Abstract
Blood concentrates like platelet rich fibrin (PRF) have been established as a potential autologous source of cells and growth factors with regenerative properties in the field of dentistry and regenerative medicine. To further analyze the effect of PRF on bone tissue regeneration, this study investigated the influence of liquid PRF matrices on human healthy primary osteoblasts (pOB) and co-cultures composed of pOB and human dermal vascular endothelial cells (HDMEC) as in vitro model for bone tissue regeneration. Special attention was paid to the PRF mediated influence on osteoblastic differentiation and angiogenesis. Based on the low-speed centrifugation concept, cells were treated indirectly with PRF prepared with a low (44 g) and high relative centrifugal force (710 g) before the PRF mediated effect on osteoblast proliferation and differentiation was assessed via gene and protein expression analyses and immunofluorescence. The results revealed a PRF-mediated positive effect on osteogenic proliferation and differentiation accompanied by increased concentration of osteogenic growth factors and upregulated expression of osteogenic differentiation factors. Furthermore, it could be shown that PRF treatment resulted in an increased formation of angiogenic structures in a bone tissue mimic co-culture of endothelial cells and osteoblasts induced by the PRF mediated increased release of proangiogenic growth factors. The effects on osteogenic proliferation, differentiation and vascularization were more evident when low RCF PRF was applied to the cells. In conclusion, PRF possess proosteogenic, potentially osteoconductive as well as proangiogenic properties, making it a beneficial tool for bone tissue regeneration.
Plain Language Summary
What is the context?
The treatment of bone defects is still a challenge in the field of regenerative medicine. In this context, researchers and clinicians are continuously focusing on developing new therapeutic strategies like the use of autologous blood concentrates like Platelet rich fibrin (PRF) to improve regeneration by directly delivering wound healing promoting cells and growth factors to the defect side in order to restore the structure and functional integrity of damaged hard tissue in combination with adequate tissue regeneration.
What is new?
Focus of the present in vitro study was to further evaluate the potential of PRF paying particular attention to the PRF-mediated effect on osteogenic differentiation and angiogenesis of human primary osteoblasts as well as on a more complex tissue like co-culture consisting of osteoblasts and microvascular endothelial cells. We could demonstrate that PRF is able to support and affect a variety of processes involved in bone tissue regeneration including osteogenic proliferation, osteogenic differentiation as well as angiogenic structure formation.
Treatment of PRF resulted in:
- increased cell viability*
- higher expression of osteogenic differentiation factors*
- higher release of osteogenic growth factors*
- increased formation of microvessel-like structures*
(*compared to untreated control)
What is the impact?
PRF represents a beneficial autologous tool for regenerative purposes combining proosteogenic and proangiogenic properties. Therefore, PRF might be used for applications in versatile fields of medicine in the context of improving bone tissue regeneration.
Introduction
Tissue regeneration is of crucial importance in medical interventions and in the restoration of health, thereby being of great significance in all medical fields. Consequently, there is a need to therapeutically increase and control the process of regeneration to minimize complications and to maintain the patient’s comfort while achieving an optimal and long-term clinical outcome.Citation1,Citation2 A particular challenge in the field of regenerative medicine is the treatment of bone defects since autologous bone transplants are associated with major risks such as infections, pain, hematoma, nerve damage, and scarring. Therefore, clinicians are continuously focusing on finding alternatives to restore the structure and functional integrity of damaged hard tissue in combination with adequate tissue regeneration.Citation3,Citation4 While the interaction of immune cells, the assembly of extracellular matrix components and the release of growth factors and cytokines have a significant impact on the process of wound healing and regeneration process, researchers have started to develop new therapeutic strategies like the use of autologous blood concentrates improving regeneration by directly delivering wound healing promoting cells and growth factors to the defect side. In recent years, a variety of blood concentrates such as platelet rich plasma and platelet rich fibrin gained significant attention in the field of dentistry and regenerative medicine.Citation5–8 Platelet rich fibrin is a second-generation blood concentrate obtained by collecting and centrifuging the patient´s own blood