Abstract
There is a large inter-individual variation in response to clopidogrel treatment, and previous studies have indicated higher risk of thrombotic events in those with high residual platelet reactivity (HPR). Less is known about individual variation over time. The aim of this prospective cohort study was to investigate intra-individual variation in platelet reactivity. Platelet aggregation in whole blood was assessed in 77 patients, at 3 days, 8 days and 6 months after admission for acute myocardial infarction and loading dose of clopidogrel. All patients were treated with aspirin and clopidogrel through 6-month follow-up. We found a significant increase in median ADP-stimulated aggregation from third to eighth day (195 vs. 250 AU*min, p-value = 0.001) but not from day 8 to 6 months (250 vs. 223 AU*min, p-value = 0.666). There was no significant change in the overall rate of HPR (15.6% vs 20.8%, p-value 0.503) or low platelet reactivity (LPR) (37.7% vs 33.8%, p-value = 0.609) from day 8 to 6-month follow-up. In contrast, more than one in four changed HPR status, 15.6% from non-HPR to HPR and 10.4% HPR to non-HPR. A shift in LPR status appeared even more frequent, occurring in about one of three patients. In spite of similar median aggregation and rate of HPR during 6-month follow-up, about one in four of the patients changed HPR status and one in three changed LPR status. This may be important information for a concept of risk stratification based on a single aggregation value early after an acute coronary syndromes.
Introduction
Platelet activation, adhesion, aggregation and subsequent clot formation plays an important role in the pathogenesis of acute coronary syndromes (ACS) and in thrombotic complications following ACS and percutaneous coronary intervention (PCI). Dual antiplatelet therapy (DAPT) with aspirin (Citation1) and clopidogrel (Citation2) has been standard of care for acute and long-term treatment in ACS to decrease incidence of death and myocardial infarction. Also, DAPT has proven beneficial in prevention of short- and long-term thrombotic complications following PCI (Citation3). There is a well-known and large inter-individual variation in response to clopidogrel treatment (Citation4,Citation5), which has been explained by genetic differences in clopidogrel metabolizing enzymes, patient demographic and clinical factors including co-medication. Moreover, studies have indicated higher risk of thrombotic events in patients with high residual platelet reactivity (HPR) on clopidogrel treatment, and higher bleeding risk with low residual platelet reactivity (LPR) (Citation6–Citation8). However, the trials performed so far have given limited support to a personalized antiplatelet treatment strategy based on platelet activity testing (Citation9–Citation12). While inter-individual variation has been well studied, less attention has been paid to intra-individual variation over time, and the few reports to date have given contradictory results (Citation13–Citation19). A concept of tailored treatment based on a single measurement assumes that a patient can be correctly defined as a high, optimal or low responder to clopidogrel treatment.
In the present study, we assessed platelet reactivity in patients with ACS (treated with aspirin and clopidogrel) at three time-points up to 6 months after admission and loading dose (LD) of clopidogrel. Our objective was to investigate the individual changes in platelet reactivity and change in individual HPR and LPR status over time.
Methods
Study population
The study protocol has been previously described in detail (Citation20). Briefly, between January 2009 and August 2011 125 patients with ST-elevation myocardial infarction (STEMI) or non ST-elevation myocardial infarction (NSTEMI), defined according to Global definition of myocardial infarction (Citation21) and scheduled for coronary angiography, were recruited at the Department of Cardiology, Heart Center, University Hospital, Linköping, Sweden. Exclusion criteria were: Participation in an intervention study, treatment with warfarin before admission, short life expectancy (less than 6 months) or unwillingness to participate. All patients received 600 mg LD of clopidogrel, followed by 75 mg once daily. When the study was planned and initiated there were no third-generation P2Y12 inhibitors (prasugrel or ticagrelor) approved in Sweden. According to clinical routine, if a patient was not on chronic aspirin treatment on admission, a LD of 300 mg aspirin was given, followed by a maintenance dose of 75 mg daily. There were no patients on direct oral anticoagulation (DOAC) on admission or at discharge. Coronary interventions were performed according to current guidelines (Citation20). Choices of stents were made according to treating physicians´ discretion.
The present analysis included 77 patients treated with DAPT through 6-month follow-up and with complete information on aggregation tests (performed 3 days, 8 days and 6 months after admission).
