Abstract
Glycosylation is a ubiquitous cellular or microenvironment-specific post-translational modification that occurs on the surface of normal cells and tumor cells. Tumor cell-associated glycosylation is involved in hematogenous metastasis. A wide variety of tumors undergo aberrant glycosylation to interact with platelets. As platelets have many opportunities to engage circulating tumor cells, they represent an important avenue into understanding the role glycosylation plays in tumor metastasis. Platelet involvement in tumor metastasis is evidenced by observations that platelets protect tumor cells from damaging shear forces and immune system attack, aid metastasis through the endothelium at specific sites, and facilitate tumor survival and colonization. During platelet-tumor-cell interactions, many opportunities for glycan-ligand binding emerge. This review integrates the latest information about glycans, their ligands, and how they mediate platelet-tumor interactions. We also discuss adaptive changes that tumors undergo upon glycan-lectin binding and the impact glycans have on targeted therapeutic strategies for treating tumors in clinical settings.
Plain Language Summary
Tumor hematogenous metastasis is a serious threat to the survival and prognosis of patients, and a variety of factors help this process to occur, and platelets are also involved. During tumor cell metastasis, platelets can adhere to each other and tumor cells, a phenomenon that leads to the immunity of tumor cells from various threats in metastasis, including immune attacks, shearing forces, etc. Scientists have shown that the adhesion effect between platelets and tumor cells is often dependent on various types of sugars, which are not the sugars we ingest. These sugars often appear as glycosylation modifications on the proteins of the cells, including normal glycosylation modifications and some abnormal structures that only appear on tumor cells, and their ligands, lectins, are also present on the surface of the tumor cells or platelets. Their combination results in the better adaptation of tumor cells to the metastatic process, where proteins such as P-selectin, CLEC-2, and Galectins have been more studied. Focusing on Glycan-Lectin interactions between platelets and tumor cells, related studies help us to further understand tumor metastasis, and intervene in this binding and develop related drugs with great potential.
Background
Platelets—the smallest anucleated cells in the human body—are traditionally believed to exert their function mainly in blood coagulation. However, research has made it increasingly clear that platelets are associated with not only hemostasis and thrombosis, but also other physiological and pathophysiological processes, including immunity, inflammation, and cancer, among others. Among the more remarkable functions of platelets is their role in tumor metastasis. The first discoveries that suggested a possible relationship between platelets and tumor cell metastasis were the observations that blood clots in cancer patients often contained cancer cells and that metastatic niches often had elevated platelet counts.Citation1
Several decades later, the major role of platelets in tumor metastasis is clearly illustrated by findings that platelets are activated by and participate in most aspects of metastasis, from their direct interaction with tumor cells to facilitating metastatic processes.Citation2 This cooperation and facilitation of platelets in pathological processes provide protective capacities for metastatic tumor cells beyond providing a simple physical barrier. In essence, platelets act like a “cloak” around metastatic tumor cells, causing them to appear “invisible” in circulation, ultimately leading to higher metastasis possibilities for tumor cells. What molecules mediate the interplay between platelets and tumor cells?
Extensive experimental evidence suggests that surface glycans serve as “bridges” or molecular fasteners between platelets and tumor cells, similar to the way a hook-and-loop fastener (i.e., Velcro®) combines to connect two surfaces. This fastening is accomplished through conjugation between glycoproteins on tumor cells and their receptors on platelets or vice versa. In particular, glycan ligands P-selectin, C-type lectin-2 receptor (CLEC2), galectins, and CD155 are believed to be involved in metastasis.Citation3–6
In this review, we provide an up-to-date overview of the current literature on the roles that glycan-ligand interactions and platelets play in each step of cancer metastasis. An in-depth understanding of this process may pave the way to clues on how to inhibit tumor metastasis in patients or at least attenuate it.
Glycosylation on tumor cells
Glycosylation
Glycosylation is present in 50–70% of proteins and is one of the most common and important forms of post-translational modification and epigenetic alteration. Covalent binding of carbohydrates to non-glycosyl aglycones, primarily the amino acids that comprise proteins, is highly regulated by glycosyltransferases and glycosidases in the Golgi apparatus or endoplasmic reticulum and occurs in a non-templated fashion. Covalent bonding is followed by further elongation through glycosidic bonds, which form oligosaccharides or polysaccharides. In mammals, nine amino acid residues can be modified by monosaccharides or glycopolymer chains.Citation7 There are ten monosaccharide building blocks, including xylose (Xyl), glucose (Glc), galactose (Gal), N-acetylglucosamine and N-acetylgalactosamine (GlcNAc/GalNAc), mannose (Man), sialic acid (Neu5Ac), fucose (Fuc), glucuronic acid (GlcA), and iduronic acid (IdoA).Citation7
In general, there are two primary forms of glycosidic modifications on proteins, N-linked glycosylation and O-linked glycosylation, in addition to glycosylations forming glycolipids, glycosylphospholipids (GPIs), and proteoglycans.Citation8 These glycan-related post-translationally modified compounds have no rigid structure and often exist in bulk. They possess a variety of biological functions, for example, glycosylated proteins are involved in structural or regulatory and recognition functions. However, various diseases result from defective or excessive addition of glycan chains to proteins, multiple types of cancers are especially associated with alterations in glycosylation, where it plays an important role in malignant transformation and cancer progression.Citation9
Most clinically used tumor biomarkers are glycoproteins or glycan-related molecules, such as alpha-fetoprotein for liver cancer, carbohydrate antigen 125 for ovarian cancer, carcinoembryonic antigen for colon cancer, prostate-specific antigen for prostate cancer, and CA19–9 for gastrointestinal and pancreatic cancer, the degree of glycosylation is also directly related to certain properties of tumors.Citation10,Citation11 Although no well-accepted metastasis-specific glycan biomarker is used currently in clinics, many studies have shown that specific glycans increase the potential for organ-tropic metastasis.Citation12
Glycans in cancer metastasis
Besides normal glycosylation, tumor cells often undergo aberrant glycosylation to adapt to environmental changes during metastasis. There are two main forms of aberrant glycosylation involved in metastasis. Abnormal expression of glycosyltransferases and chaperone genes underlie this aberration, one that comprises incomplete synthesis and neo-synthesis processes.Citation13 Incomplete synthesis refers to the impaired synthesis of normal complex glycans, leading to the production of truncated structures, for example, sialyl Tn antigen (sTn). Truncated O-glycans are rarely expressed in benign cells, but they are expressed in esophageal cancer (EC), where they promote the development of distant metastases, these kinds of truncated O-glycans are useful prognostic markers for EC and predict poor outcome.Citation14 Neo-synthesis processes refer to defects in gene regulation that leads to de novo expression of carbohydrate determinants in cancer cells.Citation15 In gastric cancer cells, for example, enhanced synthesis of sialyl-Lewis x (sLex) antigen is associated with a highly invasive phenotype.Citation16
Glycosylation alterations identified on metastatic cancer cells to date mainly include truncated O-glycans; changes in the branching of N-glycans mediated by glycosyltransferases; changes in the amount, linkage, and acetylation of sialic acids; and overexpression of “core” fucosylationCitation8,Citation17 (). Truncated O-glycans, including Tn antigen as well as its sialylated product sTn antigen, are the most interesting because they are believed to induce the production of epithelial-mesenchymal transition (EMT) in a variety of tumors, which in turn promote metastasis.Citation50 Undoubtably, N-glycan branching is also another important kind of glycosylation in tumor metastasis. For example, bisected N-glycan deficiency promotes cytoskeleton rearrangements and migration of ovarian tumor cells.Citation8 Sialic acid and fucose are also significant in tumor metastasis. This modification is prevalent in which sialic acid can be added to GalNac or Gal by means of 20 different sialyltransferases. For example, the predominance of sialoglycans over neutral O-glycoforms (Tn and T antigens) in bladder tumors has been observed.Citation51 The involvement of sialylation in tumor metastasis has also been reported, especially sLex.Citation52 In addition, both fucosylation and defucosylation are associated with metastasis in thyroid cancerCitation53 and prostate cancer,Citation46 respectively, suggesting that it is functionally heterogeneous.