that has been established as a convenient tool for a wide range of medical and dental indications such as guided bone regeneration, implantation and regeneration of periodontal intrabody defects.Citation9,Citation10 In addition, it has already been shown that PRF can be widely utilized for the biologization of biomaterials such as bone substitute materials and collagen membranes to trigger a material-induced tissue healing response.Citation11–13 In this regard, clinical trials have revealed that PRF treatment reduces pain development and improves tissue regeneration while minimizing the occurrence of complications such as scarring and inflammation.Citation13–15 Several in vitro studies regarding the composition of PRF identified PRF as a natural, endogenous source of high concentrations of growth factors, cytokines, fibrin and cells such as leukocytes, platelets and stem cells.Citation10,Citation16,Citation17 With respect to the cell content and growth factor release, previous studies have revealed that the relative centrifugation force (RCF) affect the composition of PRF. Since it could be shown that a higher concentration of cells and growth factors could be observed when the RCF was reduced, the low-speed centrifugation concept (LSCC) was introduced.Citation18 Furthermore, by providing multiple growth factors such as Platelet derived growth factor (PDGF), Transforming growth factor ß (TGF-β), epidermal growth factor (EGF) and Vascular endothelial growth factor (VEGF), PRF has been found to have a positive impact on wound healing and regeneration associated processes.Citation17,Citation19,Citation20 Although a number of studies are dealing with the evaluation of the effect of PRF in vitro, there is little recent evidence regarding the effects of indirectly applied PRF on the proliferation and differentiation capacity of human healthy osteoblasts or more complex in vitro human bone tissue mimics. During this study, the potential PRF mediated effect on human healthy primary osteoblasts (pOBs) has been evaluated with regard to cell proliferation, osteogenic differentiation, growth factor content and gene expression analyses of osteogenic differentiation factors. In addition, since a better understanding of bone healing mechanisms can be generally assisted by more complex tissue-like constructs,Citation21 the effect of PRF was also analyzed in an innovative in vitro co-culture model consisting of primary osteoblasts and primary endothelial cells with regard to angiogenesis and angiogenesis associated factors.
Materials and methods
Ethical statement
All cells that were used for this study were obtained from excess tissue and their application was in accordance with the principle of informed consent and approved by the responsible Ethics Commission of the state Hessen, Germany. In addition, the application of PRF in this study was approved by the responsible Ethics Commission of the state of Hessen, Germany (265/17) and all donors gave informed consent to the use of their blood for study purposes.
Primary cells
Human primary osteoblasts (pOB) were obtained from healthy cranial bone obtained as excess tissue during surgery. Cells were isolated from bone fragments according to an established protocolCitation22 and were cultivated in Dulbecco´s Modified Eagle´s Medium F-12 Ham (Sigma-Aldrich, St. Louis, USA) supplemented with 10% FCS and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, USA) at 37°C in a humidified atmosphere. Primary osteoblasts were utilized up to passage 5. Human dermal microvascular endothelial cells were isolated from healthy tissue remnants obtained during lip operations and according to an established protocol.Citation23 HDMECs were cultivated in T-25 cell culture flasks previously coated with gelatin (0.02%) in Endothelial Cell Growth Medium MV containing 1% penicillin/streptomycin.
Preparation of PRF
Whole blood was collected from at least 3 donors of all genders who gave informed consent to participate in the study. The donors were healthy and free of infectious diseases, did not take anticoagulants, nor did they consume alcohol or nicotine. Their ages ranged from 20 to 50 years. For PRF preparation, 10 ml of peripheral blood was drawn from the median cubital vein and collected into plastic-coated PRF tubes (Process for SYBR®, Nice, France). The blood was immediately centrifuged in a Duo centrifuge (Cologne, Mectron) with a 110 mm radius fixed angle rotor. Based on the LSCC, the high RCF PRF protocol and the low RCF PRF protocol were implemented in our studies (). After centrifugation, the top clear, yellow-colored layer of liquid PRF was collected using a syringe individually for each donor in sterile 15 ml tubes and then homogenized and the still liquid PRF was used for cell culture experiments.
Table I. Centrifugation protocols according to the low speed centrifugation concept (LSCC). In all experimental set-ups, the high (710 ×g) and the low (44 ×g) centrifugation protocols were selected for comparison.