Blood sampling and platelet reactivity testing
For this analysis, we have used venous blood samples collected on the third day after admission and LD (as a clinically convenient time-point when most patients were still hospitalized), 7–9 days after LD (median 8 days, a time-point when steady-state for aggregation was ascertained even with single doses), and 6 months after admission and LD of clopidogrel (as an end-of-trial value to assess aggregation value in stable patients). All samples were drawn into blood collection tubes containing hirudin as anticoagulant (Dynabyte Medical, Munich, Germany). According to instructions from the manufacturer, blood samples were kept at room temperature for a minimum of 30 minutes and a maximum of 120 minutes before aggregometry analyses were performed. Platelet activity was measured in whole blood using a Multiplane impedance aggregometer (Roche diagnostics, Mannheim, Germany, former Dynabyte Medical, Munich, Germany). The procedure is described in detail elsewhere (Citation22). In summary, whole blood was mixed in a 1:1 proportion with 0.9% saline in the test cuvette, and aggregation was initiated with adenosine diphosphate (ADP), arachidonic acid (ASPI) and thrombin receptor activating peptide (TRAP). The ADP test is used to measure the effect of ADP-receptor antagonists (e.g. clopidogrel), the ASPI test is used to assess the effect of cyclooxygenase inhibitors (like aspirin). TRAP is an activator developed primarily to measure the effect of very potent aggregation inhibitors (GP IIb/IIIa-inhibitors), with limited sensitivity toward ADP-receptor inhibition by clopidogrel and cyclooxygenase inhibition by aspirin. Impedance is measured between two electrodes in the test cuvettes. When platelets activate, they adhere and aggregate on the electrodes, increasing the impedance. The impedance as a function of time (the area under the curve) is proportional to the degree of platelet aggregation.
Outcome definitions
Based on earlier studies, HPR on clopidogrel treatment was defined as ADP-stimulated aggregation > 468 AUC*min and LPR was defined as <188 AUC*min (Citation23). Values between 188 and 468 were regarded as optimal platelet reactivity (OPR). We calculated the rate of HPR and LPR (with ADP-stimulated aggregation) at 3 days, 8 days and 6 months and the rate of patients that changed HPR and LPR status between the three time-points.
Based on an analysis from the ISAR-ASPI registry indicating that a cut-off of 203 AU*min to define ASPI- stimulated HPR may be used to predict clinical events, we report the rate of patients above that level at different time-points (Citation24). However, since there is no consensus of established cut-offs for HPR with ASPI and TRAP stimulation we calculated the proportion of patients within the highest quintile of platelet reactivity, with all three agonists. To compare changes in the rate of HPR depending on the aggregation agonist used, we used cut-off values for the highest quintile at 8 days and the proportion above that value at day 3 and at 6 months. Thereafter we assessed the number of patients changing status to or from the highest quintile from day 8 to 6 months. The same calculations were performed with ADP, ASPI and TRAP as agonists. Day 8 was chosen as reference to allow steady-state for clopidogrel and because of a presumed remaining effect of abciximab on the aggregation values at day 3. For the same reason, comparison between day 8 and 6 months was chosen as the main analysis.
Statistical analysis
Power calculation, made for the main analysis, previously presented (Citation20), was based on two earlier studies measuring platelet aggregation in patients with STEMI and NSTEMI. Based on these studies we postulated that 75% of clinical events after an acute coronary syndrome would occur in the quartile with highest platelet activity. With 80% power, assuming an incidence of 10% of the primary outcome, 60 patients would be needed to detect a statistically significant difference (p-value = 0.05) between the quartile with the highest platelet aggregation and the rest of the study population. Because of low number of expected events, we chose a power of 95% and accordingly to include 120 patients.
For the current analysis, only patients with complete aggregation data at all three time-points were included. This is a post-hoc analysis and the results should be regarded as hypothesis generating.
Statistical significance was tested with McNemar’s test for paired proportions and Wilcoxon Signed Rank test for related medians. A p-value of <0.05 was regarded as significant. Kappa statistics was used to assess agreement of high platelet reactivity (HPR) status (defined as allocated to the quintile with the highest platelet reactivity) between two time-points. A Kappa value below 0.4 is generally regarded as indicative of poor agreement.
