 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Microfluidic technology has emerged as a powerful tool in studying arterial thrombosis, allowing researchers to construct artificial blood vessels and replicate the hemodynamics of blood flow. This technology has led to significant advancements in understanding thrombosis and platelet adhesion and aggregation. Microfluidic models have various types and functions, and by studying the fabrication methods and working principles of microfluidic chips, applicable methods can be selected according to specific needs. The rapid development of microfluidic integrated system and modular microfluidic system makes arterial thrombosis research more diversified and automated, but its standardization still needs to be solved urgently. One key advantage of microfluidic technology is the ability to precisely control fluid flow in microchannels and to analyze platelet behavior under different shear forces and flow rates. This allows researchers to study the physiological and pathological processes of blood flow, shedding light on the underlying mechanisms of arterial thrombosis. In conclusion, microfluidic technology has revolutionized the study of arterial thrombosis by enabling the construction of artificial blood vessels and accurately reproducing hemodynamics. In the future, microfluidics will place greater emphasis on versatility and automation, holding great promise for advancing antithrombotic therapeutic and prophylactic measures.
Plain Language Summary
What is the context?
To study the mechanism of arterial thrombosis, including the platelet adhesion and aggregation behavior and the coagulation process.
Microfluidic technology is commonly used to study thrombosis. Microfluidic technology can simulate the real physiological environment on the microscopic scale in vitro, with high throughput, low cost, and fast speed.
As an innovative experimental platform, microfluidic technology has made remarkable progress and has found applications in the fields of biology and medicine.
What is new?
This review summarizes the different fabrication methods of microfluidics and compares the advantages and disadvantages of these methods. Recent developments in microfluidic integrated systems and modular microfluidic systems have led to more diversified and automated microfluidic chips in the future.
The different types and functions of microfluidic models are summarized. Platelet adhesion aggregation and coagulation processes, as well as arterial thrombus-related shear force changes and mechanical behaviors, were investigated by constructing artificial blood vessels and reproducing hemodynamics.
Microfluidics can provide a basis for the development of personalized thrombosis treatment strategies. By analyzing the mechanism of action of existing drugs, using microfluidic technology for high-throughput screening of drugs and evaluating drug efficacy, more drug therapy possibilities can be developed.
What is the impact?
This review utilizes microfluidics to further advance the study of arterial thrombosis, and microfluidics is also expected to play a greater role in the biomedical field in the future.
Introduction
Arterial thrombosis (AT) involves the abnormal coagulation of blood at the damaged inner surface of blood vessels due to various factors. It can lead to cardiovascular diseases such as acute myocardial infarction or ischemic stroke, which are associated with high rates of disability and mortality.Citation1,Citation2 Thrombosis primarily occurs when there is damage to vascular endothelial cells, hypercoagulability of blood, and hemodynamic changes, typically at sites with altered blood flow.Citation3 Most cases of arterial thrombosis are a result of arterial endothelial damage, deposition of lipid content under damaged endothelium, formation of atheromatous plaques, resulting in narrowing of the arteries, leading to changes in blood flow, and eventually, if local plaques rupture, platelet adhesion and aggregation occur. This process stimulates the generation of thrombin, which converts fibrinogen to fibrin, ultimately forming a thrombus covered by a layer of platelets and fibrin.Citation4,Citation5
Platelet adhesion and aggregation in the vasculature occur gradually, consisting of an initial adhesion phase followed by a rapid increase in platelet aggregation. When the vascular endothelium is injured or when an atheromatous plaque ruptures, subendothelial collagen becomes exposed, and free von Willebrand factor (vWF) in plasma binds to collagen, laminin, and fibronectin.Citation6 This results in structural changes in vWF, causing the exposure of the active center that binds to the platelet surface glycoprotein GPIb-V-IX. Consequently, platelets are recruited to the site of vascular injury, leading to unstable and reversible platelet adhesion.Citation7–9 Following activation, platelets undergo migration, and the GP Ib-V-IX complex, upon binding with von Willebrand factor (vWF), initiates the activation of signaling molecules within the platelet, among which is protein kinase C (PKC), a pivotal participant in the activation process. Activated PKC induces a conformational change in the GP IIb-IIIa complex, allowing it to interact with collagen, leading to the formation of an irreversible and stable adhesion. Additionally, soluble platelet agonists such as ADP, TXA2, thrombin, and epinephrine are involved in the activation of GP IIb-IIIa. When platelets adhere to the collagen matrix in the damaged vessel wall, platelet aggregation occurs, with this platelet serving as the core.Citation10,Citation11 Simultaneously, the damage to the vascular endothelium exposes intravascular tissue factors, which activate coagulation factors to trigger the development of the coagulation cascade. This cascade includes the conversion of prothrombin to thrombin, promoting fibrin polymerization and enhancing the stability of the thrombus.Citation12,Citation13
Arterial thrombosis has a high incidence in clinical practice and can cause serious complications, including acute myocardial infarction and ischemic stroke, which often result in disability or death. Consequently, arterial thrombosis stands as one of the leading causes of death attributed to cardiovascular disease. In order to prevent and treat arterial thrombosis effectively, it is essential to have a clear understanding of its mechanisms. This understanding can enable physicians to take appropriate steps to reduce a patient’s risk of thrombosis, whether through medication or surgical intervention. Furthermore, knowledge about the mechanisms of thrombosis can aid in the development of new treatments to enhance patients’ prognosis.
Experimental tools currently used for studying thrombosis include in vitro experiments, animal models, in vivo imaging techniques, and microfluidics. In vitro experiments involve isolated blood vessels or platelet samples, allowing for precise control of specific factors. However, they do not simulate the biological environment in vivo. Various systems, such as phototurbidimetry, whole blood resistometry, VerifyNow, and platelet analyzers, have been developed for platelet function analysis in vitro. However, they all test under static or in vitro shear conditions, which significantly differ from the actual flow environment in which they are actually located.Citation14–16
Animal models are the most commonly used experimental tool for studying the pathophysiology of thrombosis in laboratory settings. These models simulate the complex multifactorial mechanical conditions found in arterial thrombosis. Platelet adhesion and aggregation to form thrombi can be induced by damaging vascular endothelial cells through ferric chloride application, photochemical injury, laser irradiation, or electrical current stimulation. However, animal models do not fully replicate human physiology or human disease.Citation17 There are always differences between animal models with human physiology, and conducting operations are time-consuming and laborious.
In vivo imaging techniques, such as ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI), allow for noninvasive visualization of thrombus formation and evolution in patients. These techniques are useful for clinical diagnosis and therapeutic monitoring. However, they are more expensive, time-consuming, and carry the risk of radiation exposure. Furthermore, the resolution is limited and fails to provide detailed molecular-level information.
The emergence of microfluidics has greatly facilitated researchers in studying the relationship between thrombus formation, vascular geometry, and hemodynamic effects.Citation18 Microfluidics refers to the manipulation and control of fluids on a micrometer scale, enabling the miniaturization of sample reactions, separations, and assays onto a single chip. Microfluidics allows for controlled studies in a cost-effective and high-throughput manner, characterized by small size and high speed.Citation19 Microfluidics replicates realistic physiological conditions, including dynamic flow within blood vessels, shear forces, and interactions between platelets and vessel walls. This enables simultaneous performance of multiple experimental units in microchannels, facilitating high-throughput experiments. Additionally, integration of microfluidics with real-time monitoring methods, such as fluorescence microscopy, enables high-resolution imaging and monitoring of thrombus formation in the microchannel, yielding detailed dynamic information essential for comprehending thrombus formation mechanisms. Moreover, microfluidics utilizes miniaturized experimental systems, leading to reduced reagent and sample usage, hence minimizing the need for animal experiments. Furthermore, it supports individualized studies and precise control of parameters to ensure reproducible and stable experimental conditions.Citation20–22 In conclusion, microfluidic technology offers a platform for thrombosis research closer to in vivo conditions, overcoming limitations of traditional experimental tools.
Microfluidics, an innovative experimental platform, has made remarkable progress and found applications in biology and medicine.Citation23 In the field of biology, microfluidics has been widely used for cell research, biomolecular analysis, and protein interaction studies. Researchers can manipulate and observe individual cells precisely using microfluidic chips to reveal cell signaling, life cycle, and interaction mechanisms. Additionally, the high-throughput characteristics of microfluidic technology enable rapid detection and analysis of biomolecules, thereby promoting the development of proteomics, metabolomics, and other research fields. In the medical field, microfluidic technology plays a crucial role in disease diagnosis, drug screening, and personalized medicine. It enables rapid and sensitive detection of biomarkers, providing a new approach for early disease diagnosis and accelerating the drug discovery process while reducing research and development costs.Citation24 Researchers can study cell behavior, molecular interactions, simulated human organs, and other complex processes at the microscopic scale by combining microfluidic chips with other technologies such as microscopy, optical sensors, fluorescence imaging, and organ-on-a-chip technology.Citation25,Citation26
This paper provides an overview of the use of microfluidic technology in the study and treatment of arterial thrombosis, along with highlighting the advances in the combined application of microfluidic technology with other technologies. It accomplishes this through a comprehensive review of the construction, working principle, and application characteristics of microfluidic chips.
Fabrication and construction modeling of microfluidic chips
Fabrication of microfluidic chips
Microfluidics is a technology that utilizes microchips and micromachining to regulate fluid flow and mixing through micrometer-level flow channels and microvalves. Microfluidic chips, which play a crucial role in microfluidic devices, can be fabricated using various techniques including photolithography, 3D printing, microfluidic chip evaporation, PDMS (polydimethylsiloxane) molding, thermocompression, and microelectronic processing.Citation27,Citation28 Photolithography is a fundamental technique in microfluidic processing and has achieved widespread adoption for fabricating microstructures on silicon, glass, and quartz substrates. This technique involves coating photoresist onto silicon wafers and utilizing mask lithography or electron beam exposure technology to fabricate microfluidic chips. Its primary principle relies on controlling the irradiation of ultraviolet rays to utilize the chemical properties of photoresist and create precise microstructures and microchannels.Citation29,Citation30 The key steps involved in photolithography encompass designing the chip pattern, creating the mask, exposing the chip, etching, and cleaning. depicts the schematic of the chip manufacturing process using photolithography.