Cultivation of primary osteoblasts with PRF
For indirect PRF application, 100.000 human pOBs per well were pre-seeded in 24-well plates and cultivated in DMEM F-12 Ham containing 10% FCS and 1% penicillin/streptomycin for 24 h. For the separation of PRF and the pre-seeded cells, trans-well inserts with a pore size of 0.4 μm were utilized allowing only growth factors and signaling molecules to pass through the membrane, but not the cells. Therefore, the trans-wells were placed in the pre-seeded 24-well plates and either 100 μL of low RCF PRF or 100 μL of high RCF PRF was added directly into the trans-wells. Wells containing solely human pOBs without the addition of PRF served as controls. After the clotting process of PRF was completed, 1 ml of DMEM F-12 Ham containing 10% FCS and 1% penicillin/streptomycin was added to the lower compartment of the wells. An additional 100 μl of medium was added to the inner part of each trans-well to prevent the PRF from drying. Cells and cell/PRF complexes were cultivated for 9 days. Supernatants were collected after 2, 3, 7 and 9 days and stored for ELISA. Finally, the cells were analyzed for cell viability after 3, 7 and 9 days as well as fixed for immunofluorescence staining after 2 days and processed for gene expression analyses after 7 days of PRF treatment.
Co-culture experimentation
10.000 human pOBs/well and 10.000 HDMECs/well were mixed and co-seeded on thermanox™ cell culture coverslips (Karlsruhe, Thermo Fisher Scientific) in 24-well plates cultivated in Endothelial Cell Growth Medium MV with 1% penicillin/streptomycin. After 24 h, a trans-well filter system with a pore structure of 0.4 μm was inserted into the wells. 100 μl of PRF was added to each trans-well. Co-culture/PRF complexes as well as controls without PRF were cultured for 7 and 14 days. Supernatants were collected and stored for ELISA after 4, 7, 10 and 14 days. In addition, cells were fixed for immunofluorescence staining after 7 and 14 days of cultivation.
Cell viability assay
The effect of indirect PRF treatment on cell viability of pOBs was examined after 3, 7 and 9 days of cultivation. After washing the wells with PBS, DMEM F-12 Ham containing 10% FCS and 1% penicillin/streptomycin and 100 µl CellTiter 96ⓇAQueous One Solution Reagent (Promega, Madison, USA) were added followed by an incubation period of 1 h at 37°C. 100 μl from each well was transferred to a separate 96-well plate and absorbance was measured at 490 nm in a microplate reader (GENios plus, TECAN, Crailsheim, Germany).
Immunofluorescence staining
For immunofluorescence staining cells were fixed in 4% buffered formalin (Roti-Histofix 4%, acid-free pH7, Carl-Roth, Germany) washed 3 times with phosphate-buffered saline (PBS), permeabilized for intracellular antigens with 0.1% Triton-X100/PBS and incubated with the specific primary antibody (mouse alpha-smooth muscle actin) diluted in 1% BSA/PBS solution (1:2000, Dako), for 1 h at RT (room temperature). Fluorescently labeled secondary antibodies (Alexa fluor 488 anti-mouse) were diluted in 1% BSA/PBS and incubated in a dark environment for 1 h at RT. Thereafter, the cell nuclei were counterstained with DAPI diluted in 1% BSA/PBS (1:1000). The fluorescent D-LEDI LED illumination system of the Eclipse Ni/E with the Nikon DS-Ri2 camera (Nikon eclipse Ni/E, Düsseldorf, Germany) was used for evaluation.
Image quantification
Three randomly chosen immunofluorescently stained (CD31) images from each experimental group of the co-culture experiments were examined at 20-fold magnification for the presence of CD31-positive stained vascular structures. The Eclipse Ni/E fluorescence microscope with a DS-Ri2 camera (Nikon, Düsseldorf, Germany) was used to acquire the images. When quantifying the images in Nikon´s NIS-elements program (Nikon, Düsseldorf, Germany), CD31-positive stained vascular structures were manually selected with the measurement tool and total length of vascular structures in pixels were exported to MS Excel. The values were calculated in percentage by setting the control cultures to 100% as a reference value. Samples without the addition of PRF served as controls.
Enzyme-linked immunosorbent assay (ELISA)
The collected cell culture supernatants were quantified for relative growth factor concentrations of TGF-β, PDGF-BB and VEGF using ELISA-DuoSet development system (R&D Systems) according to the manufacturer´s instructions. A microplate reader (Infinite M200, Tecan, Crailsheim, Germany) detected the optical density of each well at a wavelength of 450 nm.
