Platelets, normal as well as dyspoietic, are known to arise from bone marrow megakaryocytes. Normal platelet morphogenesis, however, has been shown to take place in the blood stream from megakaryocyte cytoplasmic fragments released from the bone marrow Citation[1]. We present a case of transient myeloproliferative disorder in Down syndrome (DS) that showed dysplastic platelets arising from circulating megakaryoblasts. This sheds light on an alternative mechanism of how dysplastic platelets may be produced.
Transient myeloproliferative disorder in Down syndrome is characterized by clonal megakaryoblasts, presentation primarily in neonatal period, often at birth or shortly thereafter, common spontaneous regression and death due to extensive hepatic and visceral fibrosis.
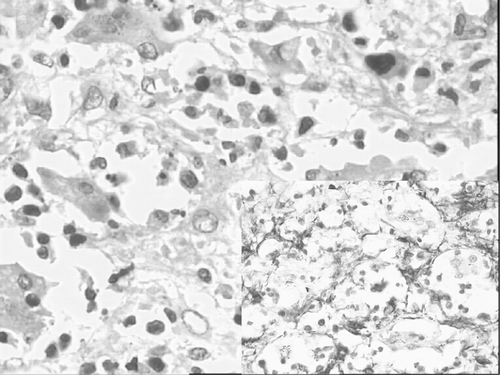
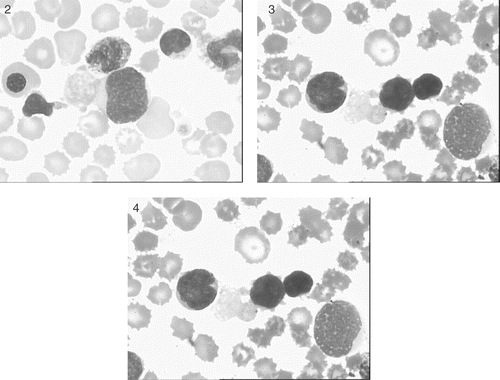
We made this diagnosis in our patient, a term female infant who died two days after birth. She presented with hepatosplenomegaly and dysmorphic facies characteristic of DS. DS was confirmed cytogenetically. Sections from liver, taken at autopsy, showed diffuse sinusoidal fibrosis breaking up the parenchyma into small groups of liver cells and showing interspersed hematopoietic cells (). Sinusoidal spindle cells and fibrous septae were positive immunohistochemically for alpha smooth muscle actin (, inset). Total leukocyte count was 70 000/cu mm. Peripheral blood smear had a leukoerythroblastic picture with 35% recognizable megakaryoblasts and 5% maturing megakaryocytic cells (micromegakaryocytes). The megakaryoblasts and the maturing megakaryocytic cells were easily identified by their characteristic nuclear and cytoplasmic morphology, and more germane to this report, by their possessing highly dysplastic large cytoplasmic projections identical to the abundantly present circulating, large, agranular or poorly granular platelets (). CD 41 was positive.
Figure 1. Section from liver showing diffuse sinusoidal fibrosis and interspersed hematopoietic cells. Inset shows sinusoidal spindle cells and fibrous septae are positive for smooth muscle actin.
Figures 2–4 Peripheral blood smear showing megakaryoblasts with highly dysplastic large cytoplasmic projections identical to the abundantly present circulating, large, agranular or poorly granular platelets.
Dysplastic platelets, recognized morphologically, among other things, as poorly granular or agranular forms seen as blue cytoplasmic fragments Citation[2] are observed in several hematological disorders. Most of the existing literature and textbooks, however, do not expressly mention the origin of these dysplastic forms, implying a bone marrow origin. This need not always be the case, however, as exceptionally, dysplastic platelets have been shown to arise from peripheral blood megakaryoblasts. This has been vividly described in myelofibrosis Citation[3] and clearly illustrated in chronic myeloid leukemia blast crisis Citation[4].
Our observation in the present case is significant for two reasons. First, it shows that identification of megakaryocytic lineage of blasts is possible in some cases even without immunocytochemistry Citation[4]. Second, it sheds light on the process of megakaryocytic maturation and platelet formation. Our observation shows that megakaryoblastic cytoplasm can fragment prematurely, without the cell undergoing maturation, leading therefore to the formation of dysplastic platelets. Equally importantly, this process takes place in the circulating blood rather than in the bone marrow, the normal home of megakaryocytes. The same process may be occurring in the bone marrow but is much more difficult to demonstrate on morphology, compared to the less cellular, less cluttered and less heterogeneous peripheral blood.
We conclude that origin from circulating megakaryoblasts is one way, hitherto not described in texts that dysplastic platelets may arise. This model of platelet formation needs further study.
References
- Behnke O, Forer A. From megakaryocytes to platelets: Platelet morphogenesis takes place in the bloodstream. Eur J Haematol Suppl 1998; 61: 3–23
- Maldonado JE. Dysplastic platelets and circulating megakaryocytes in chronic myeloproliferative diseases. II. Ultrastructure of circulating megakaryocytes. Blood 1974; 43: 811–820
- Carpenter G, Flory CM. Chronic nonleukemic myelosis. Report of a case with megakaryocytic myeloid splenomegaly, leukoerythroblastic anemia, generalized osteosclerosis and myelofibrosis. Arch Intern Med 1941; 67: 489–508
- Anand M, Kumar R, Singh S. Megakaryoblastic lineage of peripheral blood blasts of CML blast crisis identified by budding dysplastic platelets. Am J Hematol 2004; 75: 239–240