Abstract
Platelet function tests (PFT), such as the Multiple Electrode Analyzer (Multiplate) and VerifyNow, show little concordance in patients using antiplatelet drugs. A major difference between these tests is the use of prostaglandin E1 (PGE1) to inhibit P2Y1-platelet-receptor activation in VerifyNow and is proposed to be of influence in the discrepancy between these tests. We aimed to investigate whether the presence of PGE1 could provide an explanation for the moderate correlation and concordance between Multiplate and VerifyNow by adding PGE1 to the Multiplate ADP assay, also known as the ADP-high sensitivity (ADP-HS) assay. We also aimed to investigate whether the difference in baseline platelet function as measured by the VerifyNow and Multiplate could (partly) explain the moderate correlation between the tests, by plotting ADP assay results against baseline function as measured by the corresponding device, which is expressed as the ‘inhibitor percentage.’ Fifty-one patients who underwent percutaneous coronary intervention (PCI) received dual antiplatelet therapy and were considered to have a high risk of ischemic or bleeding complications were included. The addition of 20 µl PGE1 in the Multiplate resulted in a significant reduction in Arbitrary Aggregation Units, but did not improve correlation with the VerifyNow. The correlation between VerifyNow and Multiplate inhibitor percentage was moderate. Based on these results, we concluded that neither PGE1 nor the calculation of the inhibitor percentage greatly influenced the correlation between PFTs.
Introduction
Platelet function tests (PFT), including the Multiple Electrode Analyzer (Multiplate) and VerifyNow, are important methods to monitor the effects of antiplatelet drugs in patients. Upon antiplatelet testing in patients, results can be classified into categories of low, optimal or high platelet reactivity based on the therapeutic window of these PFTs [Citation1]. However, concordance and correlation between Multiplate and VerifyNow are moderate at best in patients using P2Y12 inhibitors after percutaneous coronary intervention (PCI) [Citation2–4]. Correlation is defined as the strength of association between the PFTs. Several explanations for the lack of correlation have been proposed. One of the proposed explanations is the difference in the use of prostaglandin E1 (PGE1)[Citation3]. This is added to the VerifyNow assay, while PGE1 is not added to the Multiplate assay. PGE1 inhibits ADP-mediated platelet activation by the P2Y1 receptor, which makes the assay more sensitive to P2Y12 inhibition [Citation5] and is proposed to explain some of the differences seen between Multiplate and VerifyNow[Citation3]. We hypothesized that the addition of PGE1 to the Multiplate ADP assay, known as the Multiple Electrode Analyzer ADP-high sensitivity assay (Multiplate ADP-HS), would improve the correlation between the Multiplate ADP and VerifyNow P2Y12 assay. We aimed to investigate if the presence of PGE1 could provide an important explanation for the low correlation and concordance between Multiplate and VerifyNow.
Another hypothesis for the lack of correlation between the assays is the difference in baseline platelet function (or non-ADP-mediated platelet aggregation) that is measured by the Multiplate and VerifyNow. By plotting ADP assay results against the baseline platelet aggregation, this factor can be eliminated. The VerifyNow already automatically calculates this parameter for the P2Y12 assay using a thrombin receptor activating peptide (TRAP) assay to determine baseline platelet function in patients using P2Y12 inhibitors. TRAP activates platelets through PAR-1 receptors instead of ADP-dependent pathways, which are considered blocked by the P2Y12 inhibitor.
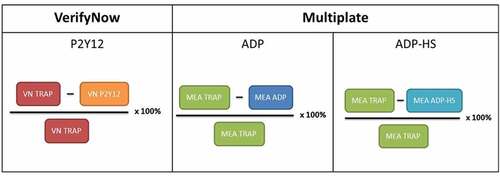
The ratio of the VerifyNow P2Y12 assay compared to the TRAP-based platelet function assay is presented as the ‘inhibitor percentage'. A low inhibitor percentage in patients on clopidogrel indicates clopidogrel resistance, currently characterized as high on treatment platelet reactivity (HTPR)[Citation6]. Even though disagreement about the HTPR cutoff exists [Citation7], in patients on DAPT with clopidogrel undergoing PCI a cutoff of 40% is generally applied [Citation8–10]. Although normally not applied, the inhibitor percentage can be calculated for the Multiplate ADP assay. To explore whether differences in the non-ADP-mediated platelet aggregation would have a great influence on the correlation between the Multiplate and VerifyNow, we investigated if the correlation between the inhibitor percentages would improve compared to the correlation between ADP assay results, since both parameters represent the percentage platelet function inhibited by the P2Y12-inhibitor opposed to the normal AU and PRU values for, respectively, the Multiplate and VerifyNow.