Table I. Types of glycosylations involved in cancer metastasis.
Glycan ligands on platelets
The earliest known ligands for glycans, called lectins, were first discovered in 1888. Proteins of the lectin family have at least one carbohydrate recognition structural domain (CRD), which binds reversibly to carbohydrates. More recently, it was proposed that glycan-binding proteins act as glycan ligands; these include glycosaminoglycan-binding proteins, lectin-like proteins, antibodies that bind glycans, and toxins,Citation7 some of which were found on platelets.
In human, a group of secreted proteins and membrane proteins that recognize glycans are called lectin-like proteins. They are divided primarily into intracellular and extracellular lectin-like proteins. The former one includes L-type lectins, P-type lectins, and calnexin. Extracellular lectin-like proteins, which play an integral role in the interaction between platelets and tumor cells in metastasis, mainly include C-type lectins (e.g., selectins, dectins, mannose-binding protein); I-type lectins (e.g., sialic acid-binding immunoglobulin-like lectins [siglecs], Ig superfamily [IgSF]); and R-type lectins and galectin.Citation17 Because a wide variety of lectins and glycans are found widely on most types of cells and interact with each other, it is not surprising that platelets also would likely interact with them.
Platelets are disc-shaped cells, having an average diameter of 2–5 µm and a thickness of 0.5 µm. They are surrounded by various pericellular matrices or dynamic glycocalyxes called glycoprotein-polysaccharide caps, which regulate platelet adherence. Although most glycoproteins are on the platelet surface during the resting state, intra-platelet organelles also contain many glycoproteins (). For instance, platelet α-granules contain some important membrane-associated proteins, such as P-selectin, fibronectin L, and CD109, that are transferred to the surface after platelet activation and α-granule release. Morphologically more diverse dense granules mainly contain pro-aggregation factors such as ATP and ADP, but almost no aggregation-associated glycoproteins. Intra-platelet lysosomes contain lysosomal-associated membrane proteins and lysosomal integral membrane proteins (also known as CD63) in addition to various hydrolases(). Besides being richly glycosylated, platelets can also provide glycosyltransferasesCitation54 and substratesCitation55 to other cells for glycosylation.
Figure 1. Schematic of platelet and surface glycoprotein expression at rest (left, blue-shaded hemisphere) and after activation (right, red-shaded hemisphere). At rest, platelets express a variety of surface glycoproteins, some of which are not activated. The internal membrane structure of platelets is also not activated and almost no content is released. After platelet activation, platelets exhibit a pseudopod-like structure, and some proteins originally located inside the cell appear on the platelet surface; Other proteins are truncated and secreted in soluble form.
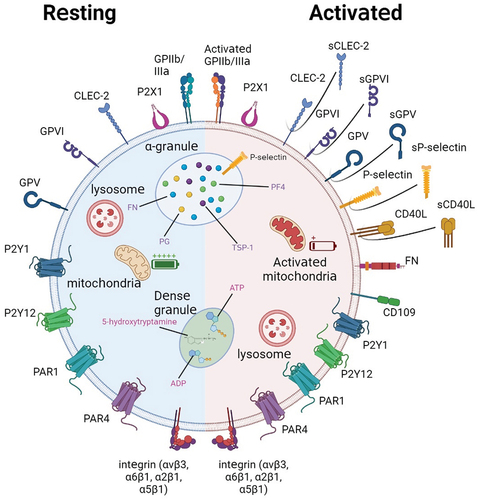
As mentioned above, the platelet surface is rich in various lectin-like receptors and other glycan complexes. More than 387 platelet O-glycosylation sites and 70 individual N-glycosylation sites for 41 platelet functional glycoproteins have been discovered, including P-selectin, GPIIb/IIIa, and GPIV.Citation55–57 Most of these are capped by sialic acid residues. What is the function of so many glycosylation sites?
Surface glycosylation is involved in platelet homeostasis and other physiological functions,Citation58 including intra-platelet calcium homeostasis, energy metabolism, and protection properties that prevent platelets from being cleared.Citation59 Platelet glycosylation also has various clinical applications, such as counteracting defects of glycosylation in Tn syndrome.Citation60 In pathological conditions, the presence of glycoside residues has a significant impact on the normal functioning of platelets.Citation61 However, in tumor metastasis, the presence of glycosylated residues confers additional alternative functions to platelets that promote metastasis.
Roles of glycoconjugation on platelets in cancer metastasis
Metastases are thought to be responsible for the majority of cancer-related deaths. Despite some progress, the vast majority of patients with recurrent or de novo metastatic solid cancers of any type still die within 5 years of diagnosis.Citation62 Metastasis is a complex multi-stage process, and tumor cells encounter various microenvironments. This complexity of metastasis makes it necessary for cancer cells to take on different characteristics precisely at certain time points, as they encounter extracellular shear stress, immune scavenging, oxidative stress, anoikis, and lack of growth factors and cytokines, among others.Citation63 Thus, platelets that surround or “encase” tumor cells not only enhance the rigidity of a tumor embolus, but they also cloak the metastatic tumor cells so they appear as “normal” platelets. This cloaking mechanism associated with platelet-tumor cell interactions essentially makes tumor cells “invisible” to circulating immunocytes.