RNA isolation and gene expression analyses
RNA isolation was performed using the RNeasy Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer´s instructions. Using the standard protocol of Qiagen´s Omniscript reverse transcription kit, 1 μg of the extracted RNA per sample was transcribed into complementary DNA (cDNA). The relative gene expression of osteogenic differentiation factors was analyzed using specific primers for BMP-1, BMP-2, col1, osteonectin (ON), osteopontin (OPN) and alkaline phosphatase (ALP). For quantitative reverse transcription polymerase chain reaction (qRT-PCR), the RT-PCR cycler StepOnePlus by Applied Biosystems was implemented. SYBR® green was utilized as a DNA-binding fluorescent dye. Analyses were performed in triplicates with a standardized cycler program: 94°C for 2 minutes, 94°C for 15 s and 60°C for 1 minute. The reactions were run for 40 cycles. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the endogenous standard. The relative gene expression was determined using the ∆∆Ct method. As a reference value, the control cultures were set to one.
Statistical evaluation
All experiments were performed with at least three different donors. The data presented were evaluated as mean ± standard deviation (SD). Statistical significance was calculated with the one-way multifactorial variance analysis ANOVA test or t-test using graphed pad prism 9 (GraphPad Software Inc.). Statistical significance was assessed when * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001 and documented in the figures.
Results
PRF-mediated effect on cell morphology and cell viability of pOBs
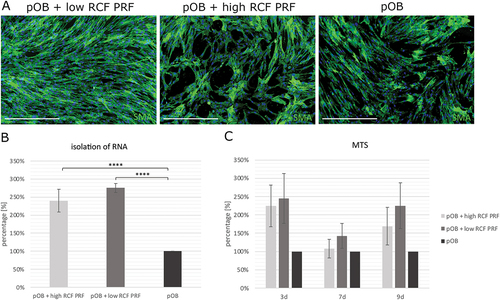
Indirect application of PRF aimed to investigate the PRF mediated effect on the morphology and proliferation of pOBs. In this regard, monocultured pOBs were stained for smooth muscle actin antibody (SMA) by immunofluorescent staining and counterstained with 4′,6-Diamidino-2-phenylindole (DAPI) (). In general, a higher cell amount could be observed in pOBs treated with PRF compared to pOBs without indirect PRF treatment. Primary osteoblasts treated with low RCF PRF formed a confluent cell layer covering the entire bottom of the cell culture well. In low RCF PRF treated samples, the cell number was observed to be higher than in pOBs treated with high RCF PRF. In pOBs without PRF treatment and pOBs treated with high RCF PRF, the cell layer was found to be semiconfluent and cell distribution and cell arrangement resembled a structure with gaps. In addition, the determination of the relative number of viable cells revealed a significant higher amount of pOBs when treated indirectly with PRF compared to pOBs without PRF treatment at all time points (). When measuring the total RNA concentration isolated from monocultured pOBs with and without PRF treatment after 2 days of cultivation, a statistically significant higher amount of total RNA could be detected in samples cultivated with PRF compared to pOBs without PRF treatment. In this regard, the amount of isolated RNA in samples of pOBs treated with low RCF PRF tended to be higher compared to pOBs treated with high RCF PRF ().
Figure 1. A. Analysis of the morphology of monocultured pOB with and without PRF treatment after 2 days. POBs were stained for SMA (green) and DAPI (blue) by immunofluorescence staining. B. Isolation of RNA from monocultured pOBs treated with PRF compared to untreated pOBs after 2 days. C. Effect of indirectly applied PRF on the viability of pOBs seeded in monoculture after 3, 7 and 9 days. Statistical significance was assessed by ****p < 0.0001. Scale bars: 500 μm.