Figure 1. Schematic diagram of the process of manufacturing a chip by lithography. photolithography is utilized to fabricate microchannels by applying a photoresist on materials like silicon or glass substrates, designing a chip pattern for mask creation, regulating the chip exposure to ultraviolet light, and subsequently forming microchannels through etching and cleaning processes. In the case of positive photoresists, the exposed area is softened and removed, while for negative photoresists, the exposed area is protected and retained.
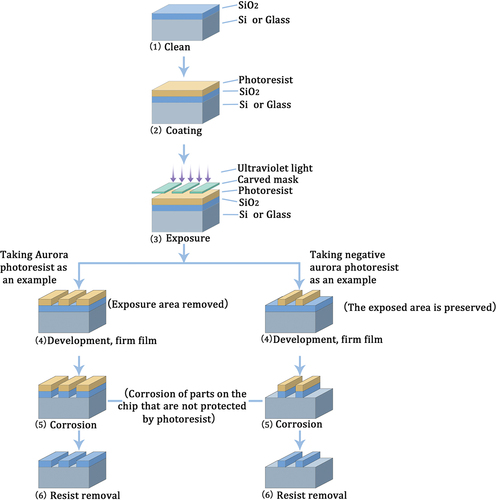
Soft lithography is a novel microfabrication technique that differs from photolithography. It is based on self-assembled monolayers, elastic stamps, and polymer molding techniques. This technique enables the creation of complex three-dimensional structures and irregular surfaces. It is applicable to a wide range of materials. Moreover, it is widely employed in the production of small-scale blood vessel models. The main techniques in soft lithography comprise micro-contact printing, nanoimprint lithography, and various molding methods, including micro-transfer molding, capillary micro-forming, and solvent-assisted micro-forming. Elastic mold stamps are typically produced through photo-etching and molding processes, with PDMS being the predominant material. Nonetheless, excessive softness or inappropriate width-to-depth ratios of the elastic mold can cause deformation or distortion of the microstructure.
Recently, 3D printing technology has attracted significant attention in the production of microdevices, particularly in the field of biomedical engineering.Citation31 3D printing technology is capable of fabricating complex three-dimensional microfluidic chip structures, making it suitable for various models like cardiovascular, cerebrovascular, hepatic vascular, and vascular branching models, among others, used for simulating real blood vessels.Citation32,Citation33 The commonly used 3D printing technologies include micro stereolithography technology (MSLA), two-photon absorption (TPA or TPP), digital light processing (DLP)-based 3D printers, fused deposition modeling (FDM), inkjet 3D printing, microselecting laser sintering (MSLS), and laminated object manufacturing (LOM).Citation30,Citation32–38 Microstereolithography technology involves controlling the exposure light source to expose and cure the photosensitive resin layer by layer, achieving a resolution of a few micrometers.Citation32 MSLA boasts high resolution and is well-suited for producing small-scale structures; nevertheless, the fabrication process is intricate and time-consuming. The two-photon polymerization effect occurs in the photosensitive resin upon focusing a laser, with a computer-controlled platform moving in three directions at nanometer-sized micrometric distances.Citation34 The two-photon polymerization (TPP) process exhibits high resolution and is well-suited for fabricating microtissues with intricate structures. Nevertheless, the machinery required for TPP fabrication is intricate and costly. Digital light processing-based 3D printer technology utilizes dynamic digital mask exposure and laser light to the micromirror array, enabling the control of the deflection of its reflective micromirrors for fully digital imaging.Citation35 Digital light processing (DLP) 3D printing technology produces objects quickly, but uses less material. Fused deposition modeling technology prints heated and softened fused filaments of polymer materials layer by layer, with subsequent cooling and curing on a substrate to form 3D structures.Citation33,Citation36 Fused Deposition Modeling (FDM) offers a diverse selection of materials, a straightforward manufacturing process, and is particularly well-suited for producing large-scale structures. Nevertheless, FDM exhibits limitations in precision and surface flatness, making it unsuitable for manufacturing tiny structures. Inkjet 3D printing technology sprays small droplets of ink from an array of printer nozzles onto a substrate, where a light source cures the resin as it is being printed.Citation37 Inkjet 3D printing technology is well-suited for bioprinting and is capable of utilizing a diverse array of biomaterials. However, its resolution is restricted, making it unsuitable for applications with high precision requirements. Micro-laser sintering uses a high-temperature laser or electron beam to selectively melt metal powders, which are then sintered and molded by computer-controlled stacking.Citation38 Micro-stereolithography (MSLS) offers exceptional precision, making it well-suited for creating intricate structures and metal products. However, its high cost and limited production capacity make it less suitable for mass production. Despite these limitations, MSLS is valuable for creating prototypes and small batches of specialized products where precision is crucial. The layered solid manufacturing method primarily utilizes paper or sheet as raw material, which is laser-cut and then thermobonded using a compression device to bond and stack the cut layers.Citation29 LOM is characterized by its low cost and efficiency, and it is not constrained by the support structure. However, its low resolution renders it unsuitable for high-precision manufacturing processes. For a summary of the characteristics of several common 3D printing techniques, refer to .Citation29,Citation32,Citation34–38Future research in arterial thrombosis using 3D printing techniques is likely to focus on the use of biocompatible materials.Citation39 Given the structural intricacy of blood vessels, the technology employed must possess a sufficiently high resolution. Moreover, the capability to employ a diverse range of materials that can accurately replicate various structures and functionalities within the body is crucial. Additionally, attention should be given to efficient fabrication speeds and equipment costs to develop a method suitable for specific requirements.
Table I. Common 3D printing technologies.
The microfluidic chip evaporation method is a specialized technique for the preparation of microfluidic chips. It involves controlled evaporation of liquids to create minute channels and microchambers. These microchannels are used for various purposes, such as regulating liquid flow, sample mixing, bioanalysis, and micro-experiments. Additionally, this method enables the precise control and concentration of micro-volume droplets by regulating the temperature, humidity, and atmosphere to control the evaporation rate. This leads to improved sensitivity and efficiency of experiments.Citation40 This technique exhibits significant efficacy in high-throughput drug screening, single-cell sequencing, and various other domains. However, it is important to note that the evaporation process requires sophisticated instruments and precise experimental conditions. If not carefully controlled, it can result in sample loss or uneven concentration.
In addition, the PDMS (polydimethylsiloxane) molding method is used to form microchannel structures by combining PDMS with a mold. PDMS is a commonly used biocompatible material, and the method is simple and low-cost for rapid fabrication of small-scale microfluidic chips. thermocompression technique, on the other hand, combines two glass or polymer plates by heat and pressure to form microchannels, thus fabricating some simple microfluidic chips. This method can be reproduced in large quantities with simple equipment, but the materials used are limited. Microelectronic processing technology allows the formation of tiny structures on silicon wafers with the help of microelectronic manufacturing techniques similar to those used in the semiconductor industry. This method is suitable for large-scale manufacturing and allows for a high degree of integration. Processing techniques such as soft lithography, micro-laser sintering and molding methods are commonly used for mainstream microfluidic chips. Various types of polymer materials have also replaced silicon and glass materials to become the mainstream matrix materials for microfluidic systems, and materials such as hydrogels, nanoparticles and colloids are also being studied. In the future, microfluidic chips will be more diversified and automated, integrating multi-functional modules for multi-parameter detection to provide more comprehensive thrombus studies. It is also expected that nanotechnology will be integrated to improve the sensitivity and specificity of the chip in the detection of biomolecules.Citation41
Microfluidic integration system and modular microfluidic system working principle
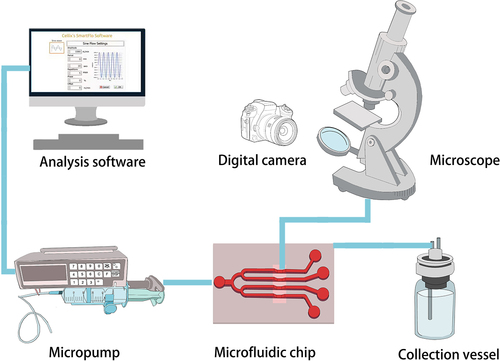
Existing microfluidic chip systems are intricately designed to integrate micro- and nanoscale fluid synthesis processing and analysis within a single chip, encompassing operations from sampling and processing to reactions and separations. The microfluidic integrated system is commonly used to simulate in vivo shear stress and study thrombosis and antithrombotic therapy. It utilizes a micropump to deliver cell samples or whole blood at constant or variable shear stress into the microchannels of a fabricated microfluidic chip. The chip is then placed in a temperature controlled microenvironmental chamber that replicates the physiological environment. The microenvironmental chamber is positioned on a platform equipped with a microscope and camera for automated image/video acquisition, real-time monitoring, and data analysis. illustrates the working program. The integrated microfluidic system offers fully automated liquid handling and analytical detection without human intervention. Despite its advantages, the system has limited adaptability, requiring redesign and reprocessing when experimental conditions change. Additionally, designing and processing the system in-house is challenging.
Figure 2. The operational principle of typical microfluidic integrated systems. The micropump is operated by computer software or controller connection, allowing precise control of liquid flow through piping or pressure when connected to the microfluidic chip. A liquid collection bottle is used to collect the liquid. Real-time monitoring and data analysis are enabled through automatic image/video acquisition using a microscope and camera.
