Familial platelet disorder with predisposition to acute myelogenous leukemia (FPD/AML) (OMIM #601399) is an autosomal dominant disorder characterized by quantitative and qualitative platelet defects and an increased risk of AML. FPD/AML shares phenotypic similarities with Jacobsen syndrome; platelet counts show mild to moderate reductions but are variable between individuals with the same genetic etiology of disease, and a reduction in dense granule secretion is often observed as a secondary qualitative abnormality [Citation1]. The major clinical complication of this disorder, however, is not the bleeding tendency experienced by some patients, but the propensity for a proportion of patients to develop myelodysplasia or leukemia [Citation2].
The molecular genetic cause of FPD/AML was first elucidated by linkage studies which mapped the underlying genetic defect to a region on human chromosome 21q [Citation3]. Contained within this region is the gene encoding the master regulator of hematopoiesis, Runt-related transcription factor 1 (RUNX1). Variants have been identified throughout the coding region of RUNX1 but those clustered within the region encoding the Runt homology domain (RHD), which mediates DNA binding and heterodimerization with core binding factor beta (CBF-β) [Citation4], and are most likely to be detrimental [Citation5]. RUNX1 mutation can result in haploinsufficiency of RUNX1, or reduced RUNX1 function as a result of a dominant-negative effect, that disrupts the formation of complexes with CBF-β, thereby disturbing the regulation of genes necessary for hematopoietic stem cell (HSC) maintenance, maturation, and differentiation [Citation6,Citation7].
Over 40 RUNX1 mutations associated with FPD/AML have been reported in patients to date (, ). However, the prevalence of RUNX1 defects is believed to be underestimated and as sequencing technologies improve an increasing number of patients are being reported [Citation8,Citation9]. The mutations reported are predominantly missense and phenotypically platelets from patients present with dense granule secretion defects and persistence of MYH10 expression which can be used as a biomarker of genetic variation [Citation1,Citation10]. It has been suggested that the risk of malignancy is reduced in those cases having RUNX1 defects that cause haploinsufficiency when compared to those patients with dominant-negative RUNX1 defects. Due to the associated predisposition to myeloid malignancy with some variants in RUNX1, it is critical to establish diagnosis as early as possible to aid in patient management and guidance.
Table I. RUNX1 variants reported to date in patients with an FPD/AML inherited bleeding disorder. Heterozygous RUNX1 nucleotide changes present in patients with inherited bleeding and their predicted effects on the resulting RNA or protein are also shown. Genomic variations are numbered according to positions in the NM_001001890 transcript for RUNX1. The references where they were initially reported is also indicated.
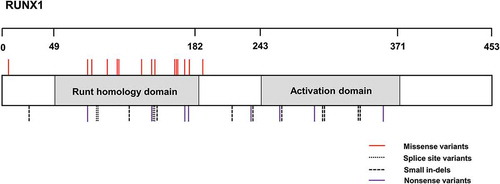
Figure 1. Schematic showing the protein location of all previously published variants within RUNX1 which are implicated in FPD/AML. The Runt-homology DNA-binding domain spanning amino acids 49 to182 and the Activation domain spanning from amino acid 243 to 371 is also displayed. Alterations are numbered according to positions in the NM_001001890 transcript for RUNX1.
Main findings
RUNX1 defects are associated with mild to moderately reduced platelet counts.
RUNX1 defects are associated with reduced responses to several platelet agonists and decreased platelet secretion.
RUNX1 missense mutations are almost exclusively located in the Runt homology DNA-binding domain.
RUNX1 defects causing haploinsufficiency are thought to be associated with a lower incidence of myeloid malignancies when compared to those patients with dominant-negative RUNX1 defects.
Declaration of interest
The authors report no conflict of interest.
Funding
The work in the author’s laboratories is supported by the British Heart Foundation (PG/13/36/30275; FS/15/18/31317).
Additional information
Funding
References
- Stockley J, Morgan NV, Bem D, Lowe GC, Lordkipanidze M, Dawood B, Simpson MA, Macfarlane K, Horner K, Leo VC, et al. Enrichment of FLI1 and RUNX1 mutations in families with excessive bleeding and platelet dense granule secretion defects. Blood 2013 Dec 12;122(25):4090–4093.
- Walker LC, Stevens J, Campbell H, Corbett R, Spearing R, Heaton D, Macdonald DH, Morris CM, Ganly P. A novel inherited mutation of the transcription factor RUNX1 causes thrombocytopenia and may predispose to acute myeloid leukaemia. Br J Haematol 2002 Jun;117(4):878–881.
- Song WJ, Sullivan MG, Legare RD, Hutchings S, Tan X, Kufrin D, Ratajczak J, Resende IC, Haworth C, Hock R, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet 1999 Oct;23(2):166–175.
- Kamachi Y, Ogawa E, Asano M, Ishida S, Murakami Y, Satake M, Ito Y, Shigesada K. Purification of a mouse nuclear factor that binds to both the A and B cores of the polyomavirus enhancer. J Virol 1990 Oct;64(10):4808–4819.
- Johnson B, Lowe GC, Futterer J, Lordkipanidze M, MacDonald D, Simpson MA, Sanchez Guiu I, Drake S, Bem D, Leo V, et al. Whole exome sequencing identifies genetic variants in inherited thrombocytopenia with secondary qualitative function defects. Haematologica 2016 Jun 16.
- Jalagadugula G, Mao G, Kaur G, Goldfinger LE, Dhanasekaran DN, Rao AK. Regulation of platelet myosin light chain (MYL9) by RUNX1: implications for thrombocytopenia and platelet dysfunction in RUNX1 haplodeficiency. Blood 2010 Dec 23;116(26):6037–6045.