Platelets are involved in tumor metastasis of a variety of tumors, including osteosarcoma,Citation64 lung cancer,Citation65 and others.Citation66 The interaction between platelets and tumor cells is reciprocal, forming a positive feedback loop.Citation67 Tumor cells directly stimulate platelet production, activation, and aggregation (i.e., tumor cell-induced platelet aggregation or TCIPA) through various mechanisms, after which they can use platelets to promote their own metastasis. The process of platelet activation by tumor-cell stimulation is dependent on platelet surface glycoproteins, such as integrin α6β1.Citation68 The aggregation of activated platelets on the surface of circulating tumor cells (CTCs) can form thrombi, which are mediated mainly by the interaction of P-selectin with mucins. The binding of GPIb-IX-V and GPIIb-IIIa on platelets with tumor cell integrin αvβ3 also plays a role in this process.Citation69 These surface molecule interactions prevent CTCs from being destroyed by shear stress, and they reduce the exposed CTCs surface area that can be recognized and subsequently attacked by natural killer (NK) cells.Citation70
In addition to providing the physical barrier described above, platelets can also transfer their own glycoproteins to CTCs. When co-cultured with tumors, platelets transfer their own MHC-I and integrin subunit β3 (ITGB3) to the surface of CTCs, and this translocation helps CTCs to mimic platelets and avoid immune clearance.Citation71 Thus, ITGB3 plays an essential role in helping CTCs to complete subsequent EMT. Platelets can also actively release other soluble glycoproteins to impede the anti-tumor effects of NK cells. One of these releasates is transforming growth factor-β (TGF-β), which reduces the expression of immune receptor natural killer group member D (NKG2D), thus inhibiting the anti-tumor activity of NK cells.Citation72
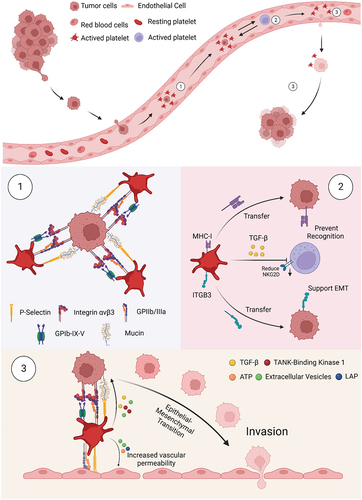
After helping CTCs to survive, platelet surface molecules can also help tumor cells adhere to and penetrate the endothelium. In hematogenous metastases, one of the most important factors in accomplishing the size-limiting arrest of tumor cell-platelet aggregates in the vascular lumen is P-selectinCitation73; glycoprotein IIb on the surface of platelets is also involved.Citation74 After promoting adhesion, platelets also help CTCs overcome the endothelial barrier, mainly by enhancing tumor invasiveness and vascular permeability of CTCs. For example, CLEC-2 on the platelet surface and TGF-β,Citation75 ATP, and other secreted factors can induce the EMT phenotype in tumor cells (). Last but not the least, besides the direct role in metastasis just described, platelet glycoproteins are likely to be associated in some way with site-specific metastasis. Organs or tissues that contain more specific glycoproteins can promote tumor metastasis to them after the glycoproteins bind to the matching lectins on platelets. For example, glycoprotein expression on the lungs has been related to platelet-associated metastasis of osteosarcoma to the lungs.Citation12
Figure 2. Platelet involvement in cancer metastasis. Once tumor cells enter the bloodstream, a 3-step sequence of events occurs. (1) Platelet-tumor cell aggregates are formed, facilitated through the recognition of multiple glycoproteins. (2) Platelets aggregated on the tumor surface protect CTCs from NK cell-associated cell death. (3) Platelets and their secretion stimulate and help cancer cells to adhere to and penetrate the endothelium, thus supporting cancer cell migration and metastasis formation.
Among all the glycan and ligands, several pairs should receive more research attention for their critical roles in the interaction of platelets and tumor cells. These will be discussed in the next section.
Surface lectins-ligands pairs on platelets or CTCs are involved in metastasis
P-selectin and PSGL-1 or CD24
As mentioned, the most important glycan ligand on activated platelets is P-selectin. Its extracellular structure comprises an N-terminal C-type lectin domain, an epidermal growth factor-like domain, and complement-binding protein-like domains with nine consensus repeats, and it has become clear that P-selectin dimers mediate the adhesion of leukocyte in vivo and in vitro recently. On the tumor surface, sLex and its isomer sialyl-Lewis a (sLea) possess a minimal sugar structure to which P-selectin can bind. sLex is produced by fucosylation of sialylated LacNAc, a reaction catalyzed by glycosyltransferases called α(1,3)-fucosyltransferases (α(1,3)-FUTs).Citation76 However, sLex needs to bind proteins and not just act as an oligosaccharide to exhibit the highest affinity for P-selectin. On tumor cells, this high affinity mainly occurs at P-selectin glycoprotein ligand-1 and CD24. These play a role in metastasis of various tumor cell lines, including melanoma,Citation77 and breast cancer.Citation78 P-selectin promotes metastasis by participating in the adhesion and interaction between platelets and tumor cells, and this interaction leads to the formation of a “platelet cape” or cloak.Citation70 This cloak shields tumor cells from damage, such as that caused by shear forces and immune attack. This binding is the first step in the nonphysical binding of platelets and tumor cells.Citation79 In addition to promoting the adhesion of platelets and tumor cells, P-selectin also promotes the adhesion of tumor cell-platelet aggregates to the vascular endothelium.Citation73
CLEC-2 and podoplanin
Another important glycan ligand enriched on platelet membranes is CLEC-2, a type II transmembrane receptor. CLEC-2 potently induces platelet activation, and binding to ligands triggers phosphorylation of CLEC-2 hemITAM and recruitment of Syk.Citation80 This ultimately leads to platelet activation, aggregation, and thrombosis in blood vessels.Citation80 The only known endogenous ligand for CLEC-2 is podoplanin (PDPN), a small transmembrane mucin-like sialoglycoprotein expressed in a variety of cancers.Citation80 The extracellular structural domain of PDPN contains three tandemly repeated platelet aggregation stimulating (PLAG) domains.Citation81 Sialylated O-glycosylation at T-52 in the human PLAG3 domain may be the recognition site for CLEC-2; a separate CLEC-2 binding site has been identified on PLAG4.Citation81 When CLEC-2 is deficient, metastasis is inhibited in PDPN-positive tumor cells, confirming that CLEC-2 binding to PDPN can promote metastasis.Citation82 Metastasis is not significantly altered in PDPN-negative cells.Citation82 Targeting the PLAG4 domain, as described above, significantly inhibits PDPN binding to CLEC-2 and metastasis in osteosarcoma.Citation83
PDPN-mediated platelet aggregation (PMPA) occurs when PDPN binds to CLEC-2 on the surface of tumor cells,Citation84 which is one of the prerequisites for thrombus formation on the surface of CTCs. PMPA can also induce platelets to secrete various factors that promote tumor cell metastasis. For example, PDPN can induce platelets to release TGF-β, which in turn promotes the induction of EMT in bladder squamous cell carcinoma cells.Citation85 In addition to activating platelets, CLEC-2/PDPN binding promotes megakaryocyte expansion and proplatelet formationCitation86 and also supports platelet capture at arteriolar shear ratesCitation87 in the bloodstream. Whether this promotes platelet production and tumor metastasis in tumors awaits further investigation.