Recently, there has been increasing research and application of modular microfluidic systems; however, their development is still in the early stages and is far from being standardized. Modular microfluidics refers to the integration of various standardized modules into a comprehensive and intricate platform that offers portability, field deplorability, and high customizability.Citation42 These modules are typically comprised of flow channels, valves, reaction chambers, detection systems, transport mechanisms, storage compartments, and control units. Flow channel modules serve the purpose of guiding and regulating fluid flow, encompassing main flow channels, branched flow channels, mixed flow channels, and more. Valve modules are employed to control and adjust fluid flow rate, direction, and distribution, with options including active and passive valves.Citation43 Active micro-valves are particularly sensitive and allow for precise fluid control,Citation44 whereas passive micro-valves are cost-effective, consume less power, and boast simpler designs. Reaction chamber modules are utilized to facilitate chemical and biological reactions, often in experiments like drug screening or cell culture. Detection modules are responsible for analyzing fluid parameters, cell growth states, chemical reaction products, and other such variables. Fluorescence detection, electrochemical detection, and miniature biosensors are commonly employed for this purpose. Transmission modules enable the transport of micro samples, cells, or molecules through methods such as micropumps and electrophoresis systems. Micropumps are classified into mechanical and non-mechanical types, with the former featuring moving components for liquid pumping, while the latter relying on physical fields to drive fluid motion. Storage modules, typically microchannels or micro reaction chambers, are devoted to housing samples, reagents, biological cells, and similar entities. Lastly, the control module oversees the operation, regulation, and monitoring of the microfluidic system, offering functions like temperature control, pressure control, and liquid level control, among others.
Each individual microfluidic module is interconnected to form a complete microfluidic system. The specific number and type of modules used depend on the design and requirements of the model. The microfluidic model can be connected to the detection equipment either through a pressure port or a pipe. While this connection method is simple and easy to operate, there is a risk of air leakage, thus necessitating a tightly sealed port. Alternatively, an integrated connection can be established between the microfluidic model and the testing device as a whole. This type of connection ensures a tight seal with no risk of air leakage, but it is more expensive to manufacture and not easily maintained. Additionally, plug-and-play connections using specially designed connectors can be employed. Although this method is simple to maintain, it requires specialized connectors that come at a higher cost. Consequently, the selection of the connection method should be made based on the specific requirements of the application and the manufacturing capabilities of the equipment. Furthermore, attention must be given to factors such as the material, sealing, and connection method of the connectors to guarantee the reliability and stability of the connection.Citation45
Construction and application characteristics of common microfluidic chip runner modules
Typically, microfluidic chips utilize semiconductor-like MEMS (microelectromechanical systems) technology for constructing a microfluidic channel system on a chip. The chips are fabricated using inorganic materials such as silicon wafers and glass, as well as organic polymers including polycarbonate (PC) and polydimethylsiloxane (PDMS). Organic polymer materials offer advantages such as simplified preparation, good elasticity, and easy deformability.Citation46 Consequently, organic polymers are commonly employed for fabricating microfluidic chips with complex flow paths, and soft lithography techniques are utilized to achieve the fabrication of three-dimensional structures with irregular surfaces.
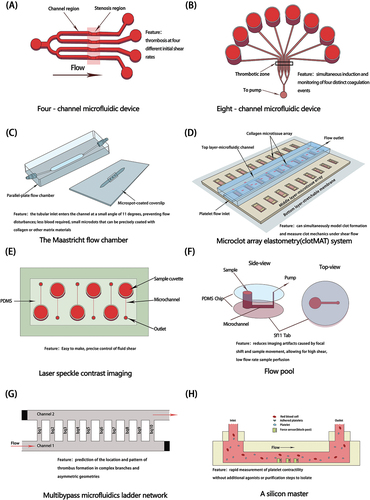
Effective devices have been developed to replicate thrombus formation after vascular injury. This is done by perfusing whole blood through microfluidic channels at a set shear rate and applying appropriate surface coatings, such as type I collagen and laminin, for platelet adhesion, activation, and formation of mural thrombi.Citation47 Multi-channel models enable the simultaneous measurement of platelet aggregation at multiple shear rates. They also allow the simultaneous induction and monitoring of four different types of coagulation events to evaluate the platelet response to various inhibitors. provides a summary of the construction and characteristics of several typical microfluidic flow channel modules.
Figure 3. Construction and characteristics of common microfluidic devices. (A) Four-channel microfluidic device (B) Eight-channel microfluidic device (C) The Maastricht flow chamber (D) Microclot array elastometry (clotMAT) system (E) Laser speckle contrast imaging (F) Flow pool (G) Multibypass microfluidics ladder network (H) A silicon master.
Application of microfluidics in thrombosis research
Simulating the flow environment within human blood vessels
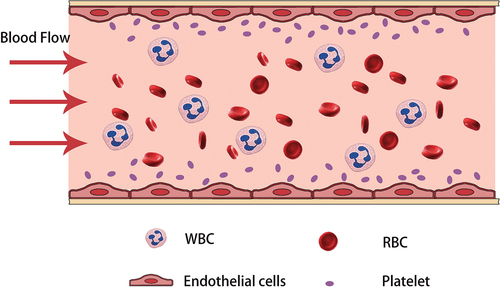
Microfluidic technology can replicate the flow conditions within healthy or constricted human blood vessels using in vitro microfluidic models. This enables the observation of intravascular cell flow, platelet behavior, and the process of thrombus formation under various conditions. Under normal circumstances, blood flow within blood vessels is laminar, with slower flow near the vessel wall and faster flow in the central region. Shear refers to the force created by the movement between adjacent layers in a fluid, specifically caused by variations in blood flow rates at different levels. Shear rate represents the difference in relative velocity between adjacent layers of blood flow within vessels. The magnitude of the shear rate is influenced by the gradient of blood flow velocity and blood viscosity, with the shear rate increasing when there is a substantial flow velocity gradient or high blood viscosity. Usually, the flow velocity is faster in the central region of the vessel, but the flow velocity gradient between layers is not large, so the central region has the smallest shear rate. The region near the vessel wall, on the other hand, has a slower flow rate due to viscous resistance, but the flow rate gradient is large and the wall shear rate can instead be maximized. In normal physiological conditions, red blood cells flow within the central region of the vasculature, while platelets move near the sidewalls due to the interaction with red blood cells (As shown in ).
Figure 4. The environment of intravascular flow in the human body under normal physiologic conditions. Blood exists in a laminar flow within the vessel, usually with the highest flow rate in the center and a gradual decrease in flow rate around the periphery. This velocity distribution allows red blood cells to flow in the center of the vessel, while platelets travel close to the sidewalls due to the impingement of the red blood cells.
Shear forces play a crucial and intricate role in thrombosis. On one hand, shear forces prevent blood pooling by swiftly rinsing the inner walls of blood vessels, dispersing platelets that have just started to aggregate but are still unstable, and diluting soluble agonists. Consequently, they exert an antithrombotic effect, contributing to the maintenance of normal vascular patency.Citation48 On the other hand, in regions where blood flow exhibits low shear, the flow rate decreases and the blood moves smoothly within the vessels. In this scenario, the shear force is not strong enough to overcome the force of cell-to-cell interaction, which can lead to an increase in blood viscosity and promote the formation of a thrombus. Additionally, shear forces can cause damage to endothelial cells, prompting the secretion of inflammatory factors and exacerbating the development of thrombosis.Citation49
Nesbitt et al. utilized microfluidics to investigate the impact of high shear rates on platelets. Their findings revealed that a high shear rate lowers the platelet activation threshold and increases their sensitivity to soluble platelet activators. Additionally, the shear rate induces platelets to adhere through GP Ib-V-IX, followed by platelet aggregation that relies on platelet activation.Citation47,Citation50 However, Ruggeri et al. proposed that aggregation, dependent solely on the interaction between GP Ib-V-IX and vWF and not on platelet activation, could directly occur at extremely high shear rates (>20000 s−1).Citation51–53 Li et al. conducted in vitro experiments using porcine blood and varied shear rates to assess platelet aggregation. The results demonstrated that at low shear rates (<1500 s−1), platelet accumulation was not rapid; however, pathological shear rates (4000 s−1) resulted in the formation of platelet-rich occlusive thrombi.Citation54 Rahman et al. developed a microfluidic model to simulate the hemodynamic conditions in patients with upstream stenosis. Their model incorporated wall shear rates ranging from 1620 s−1 to 11560 s−1. The experiments revealed that under elevated shear flow conditions, downstream platelets exhibited increased adhesion to three platelet agonists (fibrinogen, collagen, or vascular hemophilic factor) and a significant upregulation of four platelet activation markers (P-selectin, GP IIb/IIIa, lysosomal glycoprotein, and phosphatidylserine).Citation55
Microfluidic technology utilizes microfluidic chip fabrication to construct precise microchannel structures that simulate the flow environment in blood vessels. By precisely controlling and regulating fluid flow rate and pressure, this technology allows for the study of physiological and pathological processes of blood flow.Citation56 Additionally, it can be used to investigate biological processes such as the physiological function of vascular endothelial cells, platelet-vessel wall interactions, and thrombus formation. Citation15,Citation47,Citation54,Citation57,Citation58 provides a summary of representative microfluidic devices that simulate the intravascular flow environment, focusing on the impact of shear rate on thrombus formation. The table includes information on construction, principles, experimental results, as well as advantages and disadvantages of each device.
Table II. Microfluidic device for simulating in vivo vascular flow environment.
Study of platelet adhesion aggregation and coagulation processes
Microfluidics can be utilized to investigate platelet adhesion, aggregation, coagulation factor activation, as well as thrombosis processes by designing microfluidic chips and systems to gain insights into platelet function and coagulation risk. In vitro microfluidic channels fabricated or damaged through mechanical extrusion feature narrowed passages that replicate the idealized geometry of microconstrictions. Research has shown that healthy vessel models exhibit no signs of thrombosis, but when vessels endure extruded stenosis, such as atherosclerosis, tumor compression, vascular obstruction, or other pathological factors, the wall shear rate rapidly increases, thereby hastening platelet aggregation. depicts a schematic illustration of discoid platelet aggregation at a stenosis. Initially, platelet aggregation takes place at the tip of the stenosis, followed by the formation of discoid platelet aggregates in the downstream low-shear region. This process relies on the dynamic reorganization of membrane tethers. These tethers are minute, elongated protrusions typically comprised of phospholipid molecules and proteins on the platelet membrane’s surface.Citation59 Membrane tethers play a vital role in maintaining and stabilizing platelet interconnections and aggregation during platelet activation and adhesion.