Methods
Study Population
For the current analysis 51 consecutive patients were included as a substudy from Vries et al. [Citation3] based on the mentioned in- and exclusion criteria in this article. In short, between November 2015 and March 2016, 51 high-risk patients were included who underwent PCI at the Maastricht University Medical Centre+ (MUMC+). Patients had suffered from an acute coronary syndrome or underwent an elective PCI, received dual antiplatelet therapy (DAPT) consisting of aspirin and a P2Y12-inhibitor and were defined as high-risk patients based on ≥3 of the following risk factors: old age (≥75 years), female gender, renal dysfunction (eGFR <60 mL/min), anemia at the time of PCI (hemoglobin level <13.2 g/dL for men and <11.8 g/dL for women), low body weight (<60 kg), hypertension, diabetes mellitus, previous stroke, previous in-stent thrombosis and/or high-risk stenting.
Exclusion criteria were either the use of anticoagulation or antiplatelet drugs besides dual antiplatelet therapy (DAPT), the use of non-steroidal anti-inflammatory drugs (NSAIDs) and missing data from either Multiplate or VerifyNow. The medical ethical committee of the MUMC+ approved this study.
Laboratory Analysis
For the Multiplate ADP (Roche®), blood was collected in a hirudin blood tube and the assay was performed within 30–180 min. Initially, 300 µL NaCl solution (0.9%) was added to the test cell, followed by 300 µL whole blood to create a 1:1 saline dilution. After 3 min of incubation, 20 µl ADP was added, which resulted in a final ADP concentration of 6.5 µM. The Multiplate ADP-HS was performed as the ADP assay with the addition of 20 µl PGE1 in the test cell. The final ADP and PGE1 concentration in the Multiplate ADP-HS were, respectively, 6.3 µM and 9.4 nM. Platelet function is expressed as arbitrary aggregation units (AU). We defined an AU of 19–46 as the therapeutic window for the Multiplate ADP assay, with HTPR defined as >46 and low on-treatment platelet reactivity (LTPR) as <19, according to Tantry et al. [Citation1]. For the Multiplate ADP-HS assay HTPR is defined as ≥43 and LTPR as <18, according to Amann et al. [Citation11].
For the VerifyNow P2Y12 (Accumetrics®) assay blood was collected in a Greiner Bio-One (VACUETTE ®) 3,2% sodium citrate partial fill tube (2 mL) and rested for at least 10 min. The ADP concentration in the assay was 20 µM and the PGE1 concentration 22 nM. Within 4 h of sampling, the VerifyNow P2Y12 assay was performed and platelet function was measured in platelet reaction units (PRU). We defined a PRU of 85–208 as the therapeutic window, with HTPR defined as >208 and LTPR as <85, according to Tantry et al. [Citation1].
Inhibitor Percentage
The inhibitor percentage is calculated according to the following formula: ((Base-PlateletAggregation)/Base) × 100%. The inhibitor percentage formula for each assay is shown in and represents a normalization of the P2Y12 and ADP assays by comparison to base platelet function (or non-ADP-mediated platelet aggregation) as measured by TRAP. Inhibitor percentage can be seen as the percentage of platelet function that is blocked by a P2Y12-inhibitor.
Statistical Analysis
The statistical analysis for the results was performed in IBM SPSS Statistics 25.0 for Windows. Distribution of the obtained data was assessed visually and by the Shapiro–Wilk test. Normally distributed baseline data are presented as mean with standard deviation. Not normally distributed data are presented as median ± interquartile range (IQR). Wilcoxon signed-rank test for not normally distributed paired data was used for comparison between medians.