Ethical considerations
The study was performed according to good clinical practice, complies with the Declaration of Helsinki and was approved by the Regional Ethical Review Board in Linköping (Dnr M45-08). All patients gave written informed consent.
Results
We included 77 patients; median age was 66 years, 74% were male, 29% were smokers, 12% had diabetes, 17% had a history of myocardial infarction (MI) and 16% had a history of revascularization with PCI or coronary artery by-pass grafting (9% and 7%, respectively). A majority of the patients were admitted with STEMI, (60%), all but one were catheterized, 90% underwent PCI and 82% had a stent implanted (). During PCI, 58% were treated with a GP IIb/IIIa-inhibitor (abciximab). There were no significant differences in pharmacological treatment from discharge to 6-month follow-up. Importantly, all patients were discharged with DAPT (clopidogrel and aspirin) and continued on DAPT at least 6 months. () A total of 15 patients were discharged with a proton pump inhibitor (PPI) or H2 antagonist. Nine received pantoprazole (recommended PPI with clopidogrel treatment), three ranitidin, two omeprazol and one lanzoprazol. The majority of the patients remained on the same treatment over 6-month follow-up. Of the two patients discharged with omeprazole, one was on omeprazole for the duration of the follow-up with no change in platelet aggregation category (LPR at both time-points) and one stopped treatment before 6-month follow-up (LPR at 8 days and optimal response at 6 months). In addition, one patient discharged with ranitidine changed to omeprazole during follow-up, with no change in aggregation category (optimal response both at 8 days and 6 months).
Table I. Baseline characteristics.
Table II. Medication at discharge and 6-month follow-up.
Platelet aggregation at different time-points
An increase in platelet aggregation was observed from day 3 to day 8 with ADP (195–250 AUC*min, p = 0.001), ASPI (69–106 AUC*min, p = 0.009) and TRAP (704–912 AUC*min, p < 0.001) stimulation. In contrast, from day 8 to 6 months after admission, there were no further significant changes (250–223 AUC*min [p = 0.666], 106–100 AUC*min [p = 0.748], 912–898 AUC*min [p = 0.393] with ADP, ASPI and TRAP stimulation, respectively). Similarly, there was no significant change in the overall rate of ADP-stimulated HPR (15.6% vs 20.8%, p-value 0.503) or LPR (37.7% vs 33.8%, p = 0.609) from day 8 to 6-month follow-up.
We also calculated the proportion of patients within the highest quintile of platelet reactivity, with all three agonists. Again, we found a similar pattern with ADP, ASPI and TRAP stimulation, with an increase from day 3 to 8 and no further change from 8 days to 6 months ().
Table III. Platelet aggregation at different time-points.
Change in HPR and LPR status over time on treatment with clopidogrel
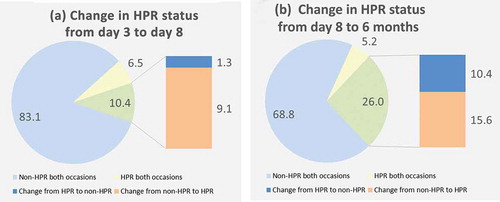
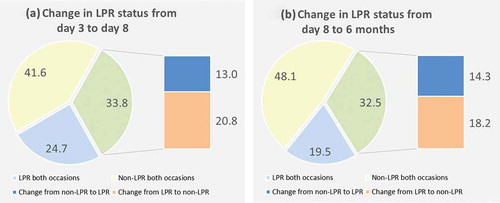
From the third to the eighth day, 9.1% of the patients shifted from non-HPR to HPR, while the majority remained in the same category (89.6%). In contrast, more than one in four changed HPR status from day 8 to 6-month follow-up, 15.6% from non-HPR to HPR and 10.4% HPR to non-HPR. (,)) A shift in LPR status appeared even more frequent, occurring in about one of three patients. From day 8 to 6 months 18.2% shifted from LPR to non-LPR and 14.3% from non-LPR to LPR. (,))
Long-term variation in platelet reactivity with ADP, ASPI and TRAP activation
Platelet reactivity changed substantially in individual patients, both increases and decreases, from day 8 to 6-months follow-up, with all three agonists, although median changes were small ().
Figure 3. Change in aggregation from 8 days after admission to 6-month follow-up with (a). ADP, (b) ASPI and (c) thrombin activating peptide (TRAP).