Figure 5. Microfluidic modeling simulates discoidal platelet aggregation at a vessel stenosis. the simulation of a microconstriction with an idealized stenosis geometry involves the formation of a shear microgradient at the stenosis when the vessel is narrowed. This process can be divided into three phases: shear acceleration, peak shear, and shear deceleration. As platelets and erythrocytes flow through the bloodstream, they pass through the stenosis. Aggregation of platelets occurs at the tip of the stenosis, leading to the subsequent formation of discoidal platelet aggregates in the low-shear region downstream.
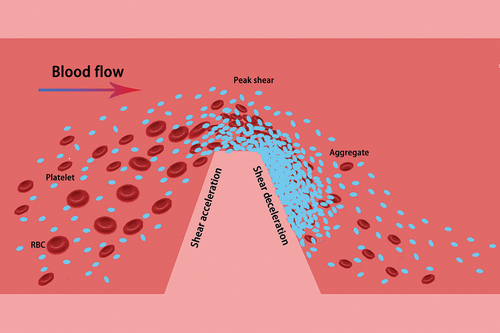
During platelet adhesion and aggregation, the application of compressive and tensile stresses under blood flow leads to a series of morphological changes in the plasma membrane and underlying cytoskeletal network of platelets.Citation7,Citation8 Nesbitt WS et al. (2007) demonstrated through microfluidic studies that initial platelet adhesion is characterized by the formation of membrane tethers, which are localized extensions of the lipid bilayer that experience hydraulic resistance from fluid extrusion at the cell surface in response to fluid resistance.Citation9–11 This process is dynamic and involves various stages of initial formation, elongation, and contractile remodeling of membrane tethers.Citation47 The formation of membrane tethers also facilitates the translocation and aggregation of disc-shaped platelets. Therefore, the formation and reorganization of membrane tethers during the early stages of thrombosis play a crucial role in the initial recruitment and aggregation of platelets.
In flowing blood, platelets adhere and aggregate by interacting with adhesion molecules on the exposed subendothelial matrix. The behavior of platelets in microfluidic channels can provide insights into the mechanisms of platelet adhesion and aggregation. One study investigated the impact of blocking the function of GP Ib or integrin GP IIb-IIIa on discoidal platelet aggregation using microfluidic modeling. The results showed that GP Ib is crucial for platelet recruitment, while blocking the integrin GP IIb-IIIa had no inhibitory effect on initial platelet recruitment but significantly hindered platelet aggregation in the deceleration zone downstream of the stenosis.Citation60 Moreover, the irreversibly aggregated adhesion of discoidal platelets relies on soluble platelet agonists (ADP, TXA2, and thrombin), which influence platelet activation at the site of vascular injury. Interestingly, microfluidic modeling demonstrated that blocked ADP, TXA2, and thrombin had no effect on platelet aggregation kinetics under shear microgradients. This suggests that stable discoidal platelet aggregates can persist for up to 10 minutes during blood perfusion, even in the presence of soluble agonists. However, they can rapidly disassemble after the cessation of blood flow, indicating that soluble agonists play a secondary role in the irreversible phase of platelet aggregation.Citation47
In addition, damage to the vascular endothelium leads to the release of tissue factor, which then forms a complex with coagulation factor VII. This complex initiates the extrinsic coagulation pathway. Simultaneously, the damaged vessel wall exposes clotting factors in the blood, initiating the endogenous coagulation pathway. Later coagulation factor Xa and coagulation factor Va in the presence of calcium ions and phospholipid membranes form the prothrombin complex, or thromboplastin, which converts prothrombin to thrombin. Thrombin converts coagulation factor I, commonly known as fibrinogen, into fibrin, initiating the coagulation pathway. Fibrin is the final product of blood coagulation and forms a network that binds platelets and other cells, resulting in the formation of thrombi. Thrombi can contribute to the growth and stability of a thrombus.Citation61
Citation1,Citation9,Citation55,Citation62–65 presents a comprehensive overview of microfluidic devices, including their construction, principles, experimental findings, as well as the advantages and disadvantages associated with studying platelet adhesion, aggregation, and coagulation processes.
Table III. Microfluidic device for studying platelet adhesion and aggregation.
Research on blood clot mechanics analysis
Using microfluidic technology, employing microfluidic technology to control the flow rate and flow conditions, the deformability and elasticity properties of blood clots in varying flow states can be replicated. By analyzing the morphological changes and deformation rate of blood clots, the deformability and elastic modulus can be evaluated, shedding light on their mechanical behavior in blood flow. This study establishes a foundation for gaining a deeper understanding of thrombosis and related diseases, and presents novel approaches to prevent and treat arterial thrombosis.
In clinical practice, Thromboelastography (TEG) or Rotational Thromboelastometer (ROTEM) are commonly used to measure clot stiffness at different stages of the coagulation process. However, their consideration of hemodynamics and platelet-matrix interactions is insufficient.Citation66 Commonly used tissue mechanics models, such as the cell-loaded block hydrogel model, are not suitable for studying clot mechanics due to their limited ability to generalize the dynamic flow environment, low experimental throughput, and solute diffusion. Microfluidics, on the other hand, can reproduce the dynamic process of clot formation in the presence of intravascular shear microgradients and monitor real-time changes in clot mechanics by controlling and measuring clot formation.Citation67 Tissue mechanics were measured and determined through a mechanosensing platform based on flexible micropillar arrays. Microclot contraction force was observed by monitoring micropillar deflection, and hardness was assessed through substrate tensile testing.Citation68 While clot contractility mainly depends on the direct adhesion of activated platelets to the collagen matrix, fibrin formation from locally released thrombin contributes mainly to clot formation and stabilization, with only slight involvement in partial contraction. Collagenous tissue and adherent platelets were subjected to immunofluorescent staining, and flowing platelets were captured by exposed collagen microtissues to form individual microclots. It was observed that with increasing shear rate, corresponding increases were observed in tissue fluorescence intensity, microclot contractility, and the hardness of microclots, while the thickness and volume of the microclots decreased. These findings suggest the promotion of platelet adhesion, microclot contractility, volume reduction, and hardening with increasing shear rate, thereby indicating a dependence of platelet adhesion on changes in shear rate.
Microfluidics is utilized not only for studying clot contraction and clot permeability, but also for detecting impaired clot formation and clot stiffness in samples from patients with von Willebrand disease (VWD). This aids in the diagnosis of coagulation disorders in VWD patients whose phenotypes cannot be determined through existing clinical trials.Citation69 Microfluidic systems have the potential to be applied in various clinical aspects in the future, such as analyzing highly reactive platelets in patients with cardiovascular diseases, real-time analysis of VWD patients with different phenotypes, predict.Citation70 Additionally, the combination of microfluidics with numerical simulation and mechanical modeling methods enables the quantitative analysis and prediction of blood clot mechanics. By establishing both a hydrodynamic model and a physical model of the blood clot, microfluidics can be used to investigate the force distribution, deformation behavior, and fracture mechanism of the clot. This provides valuable insights into the mechanical properties and response of blood clots. Citation66,Citation68,Citation71,Citation72 summarizes the construction, principles, experimental results, and advantages and disadvantages of representative microfluidic devices used for studying the mechanical analysis of blood clots.
Table IV. Microfluidic device for studying blood clot mechanics.
Microfluidics in the treatment of arterial thrombosis
Classification and rationale of current antithrombotic therapeutic agents
Antithrombotic therapeutic drugs can be categorized into three groups based on their mechanism of action and pharmacological characteristics: antiplatelet drugs, anticoagulant drugs, and fibrinolytic drugs.
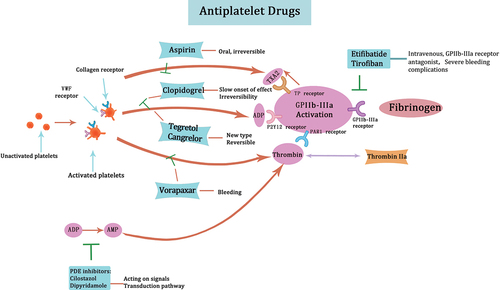
Currently, the main antiplatelet drugs in clinical practice include aspirin, P2Y12 receptor antagonists, GP IIb-IIIa receptor antagonists, phosphodiesterase (PDE) inhibitors, and PAR1 antagonists. Aspirin, a cyclooxygenase inhibitor, irreversibly acetylates platelet cyclooxygenase and inhibits the synthesis of thromboxane A2. By inhibiting platelet aggregation,Citation73 it exerts its antithrombotic effect. However, drug resistance to aspirin is a common phenomenon. The P2Y12 receptor plays a role in ADP-induced platelet aggregation. Clopidogrel is the representative drug of P2Y12 receptor antagonism, but it has a slow onset of effect. It has been found that P2Y12 receptor antagonism is not always effective due to platelets being able to directly aggregate under high shear forces without relying on soluble agonists.Citation47,Citation74 Tegretol and cangrelor are novel reversible P2Y12 receptor antagonists. Tegretol, an oral ATP structural analogue, has a faster onset of action and is often used in combination with aspirin in clinical practice.Citation75–78 GP IIb-IIIa receptor antagonists block the binding of fibrinogen and other ligands to GP IIb-IIIa and reduce aggregation under high shear forces. Eptifibatide and tirofiban are representative drugs in this class, administered intravenously. While they are effective antithrombotic drugs, the class of drugs impedes the aggregation of all circulating platelets, leading to severe bleeding complications.Citation79 PDE inhibitors regulate the hydrolytic metabolism of intracellular second messenger molecules, particularly cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). Cilostazol and dipyridamole are representative drugs that increase the concentration of cAMP and cGMP and affect intracellular signaling pathways. PAR1 antagonists block thrombin-mediated platelet activation and thereby prevent platelet aggregation.Citation80 Vorapaxar is the representative drug in this class, indicated for thrombosis-associated cardiovascular events. However, a significant increase in hemorrhage, particularly intracranial bleeding, is a major side effect.Citation81 summarizes the major antiplatelet agents and their mechanisms of action.