Correlations were assessed with Spearman correlation coefficients (ρ). Values of 0.00 to 0.30 (or 0.00 to −0.30) were considered negligible, 0.30 to 0.50 (or −0.30 to −0.50) low positive (negative), 0.50 to 0.70 (or −0.50 to −0.70) moderate positive (negative), 0.70 to 0.90 (−0.70 to −0.90) high positive (negative) and 0.90 to 1.00 (or −0.90 to −1.00) very high positive (negative) correlation[Citation12]. Cohen’s kappa was used to determine concordance between PFTs beyond that expected by chance. A p-value of <0.05 represents statistical significance.
Results
Study Population
The study population consisted of 51 patients undergoing PCI; their baseline characteristics are shown in . Most common risk factors were hypertension, old age (>75 years), renal dysfunction and female sex. Antiplatelet therapy consisting of aspirin with clopidogrel was most frequently used (64.7%), followed by aspirin with prasugrel (29.4%) and only 3 patients were treated with a combination of aspirin and ticagrelor (5.9%).
Table 1. Baseline characteristics
Multiple Electrode Analyzer ADP versus ADP-HS Assay
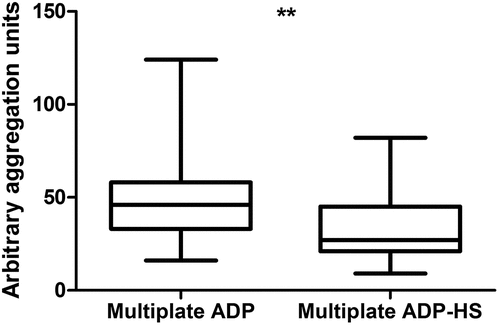
Addition of PGE1 significantly decreases the AU of the Multiplate ADP assay, as shown in . The Multiplate ADP has a median AU of 46.00 (IQR: 33.00–58.00) and the Multiplate ADP-HS has a median AU of 27.00 (IQR: 21.00–45.00), p < .001. The VerifyNow P2Y12 assay presented with a median PRU of 127.00 (IQR: 40.00–199.00).
Figure 2. Comparison of the Multiple Electrode Analyzer (Multiplate) ADP assay and the Multiplate ADP-high sensitivity assay (ADP-HS), with the addition of Prostaglandin E1 (PGE1), in patients taking aspirin and a P2Y12-inhibitor. Plotted are the median, interquartile range (IQR) and whiskers showing minimum to maximum values. **p < .001
Multiple Electrode Analyzer versus VerifyNow
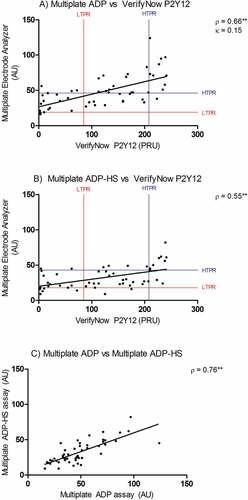
The Spearman ρ for correlation between the Multiplate ADP and VerifyNow P2Y12 assay is 0.66 (p < .001). The Spearman ρ for correlation between the Multiplate ADP-HS and VerifyNow P2Y12 assay is 0.55 (p < .001). Both values can be described as moderate positive correlations, as can be seen in . The concordance between Multiplate ADP and VerifyNow P2Y12 can be described as slight, κ = 0.15 (95% CI 0.01–0.15, p = .078). In general, the Multiplate ADP assay classified patients into higher platelet reactivity categories (). The Multiplate ADP assay classified 27 (53%) patients in different categories compared to the VerifyNow P2Y12. The two Multiplate assays showed a moderate positive correlation with Spearman ρ = 0.76 (p < .001), as presented in .
Figure 3. (A) Correlation and concordance between Multiple Electrode Analyzer (Multiplate) ADP assay and VerifyNow P2Y12 assay in patients taking aspirin and a P2Y12 inhibitor. The cutoff values for low on-treatment platelet reactivity (LTPR) and high on-treatment platelet reactivity (HTPR) are displayed. (B) Correlation between Multiplate ADP-high sensitivity assay (ADP-HS) and VerifyNow P2Y12 assay. (C) Correlation between Multiplate ADP and ADP-HS assay. AU: arbitrary aggregation units. PRU: platelet reactivity units. **p < .001
VerifyNow Inhibitor Percentage versus Multiple Electrode Analyzer Inhibitor Percentage
The following statistical analyses were performed on a study population of 48 patients, since we were unable to retrieve the VerifyNow inhibitor percentage for 1 patient. Furthermore, two patients were excluded due to a negative Multiplate inhibitor percentage. Both patients presented with a Multiplate TRAP value below the ADP assay, resulting in a negative percentage as shown in , as well as the reference range (94–156 AU) for the Multiplate TRAP assay. Additionally, both had a positive VerifyNow inhibitor percentage and light transmission aggregometry (LTA) ADP values were within the therapeutic range for patients on DAPT [Citation13,Citation14], thus resulting in exclusion for this analysis.