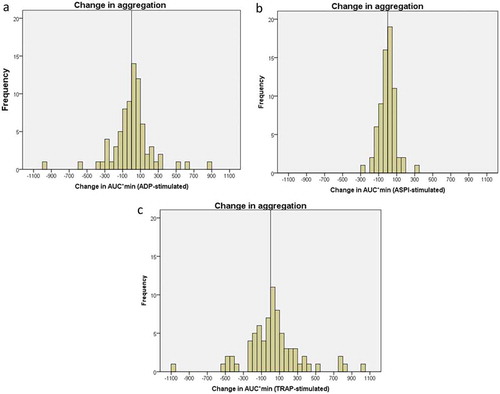
The median absolute change (increase or decrease) with ADP (122, (62–279)) stimulation was significantly higher than with ASPI (50 (28–90) (p < 0.01) or TRAP (88(44–189)) (p = 0.03) stimulation. Given the difference in median aggregation values with the three platelet activators, we also calculated the relative change in aggregation between day 8 and 6 months. Between the two time-points, almost every second patient changed more than 50% with ASPI stimulation, compared to 1 in 3 with ADP stimulation and 1 in 7 with TRAP stimulation ().
Table IV. Change in aggregation value from 8 days to 6 months after LD.
Table V. Change in platelet activity over time. Patients in the highest quintile.
Agreement in HPR status between 8 days and 6 months with different agonists
We observed a substantial shift in HPR status (here defined as the quintile with the highest reactivity) with all three agonists. The Kappa values were 0.28, 0.31 and 0.35 with ADP, ASPI and TRAP stimulation, respectively, indicating poor agreement between aggregation values 8 days and 6 months after admission, using any of the three agonists ().
Discussion
Although we found no significant change in median ADP-stimulated aggregation or proportion of patients with HPR from day 8 to 6 months after an acute MI (and treatment with DAPT) more than one in four patients changed individual HPR status. Moreover, about one in three patients changed LPR status during the same period.
Earlier trials have shown a large inter-individual variation in response to clopidogrel treatment (Citation4,Citation5). HPR has been associated with higher risk for ischemic events and LPR has been associated with higher risk of bleeding (Citation6,Citation7,Citation23). An individual treatment algorithm has therefore been proposed based on information about individual response to clopidogrel treatment (Citation25). However, the concept assumes that risk prediction, based on categorization as LPR, OPR or HPR is stable over time. This study shows that, more than half of the population cross over between LPR, OPR and HPR during follow-up, making early risk prediction based on cut-off values precarious.
The longitudinal intra-individual variation is much less studied than the inter-individual variation. Two small previous trials showed very little variation of aggregation through 2 weeks (Citation14) and 1 year (Citation13), respectively, in patients treated with clopidogrel, while another small study of patients with MI, treated with PCI and clopidogrel, showed that about one in four patients changed HPR status from baseline to 6-month follow-up. Aggregation measurements were performed with Multiplate and VerifyNow, with similar proportion of patients shifting HPR status (Citation16). Another study reported that 27% of patients changed HPR status from baseline to 1 month, mainly caused by poor responders becoming responders, with no further change from 1 to 6 months. The change occurred in both stable patients and NSTEACS patients (Citation15). Supporting our findings, a recently published paper reported that 36.6% of clopidogrel-treated patients had HPR status at any of three time-points over 6 months (at discharge, 3 and 6 months) (Citation18). In our study, 31.2% off the patients were HPR at day 8 or at 6 months. In contrast, they found that only 9.8% had LPR over 6 months, compared to about half of the patients in our study. Finally, in a large study including over 300 patients, Hochholzer et al. reported that about one in five patients changed HPR status during follow-up, without any significant difference in mean aggregation values (Citation17). We found a similar change in HPR status from 8 days to 6 months follow-up, but in contrast to earlier reports, we observed changes in both directions, from non-HPR to HPR and vice versa. Hence, although the two largest studies found that a large proportion of patients change HPR status, the direction of change differed. Campo et al. (Citation15) observed mainly a change from HPR to non-HPR while Hochholzer (Citation17), in accordance with our findings, found that a substantial number of patients also changed from non-HPR to HPR. The reason for the divergent results is not evident, but differences in study patient characteristics, (like age, co-morbidity, other medication and proportion of patients with STEMI/NSTE ACS) as well as timing of measurement and time of follow-up may be important factors. Important individual change in HPR status through 6-months follow-up, in patients treated with clopidogrel, has also been observed with other methods (Citation26).