Influence of PRF on osteogenic differentiation of pOB
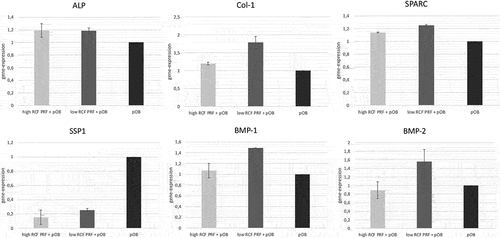
For evaluation of the influence of PRF on osteogenic differentiation, gene expression levels of osteogenic differentiation factors (ALP, col-1, SPARC, SPP1, BMP-1, BMP-2) were assessed in samples of pOBs with and without indirect PRF treatment for 7 days (). A higher expression of ALP (alkaline phosphatase), col-1 (collagen type 1), BMP-1 (bone morphogenic protein 1), BMP-2 and SPARC (osteonectin) could be determined in PRF treated pOBs compared to untreated pOBs. Primary osteoblasts treated with low RCF PRF appeared to express higher levels of col-1, BMP-1, BMP-2 and SPARC compared to pOBs treated with high RCF PRF. Evaluation of the SPP1 (osteopontin) gene expression was found to be downregulated when pOBs were combined with PRF compared to untreated pOBs.
Figure 2. Determination of the relative gene expression of osteogenic differentiation factors in cultures of pOBs with and without indirect PRF treatment using specific primers for alkaline phosphatase (ALP), collagen type 1 (col-1), osteonectin (SPARC), osteopontin (SPP1), BMP-1 and BMP-2 by real time PCR after a 2-day incubation period.
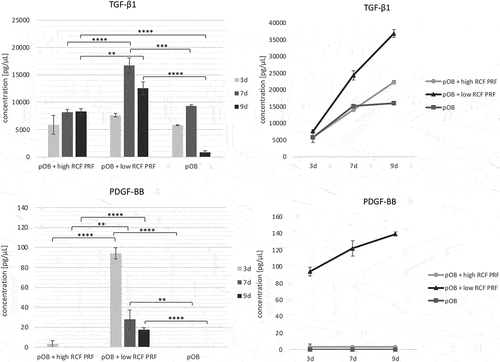
Determination of osteogenic growth factors in pOBs in response to PRF
The protein concentration of the osteogenic growth factors TGF-β1 and PDGF-BB was determined after 3, 7 and 9 days in supernatants of indirect PRF treated pOBs compared to controls (). On day 3, TGF-β1 levels did not differ significantly when supernatants of untreated pOBs were compared to supernatants of pOBs treated with PRF. On the 7th day, supernatants of pOBs treated with low RCF PRF contained statistically significant higher TGF-β1 levels compared to the other experimental groups, while TGF-β1 values of untreated pOB and pOB treated with high RCF PRF hardly differed. After a cultivation period of 9 days, TGF-β1 concentrations in supernatants of pOBs treated with low and high RCF PRF were statistically significant higher compared to supernatants of untreated pOB. In this regard, low RCF PRF treated pOBs contained statistically significant higher levels of TGF-β1 than supernatants of pOBs treated with high RCF PRF. The cumulative TGF-β1 concentration increased in all experimental groups during the course of cultivation from the 3rd to the 7th day. PDGF-BB concentration in supernatants of pOBs treated with low RCF PRF was statistically significant higher compared to supernatants of the other experimental groups at all time points. In this regard, the highest PDGF-BB concentrations could be measured after 3 days of cultivation. After a cultivation period of 7 and 9 days, the detectable PDGF concentrations decreased. While supernatants of pOBs treated with high RCF PRF contained low levels of PDGF-BB on day 3, PDGF-BB values could not be measured in supernatants at any of the other time points. In supernatants of monocultured pOBs without PRF treatment, PDGF-BB was also not detectable at any tested time point.