Galectins and GPVI
Galectins are lectins that contain one or two highly conserved carbohydrate recognition domains having an affinity for poly-N-acetyllactosamine-enriched glycoconjugate. More than 80% of human tumors have paradoxical changes in galectin expression, correlating with tumor type, tissue distribution, and cellular localization.Citation88 The galectin family is involved in many types of tumors and is involved in cell proliferation, differentiation, and metastasis, galectin-1 and 3 (Gal1 and Gal3) are particularly involved. Clinically, upregulation of multiple galectin family members occurs in multiple types of tumors, tumor tissues, or blood samples from certain cancer patients and is associated with poor prognosis.Citation89
The galectin family may also promote platelet activation, secretion, and adhesion. However, little research has been done on the association between tumor surface galectin and platelet glycans. Saha et al. discovered that galectin and platelet surface GPVI bind to each other under conditions of shearing stress.Citation90 Others have revealed that platelet GPVI and Gal3 of colon cancer cells interact to promote EMT of colon cancer cells.Citation91 Surface Gal3 on breast cancer cells was also found to bind with platelet surface GPVI, which effectively promotes platelet activation and transendothelial cell migration, ultimately leading to more aggressive tumor cell extravasation and metastasis, and pharmacologically blocking GPVI significantly reduces platelet-related metastasis without affecting hemostasis.Citation5
CD155 and CD226
CD155 is a transmembrane glycoprotein located on the surface of tumor cells; it an IgSF member and is an I-type lectin.Citation92 CD155’s ligand on the surface of platelets is CD226, which also belongs to the IgSF of glycoproteins.Citation92 CD155 can interact through the first N-terminal extracellular IgV-like structural domain of CD226.Citation92 CD155 is upregulated in a variety of human malignancies, such as bladder, colon, and lung cancer; melanoma; pancreatic cancer; and glioblastoma.Citation93 There is a clear relationship between CD155 and tumor metastasis, because CD155 deficiency leads to a significant delay in tumor growth and metastasis.Citation94 Although therapeutic approaches that target CD155 have achieved significant experimental success and anti-tumor potential, much of the relevant research has focused on tumor immunosuppression, and most of this markedly enhanced anti-tumor characteristic has been attributed to CD226.Citation95 The important role of CD155-CD226 interactions in platelet-associated tumor metastasis was first demonstrated to promote lung metastasis, and subsequent experiments demonstrated that this interaction could promote platelet activation and thus enhance metastasis.Citation6 This suggests that CD155 plays a role in the process of tumor cell metastasis. However, the mechanism and prevalence of this facilitation remain to be investigated.
Summary and perspective
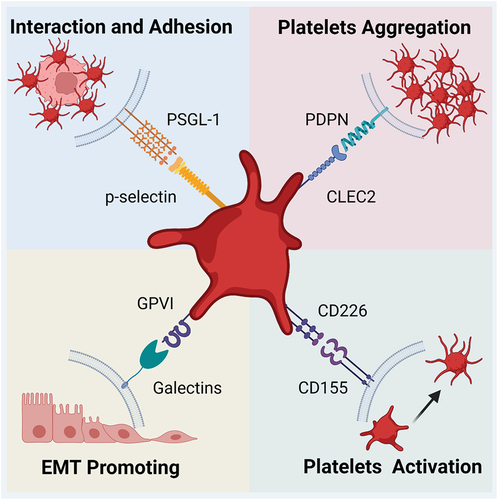
As an important functional player in the metastatic process, glycan or lectin on platelets participate in a variety of pro-metastatic mechanisms, including achieving physical barriers, and endothelial breakthrough, among others (). However, the presence of a glycan-ligand bridge between platelets and tumor cells has been overlooked for a long time. The interactions between platelets and glycans and their involvement in tumor metastasis suggest a new cancer-fighting strategy. There are several advantages in targeting glycosylation as a strategy for treating cancer. First, tumor-associated glycosylation-specific changes have been identified in multiple cancer types, providing potential new specific therapeutic targets for prevalent tumor lesions. Second, the vast majority of carbohydrate modifications occur and/or are present on the cell surface, which makes their targeting less problematic. Third, in terms of a multifactorial combination of epigenetic modifications, altering or targeting a single genetic factor in the process can have a fundamental impact. For these purposes, glycomimetics, a class of chemically synthesized glycan analogs with unique modifications, have been developed as promising candidates for glycan-based therapeutics. Glycomimetics have a higher affinity for ligands than natural glycans. Furthermore, glycomimetics are able to competitively inhibit lectin-like proteins, so they can prevent the progression of certain cancers such as multiple myeloma, and at the same time, this strategy can also be used to deliver chemotherapeutic agents for targeted therapy.Citation96 The targeting of the interaction between platelets and glycan ligands clearly emerges as a promising avenue for tumor therapy.
Figure 3. Major glycan-ligand binding present in platelet-associated metastasis. The glycan-lectin binding involves multiple steps from platelet activation to metastasis through the endothelium. The four major lectins include P-selectin, CLEC-2, Galectin, and CD155, and one of their ligands is PSGL-1, PDPN, GPVI, and CD226, respectively. They can affect both platelets and tumor cells. The former may be activated and agglutinated upon glycan-ligand binding, while the latter mainly undergoes adhesion and EMT, ultimately allowing metastasis to be promoted.
In addition to mimicking glycans, the development of antibodies that target platelet surface CLEC-2Citation97 and P-selectinCitation98 are also promising. A study with mice on anti-CLEC-2 antibodies, which blocks the critical amino acid residues on CLEC-2 that bind to PDPN, demonstrated that platelet activation could be inhibited without elevating the risk of bleeding, thereby prolonging survival.Citation97 In addition, drugs like Revacept, a competitive GPVI inhibitor comprising the ectodomain of GPVI fused to IgG Fc, which reduces platelet cyclooxygenase-2(COX-2) release and is a competitive inhibitor of EMT-mediated GPVI, and certain polysaccharide-containing compounds that target tumor cell surface lectin-like proteins were found to reduce the binding of platelets to colon or breast cancer cells.Citation5 Nonetheless, there are still some issues that need to be mentioned, such as the significance of platelets in inhibiting metastasis.Citation99
In summary, much progress has been made in understanding glycan-ligand interactions in platelet-involved tumor metastasis. Although this interaction was underestimated for a long time, rich breakthrough results await researchers. These may produce a universal antagonist to various tumors. The fundamental discoveries briefly reviewed here and the trial applications borne out of them represent great potential for blocking glycan-ligand interactions in metastasis. Hence, we firmly believe that a deeper understanding of the fundamentals of the interaction between glycans and ligands on tumor cells and platelets further broaden potential therapeutic approaches and provide new directions for the treatment of patients carrying metastatic niches in the future.
Abbreviations
ADP: adenosine diphosphate; AFP: alpha fetoprotein; ATP: adenosine triphosphate; CA125: carbohydrate antigen 125; CEA: carcinoembryonic antigen; CDG: congenital disorders of glycosylation; CLEC-2: C-type lectin-2 receptor; CLRs: C-type lectins; COX-2: Cyclooxygenase-2; CRD: carbohydrate recognition domains; CTCs: circulating tumor cells; C1GALT1: Glycoprotein-N-Acetylgalactosamine 3-Beta-Galactosyltransferase 1; EC: esophageal cancer; EGF: epidermal growth factor; EMT: epithelial-mesenchymal transition; Fuc: fucose; FUT: fucosyltransferases; GAG: glycosaminoglycan; Gal: galactose; Gal1: galectin-1; GalNAc: N-galactosamine; GBP: glycan binding protein; Glc: glucose; GlcA: glucuronic acid; GlcNAc: N-acetylglucosamine; GPIs: glycosylphospholipids; LAMP: lysosomal-associated membrane protein; LIMP: lysosomal integral membrane protein; IdoA: iduronic acid; IgSF: Ig superfamily; ITGB3: integrin subunit β3; Man: mannose; Neu5Ac: sialic acid; NK: natural killer; NKG2D: natural killer group member D; PDPN: podoplanin; PLAG: platelet aggregation stimulating; PMPA: PDPN-mediated platelet aggregation; PSA: prostate specific antigen; PSGL-1: P-selectin glycoprotein ligand-1; sLea: sialyl-Lewis a; sLex: sialyl-Lewis x; sTn: sialyl Tn antigen; TCIPA: tumor cell-induced platelet aggregation; TGF-β: transforming growth factor-β; Xyl: xylose;
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lee CH, Lin Y-J, Lin C-C, Yen C-L, Shen C-H, Chang C-J, Hsieh S-Y. Pretreatment platelet count early predicts extrahepatic metastasis of human hepatoma. Liver Int. 2015;35(10):2327–10. doi:10.1111/liv.12817.
- Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018;33(6):965–83. doi:10.1016/j.ccell.2018.03.002.
- Gong L, Cai Y, Zhou X, Yang H. Activated platelets interact with lung cancer cells through P-selectin glycoprotein ligand-1. Pathol Oncol Res. 2012;18(4):989–96. doi:10.1007/s12253-012-9531-y.
- Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, Yamazaki Y, Narimatsu H, Ozaki Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282(36):25993–6001. doi:10.1074/jbc.M702327200.
- Mammadova-Bach E, Gil-Pulido J, Sarukhanyan E, Burkard P, Shityakov S, Schonhart C, Stegner D, Remer K, Nurden P, Nurden AT, et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood. 2020;135(14):1146–60. doi:10.1182/blood.2019002649.
- Morimoto K, Satoh-Yamaguchi K, Hamaguchi A, Inoue Y, Takeuchi M, Okada M, Ikeda W, Takai Y, Imai T. Interaction of cancer cells with platelets mediated by Necl-5/poliovirus receptor enhances cancer cell metastasis to the lungs. Oncogene. 2008;27(3):264–73. doi:10.1038/sj.onc.1210645.
- Cummings RD, Pierce JM. The challenge and promise of glycomics. Chem Biol. 2014;21(1):1–15. doi:10.1016/j.chembiol.2013.12.010.
- Lin S, Zhou S, Yuan T. The “sugar-coated bullets” of cancer: tumor-derived exosome surface glycosylation from basic knowledge to applications. Clin Transl Med. 2020;10(6):e204. doi:10.1002/ctm2.204.
- Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–555. doi:10.1038/nrc3982.
- Wang M, Zhu J, Lubman DM, Gao C. Aberrant glycosylation and cancer biomarker discovery: a promising and thorny journey. Clin Chem Lab Med. 2019;57(4):407–16. doi:10.1515/cclm-2018-0379.
- Gratacós-Mulleras A, Duran A, Asadi Shehni A, Ferrer-Batallé M, Ramírez M, Comet J, de Llorens R, Saldova R, Llop E, Peracaula R. et al. Characterisation of the main PSA glycoforms in aggressive prostate cancer. Sci Rep. 2020;10(1):18974. doi:10.1038/s41598-020-75526-3.
- Bindeman WE, Fingleton B. Glycosylation as a regulator of site-specific metastasis. Cancer Metastasis Rev. 2022;41(1):107–129. doi:10.1007/s10555-021-10015-1.
- Hakomori S, Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst. 1983;71:231–51.
- Cotton S, Ferreira D, Soares J, Peixoto A, Relvas-Santos M, Azevedo R, Piairo P, Diéguez L, Palmeira C, Lima L, et al. Target score—a proteomics data selection tool applied to esophageal cancer identifies GLUT1-sialyl tn glycoforms as biomarkers of cancer aggressiveness. Int J Mol Sci. 2021;22(4):1664. doi:10.3390/ijms22041664.
- Kannagi R, Yin J, Miyazaki K, Izawa M. Current relevance of incomplete synthesis and neo-synthesis for cancer-associated alteration of carbohydrate determinants—Hakomori’s concepts revisited. Biochim Biophys Acta. 2008;1780(3):525–31. doi:10.1016/j.bbagen.2007.10.007.
- Gomes C, Osório H, Pinto MT, Campos D, Oliveira MJ, Reis CA. Expression of ST3GAL4 leads to SLe(x) expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS One. 2013;8(6):e66737. doi:10.1371/journal.pone.0066737.
- Taylor ME, Drickamer K, Imberty A, van Kooyk Y, Schnaar R, Etzler M, Varki A. Discovery and classification of glycan-binding proteins. In: Varki A, editor. Essentials of glycobiology. Cold Spring Harbor Laboratory Press; 2015. p. 361–72.
- Tan Z, Cao L, Wu Y, Wang B, Song Z, Yang J, Cheng L, Yang X, Zhou X, Dai Z, et al. Bisecting GlcNAc modification diminishes the pro-metastatic functions of small extracellular vesicles from breast cancer cells. J Extracell Vesicles. 2020;10(1):e12005. doi:10.1002/jev2.12005.
- Yoshimura M, Nishikawa A, Ihara Y, Taniguchi S, Taniguchi N. Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc Natl Acad Sci USA. 1995;92(19):8754–8. doi:10.1073/pnas.92.19.8754.
- Zhang H, Meng F, Wu S, Kreike B, Sethi S, Chen W, Miller FR, Wu G. Engagement of I-Branching β-1, 6- N -Acetylglucosaminyltransferase 2 in breast cancer metastasis and TGF-β signaling. Cancer Res. 2011;71(14):4846–56. doi:10.1158/0008-5472.CAN-11-0414.
- Dong X, Chen C, Deng X, Liu Y, Duan Q, Peng Z, Luo Z, Shen L. A novel mechanism for C1GALT1 in the regulation of gastric cancer progression. Cell Biosci. 2021;11(1):166. doi:10.1186/s13578-021-00678-2.
- Chugh S, Barkeer S, Rachagani S, Nimmakayala RK, Perumal N, Pothuraju R, Atri P, Mahapatra S, Thapa I, Talmon GA, et al. Disruption of C1galt1 gene promotes development and metastasis of pancreatic adenocarcinomas in mice. Gastroenterology. 2018;155(5):1608–1624. doi:10.1053/j.gastro.2018.08.007.
- Kuo TC, Wu M-H, Yang S-H, Chen S-T, Hsu T-W, Jhuang J-Y, Liao Y-Y, Tien Y-W, Huang M-C. C1GALT1 high expression is associated with poor survival of patients with pancreatic ductal adenocarcinoma and promotes cell invasiveness through integrin αv. Oncogene. 2021;40(7):1242–54. doi:10.1038/s41388-020-01594-4.
- Liu J, Xu F, Li J, Jiang H. Overexpression of Cosmc suppresses cell migration and invasion in different subtypes of breast cancer cells via Tn and T glycans. Biosci Rep. 2020;40(6). doi:10.1042/BSR20191062.
- Liu Z, Liu J, Dong X, Hu X, Jiang Y, Li L, Du T, Yang L, Wen T, An G, et al. Tn antigen promotes human colorectal cancer metastasis via H-Ras mediated epithelial-mesenchymal transition activation. J Cell Mol Med. 2019;23(3):2083–2092. doi:10.1111/jcmm.14117.
- Ding Y, Gelfenbeyn K, Freire-de-Lima L, Handa K, Hakomori S-I. Induction of epithelial-mesenchymal transition with O-glycosylated oncofetal fibronectin. FEBS Lett. 2012;586(13):1813–20. doi:10.1016/j.febslet.2012.05.020.