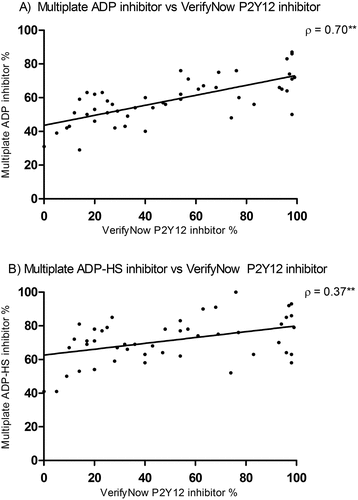
The inhibitor percentage represents the amount of baseline platelet function that is blocked by a P2Y12 inhibitor. A median inhibitor percentage of 44.0% (IQR: 20.0–76.3) was found for the VerifyNow (). The Multiplate presented with significantly (p = .020) higher median inhibitor percentages of 58.5% (IQR: 50.0–66.0) and 71.0% (IQR: 63.0–79.0) for the ADP and ADP-HS assays, respectively. These results indicate that the Multiplate might be more susceptible to P2Y12 receptor inhibition. Applying a cutoff of 40% for VerifyNow inhibitor percentage on the clopidogrel subgroup identifies 66,7% of patients as HTPR.
Figure 4. VerifyNow P2Y12-inhibitor percentage and comparison of the Multiple Electrode Analyzer (Multiplate) ADP inhibitor percentage and the Multiplate ADP-high sensitivity (HS) assay in patients taking aspirin and a P2Y12-inhibitor. Plotted are the median, interquartile range and whiskers showing the minimum to maximum values. **p < .001
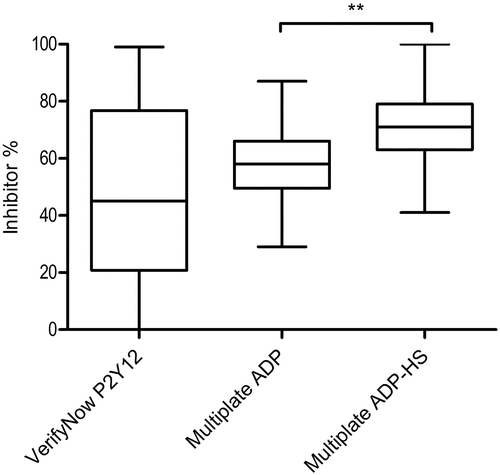
The ADP-HS inhibitor percentage significantly increased (p < .001) compared to the ADP assay. A smaller IQR between the VerifyNow compared to the Multiplate assays was noticed, whereas the VerifyNow inhibitor showed a difference between the 75th and 25th percentile of 56.3% and the Multiplate ADP and ADP-HS 16.0%. Therefore, little variation in P2Y12 inhibition between patients was seen for the Multiplate.
The Spearman ρ between the VerifyNow P2Y12-inhibitor percentage and the Multiplate ADP inhibitor percentage is 0.70 (p < .001), which can be described as a moderate positive correlation. The Spearman ρ between the VerifyNow P2Y12-inhibitor percentage and the Multiplate ADP-HS inhibitor percentage is 0.37 (p < .001) and is classified as a low positive correlation. Both correlations are plotted in, respectively, .
Discussion
This paper aimed to investigate whether the discrepancy between the Multiplate ADP and VerifyNow P2Y12 assay in patients on DAPT could partly be explained by (1) difference in the use of PGE1 between the assays and (2) differences in non-ADP-mediated platelet aggregation.