Figure 6. Major antiplatelet agents and mechanisms of action aspirin inhibits the synthesis of thromboxane A2, thereby exerting its antithrombotic effect. P2Y12 receptor antagonists such as clopidogrel are used to block ADP-induced platelet aggregation. Tegretol and cangrelor, being newer P2Y12 receptor antagonists, exhibit a more rapid onset of action while also being reversible. GP IIb-IIIa receptor antagonists, including eptifibatide and tirofiban, inhibit the binding of fibrinogen to GP IIb-IIIa. PDE inhibitors modulate intracellular signaling pathways by elevating the concentration of cAMP and cGMP. PAR1 antagonists such as vorapaxar block thrombin-mediated platelet activation.
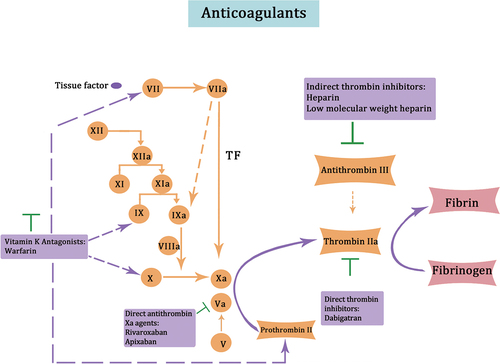
Anticoagulant drugs can be classified into indirect thrombin inhibitors, vitamin K antagonists, direct thrombin inhibitors, and direct antithrombin Xa agents.Citation82 Indirect thrombin inhibitors include heparin and low molecular weight heparin. Heparin is a natural anticoagulant that enhances the activity of antithrombin III, inhibiting the activation of coagulation factors and the coagulation process. Low molecular weight heparin, derived from the breakdown of heparin fragments, also has anticoagulant properties. Compared to heparin, it has a lower incidence of thrombocytopenia and fewer adverse effects. For vitamin K antagonists, warfarin is a representative drug that reduces the hepatic synthesis of plasminogen and factors VII, IX, and X by antagonizing vitamin K.Citation83 This drug is effective in preventing and treating thromboembolic diseases, but it has a slow and persistent effect, requiring long-term maintenance. Direct thrombin inhibitors such as dabigatran inhibit the coagulation process by directly targeting thrombin’s activity, preventing the cleavage of fibrinogen to fibrin and blocking the final step of the coagulation cascade and thrombus formation.Citation84 Direct antithrombin Xa agents like rivaroxaban and apixaban inhibit the activity of coagulation factor Xa, disrupting both endogenous and exogenous pathways of the coagulation cascade, effectively inhibiting the coagulation process. summarizes the major anticoagulant drugs and their mechanisms of action.
Figure 7. Mechanisms of action of major anticoagulant agents. indirect thrombin inhibitors, such as heparin and low molecular weight heparin, increase antithrombin III activity and inhibit the activation of coagulation factors. Vitamin K antagonists, like warfarin, antagonize vitamin K to reduce the hepatic synthesis of plasminogen, as well as factors VII, IX, and X. Direct thrombin inhibitors, such as dabigatran and sodium aprotinin, inhibit thrombin activity directly and prevent the cleavage of fibrinogen into fibrin. Direct antithrombin xa agents, such as rivaroxaban and apixaban, directly inhibit coagulation factor xa activity and inhibit the process of coagulation.
Recently, novel anticoagulants targeting coagulation factors XI and XII have emerged. There is growing evidence that FXI and FXII inhibitors prevent pathologic thrombosis and may reduce the risk of bleeding. Based on this characterization, novel FXI and FXII inhibitors are promising anticoagulants today. Currently, three different classes of FXI and FXII inhibitors are being investigated in clinical trials: (I) FXI antisense oligonucleotides (ASOs), which reduce FXI plasma levels by binding to messenger RNAs and blocking translation; (II) monoclonal antibodies and (III) small molecules, both of which act through FXI/FXII binding.Citation85 The main advantage of these novel anticoagulants is their selectivity against single molecules, which reduces the possibility of off-target adverse effects.Citation86 While most of the novel anticoagulants are still at the animal model stage, the monoclonal antibody Abelacimab is a novel, highly selective, fully human monoclonal antibody. It has a dual inhibitory effect on FXI and its active form FXIa by binding to the catalytic domain of FXI, which significantly reduces the risk of bleeding in patients with atrial fibrillation.
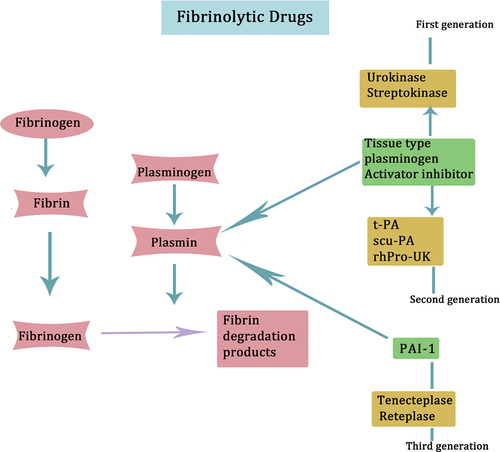
Fibrinolytic drugs directly or indirectly activate fibrinolytic zymogen, converting it into fibrinolytic enzymes. This process leads to the degradation of fibrin, the main component of thrombus, thereby promoting thrombus cleavage and effectively unclogging blood vessels. Fibrinolytic drugs can be categorized into three generations. The first generation includes urokinase and streptokinase,Citation87 which exhibit systemic fibrinolytic activation and pose an increased risk of bleeding.Citation88 The second generation comprises tissue-type plasminogen activator (t-PA), single-chain urokinase-type plasminogen activator (scu-PA), and recombinant human urokinase plasminogen (rhPro-UK). t-PA demonstrates a potent local thrombolytic effect without inducing systemic fibrinolytic activation. scu-PA has minimal antigenicity and a lower incidence of allergic reactions. rhPro-UK boasts strong thrombolytic effects, minimal bleeding risk, and a high recanalization rate. Finally, the third generation includes tenecteplase and reteplase,Citation89,Citation90 with tenecteplase exhibiting a superior ability to counteract Plasminogen Activator Inhibitor-1 (PAI-1) compared to t-PA. Reteplase, a recombinant human tissue-type plasminogen kinase derivative used in domestic clinics, boasts high recanalization rates and convenient application. provides an overview of the principal fibrinolytic medications and their respective mechanisms of action.
Figure 8. Prominent fibrinolytic drugs and their mechanisms of action are depicted. Urokinase and streptokinase represent the first generation of fibrinolytic drugs. These drugs can lead to systemic fibrinolytic activation. The second generation of fibrinolytic drugs comprises t-PA, scu-PA, and rhPro-UK. Tenecteplase and reteplase are categorized as the third generation of fibrinolytic drugs.
Understanding the mechanism of action and side effects of existing antithrombotic drugs has revealed an urgent need to investigate new antithrombotic drugs or to try to identify better combination therapy options. Laboratory monitoring is needed to help clinicians standardize the disposition of antithrombotic drug-associated bleeding and to determine whether the drug is working and whether the dose is safe. Whereas coagulation is a complex process, antithrombotic drug monitoring involves different assays. To reduce cost or time consumption and increase throughput, microfluidic microarray technology has become ideal for high-throughput drug screening. Microfluidic modeling can simulate the extracellular environment and flexibly regulate drug concentration. It helps to monitor the dose-response of drugs and avoid causing cellular damage.Citation91 The ultimate goal of functional microfluidic models is to improve patient prognosis, so future studies will analyze drug responses in different configurations of functional models to balance the risk of thrombosis and bleeding and to individualize patient treatment.Citation92
Microfluidics to assess the effects of drugs on platelets
Although tests such as the VerifyNow test, the PFA-100 test, and platelet aggregation turbidimetry to assess the inhibitory effects of drugs on platelet function are available, they suffer from the disadvantages of poor reproducibility, large sample sizes, and complexity of sample preparation, especially in terms of dose-response, which requires multiple trials.Citation73 The development of microfluidic modeling has provided a new technique for in vitro testing of antiplatelet drugs, which can be used to test the sensitivity of platelets to antiplatelet drugs and measure dose-dependent changes. Microfluidic technology can be used to study the effect of drugs on platelet activation, adhesion and aggregation, etc. by simulating the hemodynamic environment in which the drugs are administered, and it can also observe the morphological changes of platelets, such as morphological changes in the activated state and platelet stretch, etc., because the effect of drugs on platelet morphology can reflect the effect of drugs on platelet function and activation. Li R et al. explored the response of platelets to 37 kinase inhibitors in a microfluidic model. Cyclooxygenase-1 (COX-1) inhibitors, P2Y1 and P2Y12 inhibitors all reduced platelet deposition in the flow state, but individually showed “aspirin insensitivity” and non-steroidal anti-inflammatory drugs (NSAIDs) reduced the efficacy of aspirin on platelets. Rahman SM et al. evaluated the downstream adhesion effects of several antiplatelet agents and found that aspirin did not inhibit high shear-mediated platelet adhesion to the three binding proteins (fibrinogen, collagen, or vascular hemophilic factor), whereas the GP IIb-IIIa inhibitors (tirofiban and teriparatide) significantly reduced platelet adhesion to fibrinogen in the context of Antibody blockade of vWF or GP Ibα significantly inhibited downstream shear-induced platelet adhesion, suggesting that vWF binding to GP Ibα is an important event leading to irreversible platelet adhesion.Citation53 The application of microfluidic technology can assess the effects of drugs on platelets more precisely and in real time, providing an important basis for drug screening and therapeutic optimization.