- Leon F, Seshacharyulu P, Nimmakayala RK, Chugh S, Karmakar S, Nallasamy P, Vengoji R, Rachagani S, Cox JL, Mallya K, et al. Reduction in O-glycome induces differentially glycosylated CD44 to promote stemness and metastasis in pancreatic cancer. Oncogene. 2022;41(1):57–71. doi:10.1038/s41388-021-02047-2.
- Freitas D, Campos D, Gomes J, Pinto F, Macedo JA, Matos R, Mereiter S, Pinto MT, Polónia A, Gartner F, et al. O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. EBioMedicine. 2019;40:349–362. doi:10.1016/j.ebiom.2019.01.017.
- Lakshmanan I, Chaudhary S, Vengoji R, Seshacharyulu P, Rachagani S, Carmicheal J, Jahan R, Atri P, Chirravuri‐Venkata R, Gupta R, et al. ST6GalNAc-I promotes lung cancer metastasis by altering MUC5AC sialylation. Mol Oncol. 2021;15(7):1866–1881. doi:10.1002/1878-0261.12956.
- Radhakrishnan P, Dabelsteen S, Madsen FB, Francavilla C, Kopp KL, Steentoft C, Vakhrushev SY, Olsen JV, Hansen L, Bennett EP, et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci USA. 2014;111(39):E4066–75. doi:10.1073/pnas.1406619111.
- Cheng J, Wang R, Zhong G, Chen X, Cheng Y, Li W, Yang Y. ST6GAL2 downregulation inhibits cell adhesion and invasion and is associated with improved patient survival in breast cancer. Onco Targets Ther. 2020;13:903–14. doi:10.2147/OTT.S230847.
- Yuan Q, Chen X, Han Y, Lei T, Wu Q, Yu X, Wang L, Fan Z, Wang S. Modification of α2,6-sialylation mediates the invasiveness and tumorigenicity of non-small cell lung cancer cells in vitro and in vivo via Notch1/Hes1/MMPs pathway. Int J Cancer. 2018;143(9):2319–30. doi:10.1002/ijc.31737.
- Walker MR, Goel HL, Mukhopadhyay D, Chhoy P, Karner ER, Clark JL, Liu H, Li R, Zhu JL, Chen S, et al. O-linked α2,3 sialylation defines stem cell populations in breast cancer. Sci Adv. 2022;8(1):eabj9513. doi:10.1126/sciadv.abj9513.
- Guerrero PE, Miró L, Wong BS, Massaguer A, Martínez-Bosch N, Llorens RD, Navarro P, Konstantopoulos K, Llop E, Peracaula R. Knockdown of α2,3-Sialyltransferases impairs pancreatic cancer cell migration, invasion and E-selectin-dependent adhesion. IJMS. 2020;21(17):6239. doi:10.3390/ijms21176239.
- Dalangood S, Zhu Z, Ma Z, Li J, Zeng Q, Yan Y, Shen B, Yan J, Huang R. Identification of glycogene-type and validation of ST3GAL6 as a biomarker predicts clinical outcome and cancer cell invasion in urinary bladder cancer. Theranostics. 2020;10(22):10078–91. doi:10.7150/thno.48711.
- Carrascal MA, Silva M, Ramalho JS, Pen C, Martins M, Pascoal C, Amaral C, Serrano I, Oliveira MJ, Sackstein R, et al. Inhibition of fucosylation in human invasive ductal carcinoma reduces E-selectin ligand expression, cell proliferation, and ERK1/2 and p38 MAPK activation. Mol Oncol. 2018;12(5):579–593. doi:10.1002/1878-0261.12163.
- Pothuraju R, Rachagani S, Krishn SR, Chaudhary S, Nimmakayala RK, Siddiqui JA, Ganguly K, Lakshmanan I, Cox JL, Mallya K, et al. Molecular implications of MUC5AC-CD44 axis in colorectal cancer progression and chemoresistance. Mol Cancer. 2020;19(1):37. doi:10.1186/s12943-020-01156-y.
- Gomes C, Almeida A, Barreira A, Calheiros J, Pinto F, Abrantes R, Costa A, Polonia A, Campos D, Osório H, et al. Carcinoembryonic antigen carrying SLe X as a new biomarker of more aggressive gastric carcinomas. Theranostics. 2019;9(24):7431–46. doi:10.7150/thno.33858.
- Ferreira IG, Carrascal M, Mineiro A, Bugalho A, Borralho P, Silva Z, Dall’olio F, Videira P. Carcinoembryonic antigen is a sialyl lewis x/a carrier and an E‑selectin ligand in non‑small cell lung cancer. Int J Oncol. 2019;55(5):1033–48. doi:10.3892/ijo.2019.4886.
- Ganguly K, Krishn SR, Rachagani S, Jahan R, Shah A, Nallasamy P, Rauth S, Atri P, Cox JL, Pothuraju R, et al. Secretory mucin 5AC promotes neoplastic progression by augmenting KLF4-mediated pancreatic cancer cell stemness. Cancer Res. 2021;81(1):91–102. doi:10.1158/0008-5472.CAN-20-1293.
- Scheidegger EP, Lackie PM, Papay J, Roth J. In vitro and in vivo growth of clonal sublines of human small cell lung carcinoma is modulated by polysialic acid of the neural cell adhesion molecule. Lab Invest. 1994;70:95–106.
- Tu CF, Wu M-Y, Lin Y-C, Kannagi R, Yang R-B. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 2017;19(1):111. doi:10.1186/s13058-017-0904-8.
- Wang X, Inoue S, Gu J, Miyoshi E, Noda K, Li W, Mizuno-Horikawa Y, Nakano M, Asahi M, Takahashi M, et al. Dysregulation of TGF-β1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc Natl Acad Sci USA. 2005;102(44):15791–6. doi:10.1073/pnas.0507375102.
- Cheng L, Gao S, Song X, Dong W, Zhou H, Zhao L, Jia L. Comprehensive N-glycan profiles of hepatocellular carcinoma reveal association of fucosylation with tumor progression and regulation of FUT8 by microRnas. Oncotarget. 2016;7(38):61199–214. doi:10.18632/oncotarget.11284.
- Agrawal P, Fontanals-Cirera B, Sokolova E, Jacob S, Vaiana CA, Argibay D, Davalos V, McDermott M, Nayak S, Darvishian F, et al. A systems biology approach identifies FUT8 as a driver of melanoma metastasis. Cancer Cell. 2017;31(6):804–19.e7. doi:10.1016/j.ccell.2017.05.007.
- Höti N, Yang S, Hu Y, Shah P, Haffner MC, Zhang H. Overexpression of α (1,6) fucosyltransferase in the development of castration-resistant prostate cancer cells. Prostate Cancer Prostatic Dis. 2018;21(1):137–46. doi:10.1038/s41391-017-0016-7.
- Li Y, Huang X, Zhang J, Li Y, Ma K. Synergistic inhibition of cell migration by tetraspanin CD82 and gangliosides occurs via the EGFR or cMet-activated Pl3K/Akt signalling pathway. Int J Biochem Cell Biol. 2013;45(11):2349–58. doi:10.1016/j.biocel.2013.08.002.