We found that the addition of PGE1 reduced platelet reactivity as measured with Multiplate ADP, but it did not improve correlation when compared to the VerifyNow P2Y12 assay. Indicating that the difference in the use of PGE1 does not have a major influence on the correlation between Mulitplate and VerifyNow.
PGE1 is present in the VerifyNow P2Y12 assay, where it prevents ADP-dependent platelet activation via the P2Y1 receptor. In the Multiplate ADP assay PGE1 is normally not present, which gives ADP the opportunity to activate platelet aggregation through both the P2Y12 and P2Y1 receptors. When treated with a P2Y12 inhibitor, ADP activation through the P2Y1 pathway can still occur in the absence of PGE1. It was expected that improving equality between the VerifyNow and Multiplate assays would also improve the correlation. This, however, did not occur and we can only speculate about the reasons; it is probable that other differences between the VerifyNow and Multiplate overrule the effect of adding PGE1.
One of the differences concerns the used reagent concentrations between the VerifyNow P2Y12 and Multiplate ADP-HS that differ. These differences can be explained by the use of the standard manufacturer concentrations from Roche® and Accumetrics®. The ADP concentration in the VerifyNow is 20 µM compared to the 6.3 µM that is present in the Multiplate. The PGE1 concentration in VerifyNow is 22 nM versus 9.4 nM in Multiplate ADP-HS. Therefore, it is probable that the VerifyNow assay is more susceptible to ADP-dependent activation as well as exhibiting more P2Y1 inhibition of platelets compared to the Multiplate ADP-HS. It is shown that PGE1 has a dose-dependent inhibitory effect on platelet reactivity in thromboelastography[Citation15]. In the in vitro study of Khanna et al. [Citation15], concentrations of 11 nM and 22 nM PGE1 were compared and showed significant differences in ADP-mediated platelet aggregation when no active metabolite of prasugrel was present. However, when a prasugrel active metabolite was added, these differences decreased and were no longer significant[Citation15]. Since the used PGE1 concentrations are comparable to the ones used in this article, we hypothesize that the different PGE1 concentrations (9.4 nM for the Multiplate and 22 nM for the VerifyNow) did not interfere with the correlation between the assays. Thus, standardizing PGE1 concentrations is unlikely to improve correlation in patients using P2Y12 inhibitors.
Additionally, blood sampling differs between PFTs, where a sodium citrate tube was used for the VerifyNow and a hirudin tube for the Multiplate. Hirudin is a thrombin inhibitor and will not affect the calcium concentration in the blood. Citrate, however, will capture most extracellular-free calcium. The ADP-dependent platelet response in citrated blood is considered to be lower compared to hirudin blood due to a decreased Ca2+ availability required for GP IIb/IIIa, and thus, platelet activation[Citation16]. The hirudin tube gives higher Multiplate ADP values in the blood of healthy volunteers [Citation17], P2Y12 inhibitor spiked healthy volunteers [Citation18] and patients on P2Y12 inhibitors prior to coronary intervention [Citation19,Citation20]. However, the differences in AU described by Zhang et al. [Citation19] at the time of PCI were not present post-PCI (24–36 h after the first P2Y12 inhibitor loading dose). Therefore, switching to citrate tubes for the Multiplate, although improving equality between tests, does not necessarily improve the correlation. For the VerifyNow assay, citrate generally produces higher values compared to hirudin[Citation21]. This is contradictory to the proposed explanation given by Wallen et al. [Citation16] who postulated that decreased Ca2+ by citrate should result in decreased platelet aggregation. To summarize, the exact effect of different anticoagulants ex vivo used for blood sampling in patients using P2Y12 inhibitors is unclear. Currently, standard VerifyNow and Multiplate cutoffs for patients on DAPT are only reported, respectively, for citrate and hirudin blood [Citation1,Citation22] and, therefore, these corresponding blood tubes were used in our research.
Another factor that might have influenced the correlation between VerifyNow and Multiplate is time-dependent changes in reported platelet reactivity as measured by Multiplate [Citation20,Citation23,Citation24]. However, all samples were analyzed within the time frame as recommended by the firm. Nonetheless, time-dependent disturbances have to be taken into account as a possible factor interfering with the correlation between PFTs and a smaller time frame is recommended in the future research.