Our data are in line with earlier data indicating a substantial change in individual HPR status over time, in patients treated with clopidogrel. Moreover, we expand earlier knowledge by showing data indicating that individual change in LPR status may be at least as frequent as change in HPR status. Moreover, agreement in aggregation values over time appears to be low with any of the three used activators. A high rate of change in HPR and LPR status may add important information to why a strategy of personalized antiplatelet treatment, in larger multicenter trials, has not been as successful as expected (Citation9–Citation11). Patient compliance issues may explain some increased aggregation values and change in systemic inflammation from the acute to the stable phase may also explain some of the variation. Some data have shown that omeprazole treatment attenuates the antiplatelet effect of clopidogrel (Citation27). In this study, only three patients were treated with omperazol (two at discharge and one changed from ranitidine to omeprazole during follow-up). Only one of these changed platelet aggregation category between day 8 and 6 months (from LPR at 8 days, while on omeprazole, to optimal PR at 6 months when omeprazole was stopped). Hence, omeprazole treatment does not explain the large variation and frequent change in aggregation category observed in this study.
To explore whether the change in aggregation was restricted to ADP-stimulated aggregation, we measured ASPI and TRAP activated aggregation. The median change in aggregation values from day 8 to 6 months was significantly higher with ADP stimulation than with ASPI or TRAP stimulation, even though ADP-stimulated aggregation values lie in between ASPI and TRAP-stimulated values, respectively. This may indicate that ADP-receptor inhibition is more variable over time depending on changes in clopidogrel metabolism, ADP-receptor expression, patient compliance or that the ADP test is more sensitive to time-point for blood sampling and handling. A relative change, (10–50% change from day 8 to 6 months) appeared more common with ASPI stimulation and less often with TRAP stimulation compared with ADP stimulation, which is largely expected considering the median aggregation values, i.e. the stronger the activator, the less the relative change. To further investigate differences in platelet reactivity over time depending on the agonist used, we compared the agreement of HPR classification between day 8 and 6 months with the three agonists. Kappa values indicated poor agreement for all agonists, but with the lowest value for ADP activation, again indicating that ADP-stimulated aggregation measurements may be especially variable over time. A higher degree of diurnal variation with ADP-stimulated aggregation compared with ASPI-stimulated aggregation was indicated in one earlier study, which may be important for this finding (Citation28). Anyhow, these results support the notion of a substantial variation in platelet reactivity over time, a finding that has been shown also in healthy controls (Citation29). Larger trials including all patient categories with an indication for DAPT and repeated, well-defined time-points for assessment of platelet reactivity are needed to clarify the magnitude of the problem in order to move the concept of individually tailored antiplatelet treatment forward. A shift in HPR or LPR status over time substantially weakens the predictive information of platelet activity testing. Our results support the recently published European Society of Cardiology/European Society of Thoracic Surgeons update on DAPT, not recommending routine platelet function testing to tailor treatment (Citation30).
Limitations
There are some important limitations with this analysis. First, the small study size inevitably increases the risk of a chance finding and decreases the external validity. Of the initially 125 patients, 77 complied with 6 months clopdiogrel treatment and had complete aggregation data for all three time-points. However, there is a lack of data addressing intra-individual variation of platelet reactivity, so these data add information to current knowledge, especially with different agonists. Second, a large proportion of our patients received GP IIb/IIIa-inhibitors which probably had an impact on aggregation values day 3, but lack of difference in TRAP-stimulated value from day 8 to 6 months indicate that there was no influence on GP IIb/IIIa-inhibitors at these time-points. Third, even if blood tests were scheduled to the morning we did not have an exact time point, which may have impacted the aggregation values. Finally, we did not use pill count to assess compliance. Anyway, the observed change in platelet reactivity, in both directions, cannot be explained by lack of compliance.
To conclude, in spite of similar median aggregation and rate of HPR during 6-month follow-up of MI patients, about one in four change HPR status and one in three change LPR status. This may be important to understand difficulties to tailor antiplatelet treatment based on a single aggregation value, especially early after ACS.