Microfluidics assesses efficacy of anticoagulants and thrombolytics
Microfluidic technology allows precise and real-time assessment of the efficacy of anticoagulants and thrombolytics. By measuring parameters such as clotting time and prothrombin time, it is possible to assess the effectiveness of anticoagulants and understand their effect on coagulation.Citation93 By measuring the rate and extent of thrombin generation and simulating different anticoagulant concentrations and flow conditions, the appropriate anticoagulant dose can be screened to achieve the desired anticoagulant effect. In terms of thrombolytic agents, microfluidic technology can measure the rate of thrombolysis, observe the dynamic process of thrombolysis in real time, and assess the effect of thrombolytic agents.Citation94 Microfluidic technology can also be used to assess the effect of thrombolytic agents on the structure of thrombus and the stability of thrombus under different treatment conditions. In addition, the combination of anticoagulants and thrombolytics is commonly used to deal with severe thrombosis and thromboembolism, etc. By inhibiting thrombus formation and promoting thrombus dissolution, the harm caused by thrombus-related diseases can be mitigated, but there is also a certain risk of bleeding in the actual clinical application.Citation95,Citation96
Microfluidics can be used to study in vitro the treatment of hemophilia.Citation97 Yu X et al. have demonstrated that anti-Factor VIII or recombinant Factor IX (FIX) missense variants were added to maize trypsin inhibitor-treated healthy human blood to form hemophilia A or hemophilia B models, respectively, and then therapeutic drugs were added, and the efficacy of the various drugs was assessed by observing the platelet and fibrin aggregation rates.Citation98 Currently, the routine treatment of hemophiliacs is the intravenous administration of the required factors, a method that allows prophylactic on-demand treatment. However, some patients with severe hemophilia A may produce FVIII-neutralizing antibodies, and a few patients with hemophilia B produce fixed neutralizing antibodies, in which case they can only be treated with bypass therapies such as activated plasminogen complex concentrates or recombinant FVIIa,Citation99 however, how efficacious the drugs are needed to be simulated by more in vitro experiments, and microfluidic technology is an ideal support for such in vitro, high-throughput experiments. Citation73,Citation98,Citation100–103 summarizes the construction, principles, experimental results, and advantages and disadvantages of representative microfluidic devices used to study antithrombotic therapy.
Table V. Microfluidic devices for research related to antithrombotic therapy.
Microfluidics can be used to study the effects of coagulation factors on fibrin formation, as well as to screen suitable therapeutic agents in a high-throughput manner, which can help to narrow down the range of therapeutic agents and be used to assess the efficacy of therapeutic agents.Citation69,Citation104,Citation105
Conclusion
The application of microfluidic technology in the treatment of arterial thrombosis has made remarkable progress, while its application in drug development, personalized therapy, and surgical guidance provides new ideas and methods for the treatment of arterial thrombosis. The use of microfluidics to construct in vitro artificial microvascular models has unique advantages in the study of platelets and thrombus formation. This is reflected in our ability to precisely control the fluid shear rate and accurately mimic the structural characteristics of blood vessels in vivo. At the same time, we can independently introduce and control relevant variables affecting platelet adhesion and aggregation and thrombus formation, realizing real-time monitoring of thrombus formation. It also reduces the amount of blood samples. However, due to the precision and complexity of microfluidic chip technology, the processing and analysis of the model is still a great challenge. This challenge has limited the promotion and application of microfluidic technology in the clinic to a certain extent. Meanwhile, due to the complexity of arterial thrombus in vivo, the current research methods are often not standardized, and need to be solved urgently.
In the future, microfluidic technology will emphasize more on multifunctionality and undertake multiple tasks such as simultaneous processing of cells, biomolecules, drug screening, etc., to improve experimental efficiency and data accuracy. Microfluidics is expected to provide customized diagnostic and therapeutic solutions for each patient through rapid and precise detection and analysis, thus improving efficacy and reducing adverse reactions. However, to address the standardization and normalization of microfluidic technology, uniform experimental procedures, microchip fabrication standards, and data analysis methods need to be established to improve the reproducibility of experiments. The fabrication of microchips relies on the development of nanotechnology and 3D printing technology to achieve higher precision and stability, and combines with machine learning and artificial intelligence technology to realize the automated operation of experiments and the processing and analysis of high-throughput large-scale data. In addition, the promotion of microfluidic technology from laboratory to clinical application still faces some challenges, such as clinical validation, standardized processes, etc., and the establishment of a reliable clinical trial process is the key to solving these problems. Although the current application of microfluidics in medicine still faces some challenges, with the continuous innovation and progress of the technology, it is expected to play a greater role in the biomedical field and bring more opportunities for scientific research and medical applications.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Costa PF, Albers HJ, Linssen JEA, Middelkamp HHT, van der Hout L, Passier R, van den Berg A, Malda J, van der Meer AD. Mimicking arterial thrombosis in a 3D-printed microfluidic in vitro vascular model based on computed tomography angiography data. Lab Chip. 2017;17(16):2785–20. doi:10.1039/C7LC00202E.
- Jackson SP. Arterial thrombosis–insidious, unpredictable and deadly. Nat Med. 2011;17(11):1423–36. doi:10.1038/nm.2515.
- Liu ZL, Ku DN, Aidun CK. Mechanobiology of shear-induced platelet aggregation leading to occlusive arterial thrombosis: a multiscale in silico analysis. J Biomech. 2021;120:110349. doi:10.1016/j.jbiomech.2021.110349.
- KAREL MFA, Lemmens TP, TUllemans BME, WIelders SJ, Gubbins E, van Beurden D, van Rijt S, Cosemans JM. Characterization of atherosclerotic plaque coating for thrombosis microfluidics assays. Cell Mol Bioeng. 2022;15(1):55–65. doi:10.1007/s12195-021-00713-9.
- Yamashita A, Asada Y. Underlying mechanisms of thrombus formation/growth in atherothrombosis and deep vein thrombosis. Pathol Int. 2023;73(2):65–80. doi:10.1111/pin.13305.
- Shahidi M. Thrombosis and von Willebrand factor. Adv Exp Med Biol. 2017;906:285–306.
- Dopheide SM, Maxwell MJ, Jackson SP. Shear-dependent tether formation during platelet translocation on von Willebrand factor. Blood. 2002;99(1):159–67. doi:10.1182/blood.V99.1.159.
- Maxwell MJ, Dopheide SM, Turner SJ, Jackson SP. Shear induces a unique series of morphological changes in translocating platelets: effects of morphology on translocation dynamics. Arterioscler Thromb Vasc Biol. 2006;26(3):663–9. doi:10.1161/01.ATV.0000201931.16535.e1.
- Nesbitt WS, Tovar-lopez FJ, Westein E, Harper IS, Jackson SP. A multimode-TIRFM and microfluidic technique to examine platelet adhesion dynamics. Methods Mol Biol. 2013;1046:39–58.
- Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007;109(2):566–76. doi:10.1182/blood-2006-07-028282.
- Nesbitt WS, Kulkarni S, GIuliano S, GOncalves I, Dopheide SM, Yap CL, Harper IS, Salem HH, Jackson SP. Distinct glycoprotein Ib/V/IX and integrin αIIbβ3-dependent calcium signals cooperatively regulate platelet adhesion under flow. J Biol Chem. 2002;277(4):2965–72. doi:10.1074/jbc.M110070200.
- Umerah CO, Momodu II. Anticoagulation [M]. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- Sondag D, Verhoeven S, Löwik D, van Geffen M, Veer CV, van Heerde WL, Boltje TJ, Rutjes FPJT. Activity sensing of coagulation and fibrinolytic proteases. Chemistry. 2023;29(18):e202203473. doi:10.1002/chem.202203473.
- Griffin MT, Kim D, Ku DN. Shear-induced platelet aggregation: 3D-grayscale microfluidics for repeatable and localized occlusive thrombosis. Biomicrofluidics. 2019;13(5):054106. doi:10.1063/1.5113508.
- Tovar-lopez FJ, Rosengarten G, Nasabi M, Sivan V, Khoshmanesh K, Jackson SP, Mitchell A, Nesbitt WS. An investigation on platelet transport during thrombus formation at micro-scale stenosis. PloS One. 2013;8(10):e74123. doi:10.1371/journal.pone.0074123.
- Colace TV, Tormoen GW, MCcarty OJ, Diamond SL. Microfluidics and coagulation biology. Annu Rev Biomed Eng. 2013;15(1):283–303. doi:10.1146/annurev-bioeng-071812-152406.
- Karel M, Hechler B, Kuijpers M, Cosemans J. Atherosclerotic plaque injury-mediated murine thrombosis models: advantages and limitations. Platelets. 2020;31(4):439–46. doi:10.1080/09537104.2019.1708884.
- Garg S, Heuck G, IP S, Ramsay E. Microfluidics: a transformational tool for nanomedicine development and production. J Drug Target. 2016;24(9):821–35. doi:10.1080/1061186X.2016.1198354.
- Sarvepalli DP, Schmidtke DW, Nollert MU. Design considerations for a microfluidic device to quantify the platelet adhesion to collagen at physiological shear rates. Ann Biomed Eng. 2009;37(7):1331–41. doi:10.1007/s10439-009-9708-z.
- Ku CJ, D’Amico Oblak T, Spence DM. Interactions between multiple cell types in parallel microfluidic channels: monitoring platelet adhesion to an endothelium in the presence of an anti-adhesion drug. Anal Chem. 2008;80(19):7543–8. doi:10.1021/ac801114j.
- Kim D, Finkenstaedt-Quinn S, Hurley KR, Buchman JT, Haynes CL. On-chip evaluation of platelet adhesion and aggregation upon exposure to mesoporous silica nanoparticles. Analyst (Lond). 2014;139(5):906–13. doi:10.1039/C3AN01679J.
- Kotz F, Helmer D, Rapp BE. Emerging technologies and materials for high-resolution 3D printing of microfluidic chips. Adv Biochem Eng Biotechnol. 2022;179:37–66.
- Amirifar L, Besanjideh M, Nasiri R, Shamloo A, Nasrollahi F, de Barros NR, Davoodi E, Erdem A, Mahmoodi M, Hosseini V, et al. Droplet-based microfluidics in biomedical applications. Biofabrication. 2022;14(2):022001. doi:10.1088/1758-5090/ac39a9.
- Destefano P, BIanchi E, Dubini G. The impact of microfluidics in high-throughput drug-screening applications. Biomicrofluidics. 2022;16(3):031501. doi:10.1063/5.0087294.
- Regmi S, Poudel C, Adhikari R, Luo KQ. Applications of microfluidics and organ-on-a-chip in cancer research. Biosensors (Basel). 2022;12(7):459. doi:10.3390/bios12070459.