- Yamada T, Bando H, Takeuchi S, Kita K, Li Q, Wang W, Akinaga S, Nishioka Y, Sone S, Yano S, et al. Genetically engineered humanized anti-ganglioside GM2 antibody against multiple organ metastasis produced by GM2-expressing small-cell lung cancer cells. Cancer Sci. 2011;102(12):2157–63. doi:10.1111/j.1349-7006.2011.02093.x.
- Sasaki N, Hirabayashi K, Michishita M, Takahashi K, Hasegawa F, Gomi F, Itakura Y, Nakamura N, Toyoda M, Ishiwata T, et al. Ganglioside GM2, highly expressed in the MIA PaCa-2 pancreatic ductal adenocarcinoma cell line, is correlated with growth, invasion, and advanced stage. Sci Rep. 2019;9(1):19369. doi:10.1038/s41598-019-55867-4.
- Thomas D, Sagar S, Caffrey T, Grandgenett PM, Radhakrishnan P. Truncated O-glycans promote epithelial-to-mesenchymal transition and stemness properties of pancreatic cancer cells. J Cell Mol Med. 2019;23(10):6885–96. doi:10.1111/jcmm.14572.
- Cotton S, Azevedo R, Gaiteiro C, Ferreira D, Lima L, Peixoto A, Fernandes E, Neves M, Neves D, Amaro T, et al. Targeted O-glycoproteomics explored increased sialylation and identified MUC16 as a poor prognosis biomarker in advanced-stage bladder tumours. Mol Oncol. 2017;11(8):895–912. doi:10.1002/1878-0261.12035.
- Julien S, Ivetic A, Grigoriadis A, QiZe D, Burford B, Sproviero D, Picco G, Gillett C, Papp SL, Schaffer L, et al. Selectin ligand sialyl-lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 2011;71(24):7683–93. doi:10.1158/0008-5472.CAN-11-1139.
- Miyoshi E, Ito Y, Miyoshi Y. Involvement of aberrant glycosylation in thyroid cancer. J Oncol. 2010;2010:816595. doi:10.1155/2010/816595.
- Lee-Sundlov MM, Ashline DJ, Hanneman AJ, Grozovsky R, Reinhold VN, Hoffmeister KM, Lau JT. Circulating blood and platelets supply glycosyltransferases that enable extrinsic extracellular glycosylation. Glycobiology. 2017;27(2):188–98. doi:10.1093/glycob/cww108.
- Lee MM, Nasirikenari M, Manhardt CT, Ashline DJ, Hanneman AJ, Reinhold VN, Lau JTY. Platelets support extracellular sialylation by supplying the sugar donor substrate. J Biol Chem. 2014;289(13):8742–8. doi:10.1074/jbc.C113.546713.
- Lauková L, Weiss R, Semak V, Weber V. Desialylation of platelet surface glycans enhances platelet adhesion to adsorbent polymers for lipoprotein apheresis. Int J Artif Organs. 2021;44(6):378–84. doi:10.1177/0391398820968849.
- Moebius J, Walter U, Sickmann A. Elucidation of N-Glycosylation sites on human platelet proteins: a glycoproteomic approach*. Mol Cell Proteom. 2006;5(2):226–33. doi:10.1074/mcp.M500324-MCP200.
- King SL, Joshi HJ, Schjoldager KT, Halim A, Madsen TD, Dziegiel MH, Woetmann A, Vakhrushev SY, Wandall HH. Characterizing the O-glycosylation landscape of human plasma, platelets, and endothelial cells. Blood Adv. 2017;1(7):429–42. doi:10.1182/bloodadvances.2016002121.
- Li Y, Fu J, Ling Y, Yago T, McDaniel JM, Song J, Bai X, Kondo Y, Qin Y, Hoover C, et al. Sialylation on O-glycans protects platelets from clearance by liver kupffer cells. Proc Natl Acad Sci USA. 2017;114(31):8360–5. doi:10.1073/pnas.1707662114.
- Harris SB, Josephson CD, Kost CB, Hillyer CD. Nonfatal intravascular hemolysis in a pediatric patient after transfusion of a platelet unit with high-titer anti-A. Transfusion. 2007;47(8):1412–17. doi:10.1111/j.1537-2995.2007.01283.x.
- Ramírez-López A, Álvarez Román MT, Monzón Manzano E, Acuña P, Arias-Salgado EG, Martín Salces M, Rivas Pollmar MI, Jiménez Yuste V, Justo Sanz R, García Barcenilla S, et al. The importance of platelet glycoside residues in the haemostasis of patients with immune thrombocytopaenia. J Clin Med. 2021;10(8):1661. doi:10.3390/jcm10081661.
- Klein CA. Cancer progression and the invisible phase of metastatic colonization. Nat Rev Cancer. 2020;20(11):681–694. doi:10.1038/s41568-020-00300-6.
- Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529(7586):298–306. doi:10.1038/nature17038.
- Ichikawa J, Ando T, Kawasaki T, Sasaki T, Shirai T, Tsukiji N, Kimura Y, Aoki K, Hayakawa K, Suzuki‐Inoue K, et al. Role of platelet C-Type lectin-like receptor 2 in promoting lung metastasis in Osteosarcoma. J Bone Miner Res. 2020;35(9):1738–1750. doi:10.1002/jbmr.4045.
- Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez‐Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113(5):752–60. doi:10.1002/ijc.20657.
- Sabrkhany S, Kuijpers MJE, Oude Egbrink MGA, Griffioen AW. Platelets as messengers of early-stage cancer. Cancer Metastasis Rev. 2021;40(2):563–73. doi:10.1007/s10555-021-09956-4.
- Lin RJ, Afshar-Kharghan V, Schafer AI. Paraneoplastic thrombocytosis: the secrets of tumor self-promotion. Blood. 2014;124(2):184–7. doi:10.1182/blood-2014-03-562538.
- Mammadova-Bach E, Zigrino P, Brucker C, Bourdon C, Freund M, De Arcangelis A, Abrams SI, Orend G, Gachet C, Mangin PH, et al. Platelet integrin α6β1 controls lung metastasis through direct binding to cancer cell–derived ADAM9. JCI Insight. 2016;1(14):e88245. doi:10.1172/jci.insight.88245.
- Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci USA. 1998;95(16):9325–30. doi:10.1073/pnas.95.16.9325.
- Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–300.
- Rodriguez-Martinez A, Simon-Saez I, Perales S, Garrido-Navas C, Russo A, de Miguel-Perez D, Puche-Sanz I, Alaminos C, Ceron J, Lorente JA, et al. Exchange of cellular components between platelets and tumor cells: impact on tumor cells behavior. Theranostics. 2022;12(5):2150–2161. doi:10.7150/thno.64252.
- Sadallah S, Schmied L, Eken C, Charoudeh HN, Amicarella F, Schifferli JA. Platelet-derived ectosomes reduce NK cell function. J Immunol. 2016;197(5):1663–71. doi:10.4049/jimmunol.1502658.
- Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20(3):169–77. doi:10.1016/j.semcancer.2010.04.005.