Analysis of microvessel-like structure formation of HDMECs in co-culture with pOBs in response to PRF
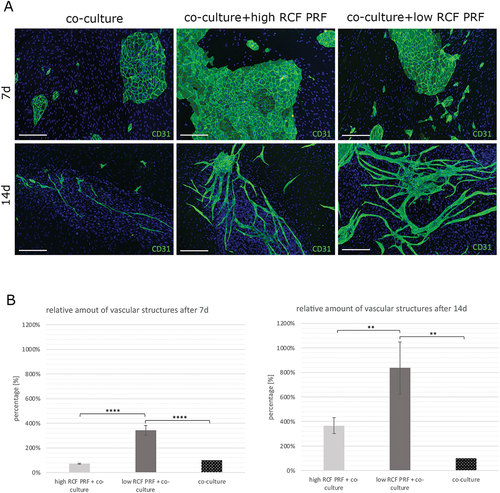
Co-culturing of human dermal microvascular endothelial cells (HDMECs) and primary osteoblasts (pOBs) followed by indirect PRF treatment aimed to investigate the effect of PRF on vascular structure formation after 7 and 14 days. The activation of endothelial cells within the co-culture system for angiogenic structure formation was visualized by immunofluorescent staining for the endothelial marker PECAM-1 (CD31) (). In co-culture systems without the addition of PRF (control group) and with the addition of high RCF PRF, the typical cobblestone-like morphology of aggregated HDMECs without the formation of vascular structures became visible after 7 days. In comparison, co-cultures cultivated with low RCF-PRF revealed the formation of vessel structures after 7 days of cultivation. After a cultivation period of 14 days, the HDMEC aggregates started to sprout out and exhibited an increasing formation of microvessel-like structures in all experimental groups. While co-cultures treated with low RCF PRF revealed the highest increase in vascular structure formation, the amount of microvessel-like structure formation was lower in co-cultures treated with high RCF PRF and co-cultures without PRF treatment. The quantitative evaluation of vascular structure formation confirmed the results (). PRF treatment resulted in an increased percentage of vascular structure formation and induced a more complex network of angiogenic structures compared to co-cultures without PRF treatment after 7 and 14 days. In this regard, the addition of low RCF PRF increased microvessel-like structure formation up to 700% and the addition of high RCF PRF increased micro-vessel like structure formation up to 250% after 14 days of cultivation. A statistically significant higher percentage of angiogenic structures could be comparatively quantified in co-cultures treated with low RCF PRF compared to co-cultures treated with high RCF PRF and untreated co-cultures after 7 and 14 days. In addition, all experimental groups showed a higher number of CD31 negative and DAPI-positive cells (pOBs) assembling around CD31 positive microvessel-like structures after 14 days of culture, whereas they were more uniformly distributed after 7 days of cultivation ().
Figure 4. Analysis of microvessel-like structure formation in an in vitro co-culture model for bone tissue engineering. A. Immunofluorescence staining for the endothelial cell type specific marker CD31 of co-cultures consisting of HDMEC and pOB. Co-cultures were either treated with PRF or without PRF (control group) for 7 (upper row) and 14 (lower row) days. B. Evaluation of the relative amount of vascular structure formation after 7 and 14 days. Statistical significance was assessed by **p < 0.01 and ****p < 0.0001. Scale bars 150 μm.
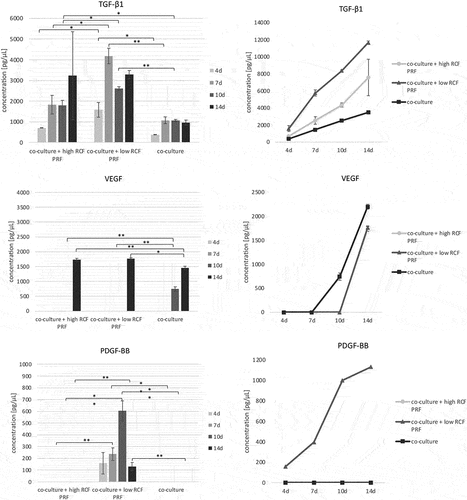
Determination of growth factor content in co-cultured pOBs and HDMECs treated with PRF
The protein concentrations of the growth factors TGF-β1, PDGF-BB and VEGF were determined in supernatants of co-cultured pOBs and HDMECs after 4, 7, 10 and 14 days (). From the 4th to the 14th day, increasing cumulative TGF-β1 concentrations could be detected in all experimental groups. In general, co-cultures treated with low RCF PRF revealed significantly higher TGF-β1 levels compared to the other experimental groups after a cultivation period of 4, 7 and 10 days, while they contained similar amounts of TGF-β1 compared to high RCF PRF after 14 days of cultivation. When comparing co-cultures treated with high RCF PRF and untreated co-cultures, co-cultures treated with high RCF PRF revealed higher concentrations of TGF-β1 in cell culture supernatants at all time points evaluated. This was statistically significant after a cultivation period of 10 days. After 10 days of cultivation, VEGF concentrations were only detectable in supernatants of the control group and were assessed as statistically significant higher compared to supernatants of co-cultures treated with PRF. In turn, VEGF concentrations were statistically significant higher in supernatants of co-cultures treated with PRF compared to supernatants of untreated co-cultures after 14 days. Statistically significant higher PDGF-BB concentrations could be observed in supernatants of co-cultures treated with low RCF PRF compared to the other experimental groups at all time points evaluated. While no PDGF-BB concentrations were detectable in supernatants of the other experimental groups, PDGF-BB content was increasingly detectable in supernatants from the 4th to the 10th day in co-cultures treated with low RCF PRF and decreased again on the 14th day.