- Galateanu B, Hudita A, Biru EI, Iovu H, Zaharia C, Simsensohn E, Costache M, Petca R-C, Jinga V. Applications of polymers for organ-on-chip technology in urology. Polym (Basel). 2022;14(9):1688. doi:10.3390/polym14091668.
- Chliara MA, Elezoglou S, Zergioti I. Bioprinting on organ-on-chip: development and applications. Biosensors (Basel). 2022;12(12):1135. doi:10.3390/bios12121135.
- Safhi AY. Three-dimensional (3D) printing in cancer therapy and diagnostics: Current status and future perspectives. Pharmaceuticals (Basel). 2022;15(6):678. doi:10.3390/ph15060678.
- Cordes DB, Lickiss PD, Rataboul F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem Rev. 2010;110(4):2081–173. doi:10.1021/cr900201r.
- Xu H, Zhu J, Ma Q, Ma J, Bai H, Chen L, Mu S. Two-Dimensional MoS(2): Structural properties, synthesis methods, and regulation strategies toward oxygen reduction. Micromachines. 2021;12(3):240. doi:10.3390/mi12030240.
- Monia Kabandana GK, Zhang T, Chen C. Emerging 3D printing technologies and methodologies for microfluidic development [J]. Anal Methods. 2022;14(30):2885–906. doi:10.1039/D2AY00798C.
- Lee H, Fang NX. Micro 3D printing using a digital projector and its application in the study of soft materials mechanics. J Vis Exp. 2012;69:e4457. doi:10.3791/4457.
- Macdonald NP, Cabot JM, Smejkal P, Guijt RM, Paull B, Breadmore MC. Comparing microfluidic performance of three-dimensional (3D) printing platforms. Anal Chem. 2017;89(7):3858–66. doi:10.1021/acs.analchem.7b00136.
- Cordeiro AS, Tekko IA, Jomaa MH, Vora L, McAlister E, Volpe-Zanutto F, Nethery M, Baine PT, Mitchell N, McNeill DW. et al. Two-Photon polymerisation 3D printing of microneedle array templates with versatile designs: application in the development of polymeric drug delivery systems. Pharm Res. 2020;37(9):174. doi:10.1007/s11095-020-02887-9.
- Zhu W, Ma X, Gou M, Mei D, Zhang K, Chen S. 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol. 2016;40:40)103–12. doi:10.1016/j.copbio.2016.03.014.
- Au AK, Huynh W, Horowitz LF, Folch A. 3D-printed microfluidics. Angew Chem Int Ed Engl. 2016;55(12):3862–81. doi:10.1002/anie.201504382.
- HE Y, Vallières C, Alexander MR, Wildman R, Avery S. Inkjet 3D printing of polymers resistant to fungal attachment. Bio Protoc. 2021;11(9):e4016. doi:10.21769/BioProtoc.4016.
- Fina F, Madla CM, Goyanes A, Zhang J, Gaisford S, Basit AW. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int J Pharm. 2018;541(1–2):101–7. doi:10.1016/j.ijpharm.2018.02.015.
- Nahak BK, MIshra A, Preetam S, Tiwari A. Advances in organ-on-a-chip materials and devices. ACS Appl Bio Mater. 2022;5(8):3576–607. doi:10.1021/acsabm.2c00041.
- Jalili A, Bagheri M, Shamloo A, Kazemipour Ashkezari AH. A plasmonic gold nanofilm-based microfluidic chip for rapid and inexpensive droplet-based photonic PCR. Sci Rep. 2021;11(1):23338. doi:10.1038/s41598-021-02535-1.
- Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014;507(7491):181–9. doi:10.1038/nature13118.
- Wu J, Fang H, Zhang J, Yan S. Modular microfluidics for life sciences. J Nanobiotechnol. 2023;21(1):85. doi:10.1186/s12951-023-01846-x.
- Qian JY, Hou CW, Li XJ, Jin Z-J. Actuation mechanism of microvalves: a review. Micromachines. 2020;11(2):172. doi:10.3390/mi11020172.
- Araci IE, Quake SR. Microfluidic very large scale integration (mVLSI) with integrated micromechanical valves. Lab Chip. 2012;12(16):2803–6. doi:10.1039/c2lc40258k.
- Lai X, Yang M, Wu H, Li D. Modular microfluidics: current status and future prospects. Micromachines. 2022;13(8):1363. doi:10.3390/mi13081363.
- Oyama TG, Oyama K, Taguchi M. A simple method for production of hydrophilic, rigid, and sterilized multi-layer 3D integrated polydimethylsiloxane microfluidic chips. Lab Chip. 2020;20(13):2354–63. doi:10.1039/D0LC00316F.
- Nesbitt WS, Westein E, Tovar-lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient–dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15(6):665–73. doi:10.1038/nm.1955.
- Ruggeri ZM. Platelet adhesion under flow. Microcirculation (New York, NY: 1994). 2009;16(1):58–83. doi:10.1080/10739680802651477.
- Tokarev AA, Butylin AA, Ataullakhanov FI. Platelet adhesion from shear blood flow is controlled by near-wall rebounding collisions with erythrocytes. Biophys J. 2011;100(4):799–808. doi:10.1016/j.bpj.2010.12.3740.
- Cranmer SL, Ashworth KJ, Yao Y, Berndt MC, Ruggeri ZM, Andrews RK, Jackson SP. High shear–dependent loss of membrane integrity and defective platelet adhesion following disruption of the GPIbα-filamin interaction. Blood. 2011;117(9):2718–27. doi:10.1182/blood-2010-07-296194.
- Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109(12):5087–95. doi:10.1182/blood-2006-12-027698.
- Casa LD, Deaton DH, Ku DN. Role of high shear rate in thrombosis. J Vas Sur. 2015;61(4):1068–80. doi:10.1016/j.jvs.2014.12.050.
- Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Cir Remodel cannot bes. 2007;100(12):1673–85. doi:10.1161/01.RES.0000267878.97021.ab.
- Li M, Ku DN, Forest CR. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab Chip. 2012;12(7):1355–62. doi:10.1039/c2lc21145a.
- Rahman SM, Hlady V. Downstream platelet adhesion and activation under highly elevated upstream shear forces. Acta Biomater. 2019;91:135–43. doi:10.1016/j.actbio.2019.04.028.
- Gracka M, Lima R, Miranda JM, Student S, Melka B, Ostrowski Z. Red blood cells tracking and cell-free layer formation in a microchannel with hyperbolic contraction: a CFD model validation. Comp Met Prog Biomed. 2022;226:107117. doi:10.1016/j.cmpb.2022.107117.
- Tovar-Lopez FJ, Rosengarten G, Westein E, Khoshmanesh K, Jackson SP, Mitchell A, Nesbitt WS. A microfluidics device to monitor platelet aggregation dynamics in response to strain rate micro-gradients in flowing blood. Lab Chip. 2010;10(3):291–302. doi:10.1039/B916757A.
- Rahman SM, Eichinger CD, Hlady V. Effects of upstream shear forces on priming of platelets for downstream adhesion and activation. Acta Biomater. 2018;73:228–35. doi:10.1016/j.actbio.2018.04.002.
- Zhussupbekov M, Méndez rojano R, Wu WT, Massoudi M, Antaki JF. A continuum model for the unfolding of von Willebrand factor. Ann Biomed Eng. 2021;49(9):2646–58. doi:10.1007/s10439-021-02845-5.
- Zhang T, Liu L, Huang X, Gao X, Chen D, Huan X, He C, Li Y. Application of microfluidic chip technology to study the inhibitory effect of tetramethylpyrazine on platelet aggregation, activation, and phosphatidylserine exposure mediated by pathological high shear rate. Blood Coagul Fib. 2023;34(1):47–60. doi:10.1097/MBC.0000000000001179.
- Muravlev IA, Dobrovolsky AB, Antonova OA, Khaspekova SG, Mazurov AV. Effects of platelets activated by different agonists on fibrin formation and thrombin generation. Platelets. 2023;34(1):2139365. doi:10.1080/09537104.2022.2139365.
- Qi QM, Dunne E, Oglesby I, Schoen I, Ricco AJ, Kenny D, Shaqfeh ESG. In vitro measurement and modeling of platelet adhesion on VWF-Coated surfaces in channel flow. Biophy J. 2019;116(6):1136–51. doi:10.1016/j.bpj.2019.01.040.
- De witt SM, Swieringa F, Cavill R, Lamers MME, van Kruchten R, Mastenbroek T, Baaten C, Coort S, Pugh N, Schulz A, et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat Commun. 2014;5(1):4257. doi:10.1038/ncomms5257.
- Jeon HJ, Qureshi MM, LEE SY, Badadhe JD, Cho H, Chung E. Laser speckle decorrelation time-based platelet function testing in microfluidic system. Sci Rep. 2019;9(1):16514. doi:10.1038/s41598-019-52953-5.
- Luna DJ, Pandian RNK, Mathur T, Bui J, Gadangi P, Kostousov V, Hui SKR, Teruya J, Jain A. Tortuosity-powered microfluidic device for assessment of thrombosis and antithrombotic therapy in whole blood. Sci Rep. 2020;10(1):5742. doi:10.1038/s41598-020-62768-4.
- Ting LH, Feghhi S, Taparia N, Smith AO, Karchin A, Lim E, John AS, Wang X, Rue T, White NJ. et al. Contractile forces in platelet aggregates under microfluidic shear gradients reflect platelet inhibition and bleeding risk. Nat Commun. 2019;10(1):1204. doi:10.1038/s41467-019-09150-9.
- Lee H, Na W, Lee BK, Lim CS, Shin S. Recent advances in microfluidic platelet function assays: moving microfluidics into clinical applications. Clin Hemorheol Microcirc. 2019;71(2):249–66. doi:10.3233/CH-189416.
- Chen Z, Lu J, Zhang C, Hsia I, Yu X, Marecki L, Marecki E, Asmani M, Jain S, Neelamegham S. et al. Microclot array elastometry for integrated measurement of thrombus formation and clot biomechanics under fluid shear. Nat Commun. 2019;10(1):2051. doi:10.1038/s41467-019-10067-6.