Addendum
J. Alfredsson, T.L. Lindahl, E. Swahn, L. Jonasson, M. Janzon, E. Logander and K.M. Gustafsson contributed to concept and design, analysis and interpretation of data; writing and revising of intellectual content and final approval of the manuscript. L. Nilsson contributed to interpretation of data, writing and revising of intellectual content and final approval of the manuscript.
Conflict of interest
J. A. reports research grant from AstraZeneca, lecture fees from AstraZeneca, Sanofi-Aventis, BMS and Eli-Lilly, and honorary for advisory board for AstraZeneca. T.L. reports research grants from Boehringer-Ingelheim, Bayer, BMS and AstraZeneca, and lecture fees from Boehringer-Ingelheim and Roche, TL reports being member of board and shareholder of Medirox and Nordic Hemostasis. K.M.G reports being shareholder of Nordic Hemostasis. E.L, E.S, M.J, L.N, L.J, report no relevant conflicts of interest
Acknowledgments
We want to thank the coronary care unit nurses who performed the aggregation analyses.
Additional information
Funding
References
- The RISC Group. Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. Lancet. 1990;336:827–830. doi:10.1016/0140-6736(90)92336-G.
- Mehta SR, Yusuf S. The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. Eur Heart J. 2000;21:2033–2041. doi:10.1053/euhj.2000.2474.
- Mehta SR. Aspirin and clopidogrel in patients with ACS undergoing PCI: CURE and PCI-CURE. J Invasive Cardiol. 2003;15 Suppl B:17B–20B. discussion 20B-21B.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45:246–251. doi:10.1016/j.jacc.2004.09.067.
- Jaremo P, Lindahl TL, Fransson SG, Richter A. Individual variations of platelet inhibition after loading doses of clopidogrel. J Intern Med. 2002;252:233–238. doi:10.1046/j.1365-2796.2002.01027.x.
- Matetzky S, Shenkman B, Guetta V, Shechter M, Bienart R, Goldenberg I, Novikov I, Pres H, Savion N, Varon D, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–3175. doi:10.1161/01.CIR.0000130846.46168.03.
- Cuisset T, Frere C, Quilici J, Barbou F, Morange PE, Hovasse T, Bonnet JL, Alessi MC. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J Thromb Haemost. 2006;4:542–549. doi:10.1111/j.1538-7836.2005.01751.x.
- Hochholzer W, Trenk D, Bestehorn HP, Fischer B, Valina CM, Ferenc M, Gick M, Caputo A, Buttner HJ, Neumann FJ. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol. 2006;48:1742–1750. doi:10.1016/j.jacc.2006.06.065.
- Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–1105. doi:10.1001/jama.2011.290.
- Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Muller U, Richardt G, Jakubowski JA, Neumann FJ. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol. 2012;59:2159–2164.
- Collet JP, Cuisset T, Range G, Cayla G, Elhadad S, Pouillot C, Henry P, Motreff P, Carrie D, Boueri Z, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367:2100–2109. doi:10.1056/NEJMoa1209979.
- Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, Delarche N, Bellemain-Appaix A, Range G, El Mahmoud R, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet. 2016;388:2015–2022. doi:10.1016/S0140-6736(16)31323-X.
- Saw J, Madsen EH, Chan S, Maurer-Spurej E. The ELAPSE (Evaluation of Long-Term Clopidogrel Antiplatelet and Systemic Anti-Inflammatory Effects) study. J Am Coll Cardiol. 2008;52:1826–1833. doi:10.1016/j.jacc.2008.08.047.
- Jaitner J, Stegherr J, Morath T, Braun S, Bernlochner I, Schomig A, Kastrati A, Sibbing D. Stability of the high on-treatment platelet reactivity phenotype over time in clopidogrel-treated patients. Thromb Haemost. 2011;105:107–112. doi:10.1160/TH10-07-0440.
- Campo G, Parrinello G, Ferraresi P, Lunghi B, Tebaldi M, Miccoli M, Marchesini J, Bernardi F, Ferrari R, Valgimigli M. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol. 2011;57:2474–2483. doi:10.1016/j.jacc.2010.12.047.
- Codner P, Vaduganathan M, Rechavia E, Iakobishvili Z, Greenberg G, Assali A, Hasdai D, Battler A, Kornowski R, Lev EI. Clopidogrel response up to six months after acute myocardial infarction. Am J Cardiol. 2012;110:321–325. doi:10.1016/j.amjcard.2012.03.029.