We found a slight (κ = 0.15) concordance between Multiplate ADP compared to VerifyNow P2Y12. For the Multiplate ADP-HS assay, no consensus is currently reached on cutoffs for HTPR and LTPR. The HTPR and LTPR values as presented in were based on calculations by Amann et al. [Citation11]. Although providing some insights into the concordance of the test, no conclusions can be drawn from one paper and, therefore, this concordance analysis was excluded from our results.
Correcting for the non-ADP-mediated platelet aggregation values of Multiplate and VerifyNow values, presented as inhibitor percentage, did not improve the correlation between both PFTs (). This indicates that the difference in measured baseline platelet function is not a major factor contributing to the discrepancy between the assays. Multiplate inhibitor percentages were higher compared to VerifyNow, suggesting a possible higher susceptibility for P2Y12 inhibition. This, however, could also be caused by the difference in ADP concentration between the Multiplate ADP and VerifyNow P2Y12 assay.
Noticeable are the mentioned excluded patients presenting negative values for both the Multiplate ADP and ADP-HS inhibitor percentages. Both patients presented with a Multiplate TRAP value below the reference range (94–156 AU). A low TRAP value could theoretically indicate a hemostatic abnormality or presence of a GpIIb/IIIa antagonist. However, the VerifyNow TRAP and LTA ADP values for both patients were within the reference range and the presence of a GpIIb/IIIa antagonist was ruled out, suggesting a test error in the Multiplate TRAP channel was more likely. Exclusion of these two patients had little effect on the reported correlations and does not affect the conclusions drawn from this analysis.
Addition of PGE1 significantly increased the median inhibitor percentage, which can be explained by the lower Multiplate ADP-HS values () that are plotted against the same Multiplate TRAP value as the Multiplate ADP results (). Presenting VerifyNow and Multiplate results as a ratio of base platelet function, the inhibitor percentage, did not improve the correlation between PFTs. Besides, the IQR of the Multiplate inhibitor percentages is relatively small compared to the VerifyNow, as seen in . This implies that little variation in P2Y12 inhibition is seen between patients in this assay. Therefore, we consider the Multiplate inhibitor percentage to be of little additional use in monitoring platelet function in patients on DAPT, although larger follow-up studies are required to confirm this hypothesis.
Conclusion
The addition of PGE1 to the Multiplate ADP assay, and the correction for the difference in baseline platelet reactivity as measured by the Multiplate and VerifyNow, did not improve the correlation between the Multiplate ADP and VerifyNow P2Y12 assay in patients on DAPT. It is therefore unlikely that these factors explain a substantial part of the discrepancy between the tests.
Disclosure statement
No conflict of interest was reported by the authors. PGE1 reagent for the Multiplate ADP-HS was sponsored by Roche® (Netherlands).
References
- Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, Stone GW, Curzen N, Geisler T, Ten Berg J, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013;62(24):2261–2273.
- Consuegra-Sanchez L, Lopez-Palop R, Cano P, Carrillo P, Pico F, Villegas M, Sanchis J, Kaski JC. Assessment of high on-treatment platelet reactivity in patients with ischemic heart disease: concordance between the Multiplate and VerifyNow assays. J Thromb Haemost 2013;11(2):379–381.
- Vries MJ, Bouman HJ, Olie RH, Veenstra LF, Zwaveling S, Verhezen PW, Ten Cate-Hoek AJ, Ten Cate H, Henskens YM, van der Meijden PE. Determinants of agreement between proposed therapeutic windows of platelet function tests in vulnerable patients. Eur Heart J Cardiovasc Pharmacother 2017;3(1):11–17.
- Ko YG, Suh JW, Kim BH, Lee CJ, Kim JS, Choi D, Hong MK, Seo MK, Youn TJ, Chae IH, et al. Comparison of 2 point-of-care platelet function tests, VerifyNow assay and Multiple Electrode Platelet Aggregometry, for predicting early clinical outcomes in patients undergoing percutaneous coronary intervention. Am Heart J 2011;161(2):383–390.
- van Werkum JW, Harmsze AM, Elsenberg EH, Bouman HJ, Ten Berg JM, Hackeng CM. The use of the VerifyNow system to monitor antiplatelet therapy: a review of the current evidence. Platelets 2008;19(7):479–488.