- Li R, Panckeri KA, Fogarty PF, Cuker A, Diamond SL. Recombinant factor VIIa addition to haemophilic blood perfused over collagen/tissue factor can sufficiently bypass the factor IXa/VIIIa defect to rescue fibrin generation. Hae J Off World Fed Hemo. 2017;23(5):759–68. 10.1111/hae.13259.
- Kornblith LZ, Kutcher ME, Redick BJ, Calfee CS, Vilardi RF, Cohen MJ. Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Sur. 2014;76(2). discussion 62-3. doi:10.1097/TA.0000000000000108.
- Govindarajan V, Zhu S, LI R, Lu Y, Diamond SL, Reifman J, Mitrophanov AY. Impact of tissue factor localization on blood clot structure and resistance under venous shear. Biophy J. 2018;114(4):978–91. doi:10.1016/j.bpj.2017.12.034.
- Judith RM, Fisher JK, Spero RC, Fiser BL, Turner A, Oberhardt B, Taylor RM, Falvo MR, Superfine R. Micro-elastometry on whole blood clots using actuated surface-attached posts (ASAPs). Lab Chip. 2015;15(5):1385–93. doi:10.1039/C4LC01478B.
- Li R, Diamond SL. Detection of platelet sensitivity to inhibitors of COX-1, P2Y₁, and P2Y₁₂ using a whole blood microfluidic flow assay. Thromb Res. 2014;133(2):203–10. doi:10.1016/j.thromres.2013.10.043.
- Castrichini M, Luzum JA, Pereira N. Pharmacogenetics of antiplatelet therapy. Annu Rev Pharmacol Toxicol. 2023;63:211–29. doi:10.1146/annurev-pharmtox-051921-092701.
- Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9(2):154–69. doi:10.1038/nrd2957.
- Marcucci R, Berteotti M, Gragnano F, Galli M, Cavallari I, Renda G, Capranzano P, Santilli F, Capodanno D, Angiolillo DJ, et al. Monitoring antiplatelet therapy: where are we now? J Cardiovasc Med (Hagerstown). 2023;24(Suppl 1):24–35. doi:10.2459/JCM.0000000000001406.
- Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, Sibbing D, So DYF, Trenk D, Alexopoulos D, et al. International expert consensus on switching platelet P2Y12Receptor–inhibiting therapies. Circulation. 2017;136(20):1955–75. doi:10.1161/CIRCULATIONAHA.117.031164.
- Pîrlog BO, Grotta JC. The applicability of thromboelastography in acute ischemic stroke: a literature review. Semin Thromb Hemost. 2022;48(7):842–9. doi:10.1055/s-0042-1753529.
- Sharifi-rad J, Sharopov F, Ezzat SM, Zam W, Ademiluyi AO, Oyeniran OH, Adetunji CO, Roli OI, Živković J, Martorell M, et al. An updated review on glycoprotein IIb/IIIa inhibitors as antiplatelet agents: basic and clinical perspectives. High Blood Press Cardiovasc Prev. 2023;30(2):93–107. doi:10.1007/s40292-023-00562-9.
- Francis LRA, MIllington-burgess SL, Rahman T, Harper MT. Q94 is not a selective modulator of proteinase-activated receptor 1 (PAR1) in platelets. Platelets. 2022;33(7):1090–5. doi:10.1080/09537104.2022.2026911.
- Xiao T, Ren S, Bao J, Gao D, Sun R, Gu X, Gao J, Chen S, Jin J, Wei L, et al. Vorapaxar proven to be a promising candidate for pulmonary fibrosis by intervening in the PAR1/JAK2/STAT1/3 signaling pathway-an experimental in vitro and vivo study. Eur J Pharmacol. 2023;943:175438. doi:10.1016/j.ejphar.2022.175438.
- Yin Q, Zhang X, Liao S, Huang X, Wan CC, Wang Y. Potential anticoagulant of traditional Chinese medicine and novel targets for anticoagulant drugs. Phytomedicine. 2023;116:154880. doi:10.1016/j.phymed.2023.154880.
- Heidbuchel H, Verhamme P, Alings M, Antz M, Diener H-C, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P, et al. updated European heart rhythm association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467–507. doi:10.1093/europace/euv309.
- Sawetaji S, Aggarwal KK. A non-competitive serpin-like thrombin inhibitor isolated from moringa oleifera exhibit a high affinity for thrombin. Protein J. 2023;42(4):305–15. doi:10.1007/s10930-023-10116-6.
- Cohen O, Ageno W. Coming soon to a pharmacy near you? FXI and FXII inhibitors to prevent or treat thromboembolism. Hematology Am Soc Hematol Educ Program. 2022;2022(1):495–505. doi:10.1182/hematology.2022000386.
- Fredenburgh JC, Weitz JI. New anticoagulants: moving beyond the direct oral anticoagulants. J Thrombosis Haemostasis. 2021;19(1):20–9. doi:10.1111/jth.15126.
- Zia MA. Streptokinase: An Efficient Enzyme in Cardiac Medicine. Ppl. 2020;27(2):111–19. doi:10.2174/0929866526666191014150408.
- Shen M, Wang Y, Hu F, Lv L, Chen K, Xing G. Thrombolytic agents: nanocarriers in targeted release. Molecules. 2021;26(22):6776. doi:10.3390/molecules26226776.
- Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327(7):662–75. doi:10.1001/jama.2022.0358.
- Mohammadi E, Mahnam K, Jahanian-najafabadi A, Sadeghi HMM. Design and production of new chimeric reteplase with enhanced fibrin affinity: a theoretical and experimental study. J Biomol Struct Dyn. 2021;39(4):1321–33. doi:10.1080/07391102.2020.1729865.
- Sun J, Warden AR, Ding X. Recent advances in microfluidics for drug screening. Biomicrofluidics. 2019;13(6):061503. doi:10.1063/1.5121200.
- Wu H, Shi S, Liu Y, Zhang Q, Lam RHW, Lim CT, Hu J. Recent progress of organ-on-a-chip towards cardiovascular diseases: advanced design, fabrication, and applications. Biofabrication. 2023;15(4). doi:10.1088/1758-5090/acdaf9.
- Harris LF, Rainey P, Castro-López V, O’Donnell JS, Killard AJ. A microfluidic anti-factor xa assay device for point of care monitoring of anticoagulation therapy. Analyst (Lond). 2013;138(17):4769–76. doi:10.1039/c3an00401e.
- Loyau S, Ho-Tin-Noé B, Bourrienne MC, Boulaftali Y, Jandrot-Perrus M. Microfluidic modeling of thrombolysis. ATVB. 2018;38(11):2626–37. doi:10.1161/ATVBAHA.118.311178.
- Meador S, Dyke S, Togami J, Kuskov B, Burnett AE. Antithrombosis stewardship efforts to de-escalate inappropriate combined therapy in outpatient clinics. J Thromb Thrombolysis. 2022;53(2):436–45. doi:10.1007/s11239-021-02551-y.
- Zhang ZX, Schroeder-Tanka J, Stooker W, Wissen S, Khorsand N. Management of combined oral antithrombotic therapy by an antithrombotic stewardship program: a prospective study. Br J Clin Pharmacol. 2022;88(9):4092–9. doi:10.1111/bcp.15346.
- Trevisan BM, Porada CD, Atala A, Almeida-Porada G. Microfluidic devices for studying coagulation biology. Sem Cell Develop Bio. 2021;112:1–7. doi:10.1016/j.semcdb.2020.06.002.
- Yu X, Panckeri KA, Ivanciu L, Camire RM, Coxon CH, Cuker A, Diamond SL. Microfluidic hemophilia models using blood from healthy donors. Res Pract Thromb Haemost. 2020;4(1):54–63. doi:10.1002/rth2.12286.
- Astermark J, Donfield SM, Dimichele DM, Gringeri A, Gilbert SA, Waters J, Berntorp E. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven comparative (FENOC) study. Blood. 2007;109(2):546–51. doi:10.1182/blood-2006-04-017988.
- Li R, Fries S, Li X, Grosser T, Diamond SL. Microfluidic assay of platelet deposition on collagen by perfusion of whole blood from healthy individuals taking aspirin. Clin Chem. 2013;59(8):1195–204. doi:10.1373/clinchem.2012.198101.
- Rahman SM, Hlady V. Microfluidic assay of antiplatelet agents for inhibition of shear-induced platelet adhesion and activation. Lab Chip. 2021;21(1):174–83. doi:10.1039/D0LC00756K.
- Jahn K, Suchodolski K, Schäfer A, Sahlmann B, Küster U, Echtermeyer F, Calmer S, Theilmeier G, Johanning K. Effect of clopidogrel on thrombus formation in an ex vivo parallel plate flow chamber model cannot be reversed by addition of platelet concentrates or vWF concentrate. Anesth Analg. 2017;124(4):1091–8. doi:10.1213/ANE.0000000000001903.
- Herfs L, Swieringa F, Jooss N, Kozlowski M, Heubel-Moenen FCJ, van Oerle R, Machiels P, Henskens Y, Heemskerk JWM. Multiparameter microfluidics assay of thrombus formation reveals increased sensitivity to contraction and antiplatelet agents at physiological temperature. Thromb Res. 2021;203:46–56. doi:10.1016/j.thromres.2021.04.014.
- Onasoga-jarvis AA, Leiderman K, Fogelson AL, Wang M, Manco-Johnson MJ, Di Paola JA, Neeves KB. The effect of factor VIII deficiencies and replacement and bypass therapies on thrombus formation under venous flow conditions in microfluidic and computational models. PloS One. 2013;8(11):e78732. doi:10.1371/journal.pone.0078732.
- Colace TV, Fogarty PF, Panckeri KA, Li R, Diamond SL. Microfluidic assay of hemophilic blood clotting: distinct deficits in platelet and fibrin deposition at low factor levels. J Thromb Haemost. 2014;12(2):147–58. doi:10.1111/jth.12457.