- Hochholzer W, Ruff CT, Mesa RA, Mattimore JF, Cyr JF, Lei L, Frelinger AL 3rd, Michelson AD, Berg DD, Angiolillo DJ, et al. Variability of individual platelet reactivity over time in patients treated with clopidogrel: insights from the ELEVATE-TIMI 56 trial. J Am Coll Cardiol. 2014;64:361–368. doi:10.1016/j.jacc.2014.03.051.
- Tello-Montoliu A, Rivera J, Hernandez D, Silvente A, Jover E, Rodriguez AI, Quintana M, Romero A, Orenes-Pinero E, Rivera-Caravaca JM, et al. Temporal changes in platelet response in acute coronary syndrome patients with prasugrel and clopidogrel after stent implantation. Circ J. 2018;82:353–360. doi:10.1253/circj.CJ-17-0471.
- Nuhrenberg TG, Stratz C, Leggewie S, Hochholzer W, Valina CM, Gick M, Kirtane AJ, Stone GW, Neumann FJ, Trenk D. Temporal variability in the antiplatelet effects of clopidogrel and aspirin after elective drug-eluting stent implantation. An ADAPT-DES substudy. Thromb Haemost. 2015;114:1020–1027. doi:10.1160/TH15-03-0257.
- Alfredsson J, Lindahl TL, Gustafsson KM, Janzon M, Jonasson L, Logander E, Nilsson L, Swahn E. Large early variation of residual platelet reactivity in Acute Coronary Syndrome patients treated with clopidogrel: results from Assessing Platelet Activity in Coronary Heart Disease (APACHE). Thromb Res. 2015;136:335–340. doi:10.1016/j.thromres.2015.05.021.
- Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi:10.1161/CIRCULATIONAHA.107.187397.
- Toth O, Calatzis A, Penz S, Losonczy H, Siess W. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thromb Haemost. 2006;96:781–788. doi:10.1160/TH06-05-0242.
- Sibbing D, Steinhubl SR, Schulz S, Schomig A, Kastrati A. Platelet aggregation and its association with stent thrombosis and bleeding in clopidogrel-treated patients: initial evidence of a therapeutic window. J Am Coll Cardiol. 2010;56:317–318. doi:10.1016/j.jacc.2010.03.048.
- Mayer K, Bernlochner I, Braun S, Schulz S, Orban M, Morath T, Cala L, Hoppmann P, Schunkert H, Laugwitz KL, et al. Aspirin treatment and outcomes after percutaneous coronary intervention: results of the ISAR-ASPI registry. J Am Coll Cardiol. 2014;64:863–871. doi:10.1016/j.jacc.2014.05.049.
- Siller-Matula JM, Trenk D, Schror K, Gawaz M, Kristensen SD, Storey RF, Huber K, European Platelet A. How to improve the concept of individualised antiplatelet therapy with P2Y12 receptor inhibitors–is an algorithm the answer? Thromb Haemost. 2015;113:37–52. doi:10.1160/TH14-03-0238.
- Freynhofer MK, Bruno V, Brozovic I, Jarai R, Vogel B, Farhan S, Hubl W, Willheim M, Wojta J, Huber K. Variability of on-treatment platelet reactivity in patients on clopidogrel. Platelets. 2014;25:328–336. doi:10.3109/09537104.2013.827781.
- Sibbing D, Morath T, Stegherr J, Braun S, Vogt W, Hadamitzky M, Schomig A, Kastrati A, von Beckerath N. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost. 2009;101:714–719.
- Kozinski M, Bielis L, Wisniewska-Szmyt J, Boinska J, Stolarek W, Marciniak A, Kubica A, Grabczewska Z, Navarese EP, Andreotti F, et al. Diurnal variation in platelet inhibition by clopidogrel. Platelets. 2011;22:579–587. doi:10.3109/09537104.2011.582900.
- Refaai MA, Frenkel E, Sarode R. Platelet aggregation responses vary over a period of time in healthy controls. Platelets. 2010;21:460–463. doi:10.3109/09537104.2010.485256.
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, et al. ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213–260. doi:10.1093/eurheartj/ehx419.