- Spiliopoulos SPG. Current status of high on-treatment platelet reactivity in patients with coronary or peripheral arterial disease: mechanisms, evaluation and clinical implications. World J Cardiol 2015;12(7):912–921.
- Nguyen TA, Diodati JG, Pharand C. Resistance to clopidogrel: a review of the evidence. J Am Coll Cardiol 2005;45(8):1157–1164.
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Barrera-Ramirez C, Sabate M, Hernandez R, Moreno R, Escaned J, Alfonso F, et al. Identification of low responders to a 300-mg clopidogrel loading dose in patients undergoing coronary stenting. Thromb Res 2005;115(1–2):101–108.
- Ari H, Ozkan H, Karacinar A, Ari S, Koca V, Bozat T. The effect of high-dose clopidogrel treatment in patients with clopidogrel resistance (the EFFICIENT trial). Int J Cardiol 2012;157(3):374–380.
- Luo Y, Li J, Liu X, Xu J, Ye Z, Yao Y, Liu X, Lai Y. Combination of P2Y12 reaction unit and percentage of platelet inhibition assessed by Verifynow P2Y12 assay is a useful predictor of long-term clinical outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Thromb Res 2016;139:114–120.
- Amann M, Ferenc M, Valina CM, Bomicke T, Stratz C, Leggewie S, Trenk D, Neumann FJ, Hochholzer W. Validation of a P2Y12-receptor specific whole blood platelet aggregation assay. Platelets 2016;27(7):668–672.
- Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J 2012;24(3):69–71.
- Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010;56(12):919–933.
- Kerneis M, Silvain J, Abtan J, Cayla G, O’Connor SA, Barthelemy O, Vignalou JB, Beygui F, Brugier D, Martin R, et al. Switching acute coronary syndrome patients from prasugrel to clopidogrel. JACC Cardiovasc Interventions 2013;6(2):158–165.
- Khanna V, Armstrong PC, Warner TD, Curzen N. Prostaglandin E1 potentiates the effects of P2Y12 blockade on ADP-mediated platelet aggregation in vitro: insights using short thromboelastography. Platelets 2015;26(7):689–692.
- Wallen NH, Ladjevardi M, Albert J, Broijersen A. Influence of different anticoagulants on platelet aggregation in whole blood; a comparison between citrate, low molecular mass heparin and hirudin. Thromb Res 1997;87(1):151–157.
- Peerschke EI, Castellone DD, Stroobants AK, Francis J. Reference range determination for whole-blood platelet aggregation using the Multiplate analyzer. Am J Clin Pathol 2014;142(5):647–656.
- Johnson A, Dovlatova N, Heptinstall S. Multiple electrode aggregometry and P2Y(12) antagonists. Thromb Haemost 2008;99(6):1127–1129.
- Zhang HZ, Yu LH, Kim MH. Effect of different anticoagulants on multiple electrode platelet aggregometry after clopidogrel and aspirin administration in patients undergoing coronary stent implantation: a comparison between citrate and hirudin. Platelets 2013;24(5):339–347.
- Johnston LR, Larsen PD, La Flamme AC, Harding SA. Methodological considerations for the assessment of ADP induced platelet aggregation using the Multiplate® analyser. Platelets 2013;24(4):303–307.
- Pittens CA, Bouman HJ, van Werkum JW, Ten Berg JM, Hackeng CM. Comparison between hirudin and citrate in monitoring the inhibitory effects of P2Y12 receptor antagonists with different platelet function tests. J Thromb Haemost 2009;7(11):1929–1932.
- Sibbing D, Schulz S, Braun S, Morath T, Stegherr J, Mehilli J, Schomig A, von Beckerath N, Kastrati A. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost 2010;8(2):250–256.
- Jilma-Stohlawetz P, Ratzinger F, Schorgenhofer C, Jilma B, Quehenberger P. Effect of preanalytical time-delay on platelet function as measured by multiplate, PFA-100 and VerifyNow. Scand J Clin Lab Invest 2016;76(3):249–255.
- Chapman K, Favaloro EJ. Time dependent reduction in platelet aggregation using the multiplate analyser and hirudin blood due to platelet clumping. Platelets 2018;29(3):305–308.