- Echtler K, Konrad I, Lorenz M, Schneider S, Hofmaier S, Plenagl F, Stark K, Czermak T, Tirniceriu A, Eichhorn M. et al. Platelet GPIIb supports initial pulmonary retention but inhibits subsequent proliferation of melanoma cells during hematogenic metastasis. PLoS One. 2017;12(3):e0172788. doi:10.1371/journal.pone.0172788.
- Guo Y, Cui W, Pei Y, Xu D. Platelets promote invasion and induce epithelial to mesenchymal transition in ovarian cancer cells by TGF-β signaling pathway. Gynecol Oncol. 2019;153(3):639–50. doi:10.1016/j.ygyno.2019.02.026.
- Trinchera M, Aronica A, Dall’olio F. Selectin ligands sialyl-lewis a and sialyl-lewis x in gastrointestinal cancers. Biology (Basel). 2017;6(4):16. doi:10.3390/biology6010016.
- Ma YQ, Geng JG. Heparan sulfate-like proteoglycans mediate adhesion of human malignant melanoma A375 cells to P-selectin under flow. J Immun (Balt Md 1950). 2000;165(1):558–65. doi:10.4049/jimmunol.165.1.558.
- Ma Y-Q, Geng J-G. Obligatory requirement of sulfation for P-selectin binding to human salivary gland carcinoma Acc-M cells and breast carcinoma ZR-75-30 cells. J Immun (Balt Md 1950). 2002;168(4):1690–6. doi:10.4049/jimmunol.168.4.1690.
- McCarty OJ, Mousa SA, Bray PF, Konstantopoulos K. Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood. 2000;96(5):1789–97. doi:10.1182/blood.V96.5.1789.
- Suzuki-Inoue K, Osada M, Ozaki Y. Physiologic and pathophysiologic roles of interaction between C-type lectin-like receptor 2 and podoplanin: partners from in utero to adulthood. J Thromb Haemost. 2017;15(2):219–229. doi:10.1111/jth.13590.
- Sekiguchi T, Takemoto A, Takagi S, Takatori K, Sato S, Takami M, Fujita N. Targeting a novel domain in podoplanin for inhibiting platelet-mediated tumor metastasis. Oncotarget. 2016;7(4):3934–46. doi:10.18632/oncotarget.6598.
- Shirai T, Inoue O, Tamura S, Tsukiji N, Sasaki T, Endo H, Satoh K, Osada M, Sato‐Uchida H, Fujii H, et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J Thromb Haemost. 2017;15(3):513–525. doi:10.1111/jth.13604.
- Takemoto A, Takagi S, Ukaji T, Gyobu N, Kakino M, Takami M, Kobayashi A, Lebel M, Kawaguchi T, Sugawara M, et al. Targeting podoplanin for the treatment of osteosarcoma. Clin Cancer Res. 2022;28(12):2633–2645. doi:10.1158/1078-0432.CCR-21-4509.
- Lee HY, Yu N-Y, Lee S-H, Tsai H-J, Wu C-C, Cheng J-C, Chen D-P, Wang Y-R, Tseng C-P. Podoplanin promotes cancer-associated thrombosis and contributes to the unfavorable overall survival in an ectopic xenograft mouse model of oral cancer. Biomed J. 2020;43(2):146–62. doi:10.1016/j.bj.2019.07.001.
- Takemoto A, Okitaka M, Takagi S, Takami M, Sato S, Nishio M, Okumura S, Fujita N. A critical role of platelet TGF-β release in podoplanin-mediated tumour invasion and metastasis. Sci Rep. 2017;7(1):42186. doi:10.1038/srep42186.
- Tamura S, Suzuki-Inoue K, Tsukiji N, Shirai T, Sasaki T, Osada M, Satoh K, Ozaki Y. Podoplanin-positive periarteriolar stromal cells promote megakaryocyte growth and proplatelet formation in mice by CLEC-2. Blood. 2016;127(13):1701–10. doi:10.1182/blood-2015-08-663708.
- Lombard SE, Pollitt AY, Hughes CE, Di Y, Mckinnon T, O’callaghan CA, Watson SP. Mouse podoplanin supports adhesion and aggregation of platelets under arterial shear: a novel mechanism of haemostasis. Platelets. 2018;29(7):716–22. doi:10.1080/09537104.2017.1356919.
- Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: a systematic review. Biochim Biophys Acta. 2015;1855(2):235–47. doi:10.1016/j.bbcan.2015.03.003.
- Demydenko D, Berest I. Expression of galectin-1 in malignant tumors. Exp Oncol. 2009;31:74–9.
- Saha B, Mathur T, Tronolone JJ, Chokshi M, Lokhande GK, Selahi A, Gaharwar AK, Afshar-Kharghan V, Sood AK, Bao G, et al. Human tumor microenvironment chip evaluates the consequences of platelet extravasation and combinatorial antitumor-antiplatelet therapy in ovarian cancer. Sci Adv. 2021;7(30). doi:10.1126/sciadv.abg5283.
- Dovizio M, Maier TJ, Alberti S, Di Francesco L, Marcantoni E, Münch G, John CM, Suess B, Sgambato A, Steinhilber D, et al. Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol Pharmacol. 2013;84(1):25–40. doi:10.1124/mol.113.084988.
- Martinet L, Smyth MJ. Regulation of immune cell functions through nectin and nectin-like receptors. 2016:404–14. doi:10.1016/B978-0-12-374279-7.02010-5.
- Ma W, Ma J, Lei T, Zhao M, Zhang M. Targeting immunotherapy for bladder cancer by using anti-CD3 × CD155 bispecific antibody. J Cancer. 2019;10(21):5153–61. doi:10.7150/jca.29937.
- Gao J, Zheng Q, Shao Y, Wang W, Zhao C. CD155 downregulation synergizes with adriamycin to induce breast cancer cell apoptosis. Apoptosis. 2018;23(9–10):512–20. doi:10.1007/s10495-018-1473-8.
- Kučan Brlić P, Lenac Roviš T, Cinamon G, Tsukerman P, Mandelboim O, Jonjić S. Targeting PVR (CD155) and its receptors in anti-tumor therapy. Cell Mol Immunol. 2019;16(1):40–52. doi:10.1038/s41423-018-0168-y.
- Azab AK, Quang P, Azab F, Pitsillides C, Thompson B, Chonghaile T, Patton JT, Maiso P, Monrose V, Sacco A, et al. P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment. Blood. 2012;119(6):1468–78. doi:10.1182/blood-2011-07-368050.
- Chang YW, Hsieh P-W, Chang Y-T, Lu M-H, Huang T-F, Chong K-Y, Liao H-R, Cheng J-C, Tseng C-P. Identification of a novel platelet antagonist that binds to CLEC-2 and suppresses podoplanin-induced platelet aggregation and cancer metastasis. Oncotarget. 2015;6(40):42733–48. doi:10.18632/oncotarget.5811.
- Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, Guthrie TH, Knight-Madden J, Alvarez OA, Gordeuk VR, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429–439. doi:10.1056/NEJMoa1611770.
- Jackson W 3rd, Sosnoski DM, Ohanessian SE, Chandler P, Mobley A, Meisel KD, Mastro AM. Role of megakaryocytes in breast cancer metastasis to bone. Cancer Res. 2017;77(8):1942–54. doi:10.1158/0008-5472.CAN-16-1084.