- Hart SM, Foroni L. Core binding factor genes and human leukemia. Haematologica 2002 Dec;87(12):1307–1023.
- Latger-Cannard V, Philippe C, Bouquet A, Baccini V, Alessi MC, Ankri A, Bauters A, Bayart S, Cornillet-Lefebvre P, Daliphard S, et al. Haematological spectrum and genotype-phenotype correlations in nine unrelated families with RUNX1 mutations from the French network on inherited platelet disorders. Orphanet J Rare Dis 2016 Apr 26;11:49.
- Johnson B, Lowe GC, Futterer J, Lordkipanidze M, MacDonald D, Simpson MA, Sanchez-Guiu I, Drake S, Bem D, Leo V, et al. Whole exome sequencing identifies genetic variants in inherited thrombocytopenia with secondary qualitative function defects. Haematologica 2016 Oct;101(10):1170–1179.
- Antony-Debre I, Bluteau D, Itzykson R, Baccini V, Renneville A, Boehlen F, Morabito M, Droin N, Deswarte C, Chang Y, et al. MYH10 protein expression in platelets as a biomarker of RUNX1 and FLI1 alterations. Blood 2012 Sep 27;120(13):2719–2722.
- Owen CJ, Toze CL, Koochin A, Forrest DL, Smith CA, Stevens JM, Jackson SC, Poon MC, Sinclair GD, Leber B, et al. Five new pedigrees with inherited RUNX1 mutations causing familial platelet disorder with propensity to myeloid malignancy. Blood 2008 Dec 01;112(12):4639–4645.
- Michaud J, Wu F, Osato M, Cottles GM, Yanagida M, Asou N, Shigesada K, Ito Y, Benson KF, Raskind WH, et al. In vitro analyses of known and novel RUNX1/AML1 mutations in dominant familial platelet disorder with predisposition to acute myelogenous leukemia: implications for mechanisms of pathogenesis. Blood 2002 Feb 15;99(4):1364–1372.
- Beri-Dexheimer M, Latger-Cannard V, Philippe C, Bonnet C, Chambon P, Roth V, Gregoire MJ, Bordigoni P, Lecompte T, Leheup B, et al. Clinical phenotype of germline RUNX1 haploinsufficiency: from point mutations to large genomic deletions. Eur J Hum Genet 2008 Aug;16(8):1014–1018.
- Preudhomme C, Renneville A, Bourdon V, Philippe N, Roche-Lestienne C, Boissel N, Dhedin N, Andre JM, Cornillet-Lefebvre P, Baruchel A, et al. High frequency of RUNX1 biallelic alteration in acute myeloid leukemia secondary to familial platelet disorder. Blood 2009 May 28;113(22):5583–5587.
- Bluteau D, Gilles L, Hilpert M, Antony-Debre I, James C, Debili N, Camara-Clayette V, Wagner-Ballon O, Cordette-Lagarde V, Robert T, et al. Down-regulation of the RUNX1-target gene NR4A3 contributes to hematopoiesis deregulation in familial platelet disorder/acute myelogenous leukemia. Blood 2011 Dec 08;118(24):6310–6320.
- Langabeer SE, Owen CJ, McCarron SL, Fitzgibbon J, Smith OP, O’Marcaigh A, Browne P. A novel RUNX1 mutation in a kindred with familial platelet disorder with propensity to acute myeloid leukaemia: male predominance of affected individuals. Eur J Haematol 2010 Dec;85(6):552–553.
- Buijs A, Poot M, van der Crabben S, van der Zwaag B, van Binsbergen E, van Roosmalen MJ, Tavakoli-Yaraki M, de Weerdt O, Nieuwenhuis HK, van Gijn M, et al. Elucidation of a novel pathogenomic mechanism using genome-wide long mate-pair sequencing of a congenital t(16;21) in a series of three RUNX1-mutated FPD/AML pedigrees. Leukemia 2012 Sep;26(9):2151–2154.
- Linden T, Schnittger S, Groll AH, Juergens H, Rossig C. Childhood B-cell precursor acute lymphoblastic leukaemia in a patient with familial thrombocytopenia and RUNX1 mutation. Br J Haematol 2010 Dec;151(5):528–530.
- Heller PG, Glembotsky AC, Gandhi MJ, Cummings CL, Pirola CJ, Marta RF, Kornblihtt LI, Drachman JG, Molinas FC. Low Mpl receptor expression in a pedigree with familial platelet disorder with predisposition to acute myelogenous leukemia and a novel AML1 mutation. Blood 2005 Jun 15;105(12):4664–4670.
- Appelmann I, Linden T, Rudat A, Mueller-Tidow C, Berdel WE, Mesters RM. Hereditary thrombocytopenia and acute myeloid leukemia: a common link due to a germline mutation in the AML1 gene. Ann Hematol 2009 Oct;88(10):1037–1038.
- Shiba N, Hasegawa D, Park MJ, Murata C, Sato-Otsubo A, Ogawa C, Manabe A, Arakawa H, Ogawa S, Hayashi Y. CBL mutation in chronic myelomonocytic leukemia secondary to familial platelet disorder with propensity to develop acute myeloid leukemia (FPD/AML). Blood 2012 Mar 15;119(11):2612–2614.
- Kirito K, Sakoe K, Shinoda D, Takiyama Y, Kaushansky K, Komatsu N. A novel RUNX1 mutation in familial platelet disorder with propensity to develop myeloid malignancies. Haematologica 2008 Jan;93(1):155–156.
- Churpek JE, Garcia JS, Madzo J, Jackson SA, Onel K, Godley LA. Identification and molecular characterization of a novel 3’; mutation in RUNX1 in a family with familial platelet disorder. Leukemia Lymphoma 2010 Oct;51(10):1931–1935.
