112(8):815–825. doi:10.1111/j.1439-0310.2006.01231.x.
- Wilden I, Herzel H, Peters G, Tembrock G. 1998. Subharmonics, biphonation, and deterministic chaos in mammal vocalization. Bioacoustics. 9(3):171–196. doi:10.1080/09524622.1998.9753394.
- Zollinger SA, Riede T, Suthers RA. 2008. Two-Voice complexity from a single side of the syrinx in northern mockingbird Mimus polyglottos vocalizations. J Exp Biol. 211(12):1978–1991. doi:10.1242/jeb.014092.