Discussion
In order to improve wound healing and regeneration processes, blood concentrates like platelet rich fibrin gained significant attention in the field of dentistry and regenerative medicine since PRF provides important immune cells and growth factors with regenerative properties.Citation5,Citation10,Citation17,Citation18,Citation24 Focus of the present study was to further elucidate the potential of PRF paying particular attention to the PRF mediated effect on osteogenic differentiation and angiogenesis. Investigating the effect of PRF on human primary osteoblasts as well as on a more complex tissue-like co-culture consisting of osteoblasts and microvascular endothelial cells, our study could demonstrate that PRF is able to support and affect a variety of processes, including osteogenic proliferation, osteogenic differentiation as well as angiogenic structure formation. Our results could assess a PRF mediated positive effect on the proliferation of pOBs resulting in generally higher cell numbers when pOBs were treated with PRF consistent other studies, which showed a PRF-mediated higher number of viable osteoblasts after 1 day of cultivation.Citation25 Based on the present results, application of low RCF PRF could be assessed as more effective in increasing osteoblast proliferation than high RCF PRF. This may be due to its higher concentrated cells such as platelets and leukocytes and and their released growth factor concentrations such as TGF-β and PDGF-BB that stimulate osteoblast to proliferate.Citation26–28 These growth factors could be found to be increased within supernatants of pOB treated with low RCF PRF. Platelets not only evoke an proosteogenic potential through the release of growth factors, but they have also been found to accelerate osteogenic proliferation through cellular components such as microparticles and cell membranes. In addition, the fibrin matrix of PRF may also attribute to an increase in proliferative capacity under certain experimental circumstances, as previously outlined in other studies.Citation29,Citation30 Besides the positive effect of PRF on osteoblast proliferation, the expression of osteogenic differentiation factors like ALP, col-1, osteonectin, BMP-1 and BMP-2 mRNA was found to be upregulated in response to PRF treatment after 7 days of culture. This phenomenon was particularly noticeable when pOBs were treated with low RCF PRF instead of high RCF PRF. This coincided with the release of platelet and leukocyte derived growth factors TGF-β and PDGF-BB, which have been shown to induce early osteogenic differentiation.Citation26,Citation31 Col-1 and ALP are characteristic markers for the early stage of osteogenic differentiation as they have already been found to be expressed in preosteoblasts. ALP activity and the expression of col-1 are increasingly upregulated according to the degree of maturity of osteoblasts.Citation32 Therefore, PRF may induce osteoblast differentiation, which in turn results in the upregulated expression of ALP and col-1. While our study revealed that ALP gene expression was upregulated in response to PRF after 7 days of cultivation, another study demonstrated a steady increase in ALP activity expressed by PRF treated osteoblast over 28 days.Citation33 Furthermore, due to the upregulated expression of col-1 mRNA, a mediating role in bone matrix formation can be ascribed to PRF, since col-1 is not only an important organic extracellular matrix component of bone, but also provides a scaffold for the attachment of apatite crystals.Citation34 Another study also confirmed that collagen-related proteins were upregulated by PRF treatment, supporting our findings.Citation35 In addition, growth factors present in PRF such as TGF-β1 support this effect by positively stimulating osteoblasts for collagen synthesis.Citation36 BMP-1 and BMP-2 are highly involved in bone formation and matrix mineralization by affecting osteoblast differentiation, collagen maturation and the accumulation of bone matrix components.Citation26,Citation37–39 Matrix mineralization is characteristic of the late stage of the osteoblastic differentiation process and is influenced by factors such as Osteonectin (ON), Osteopontin (OPN) and alkaline phosphatase (ALP).Citation32 The non-collagenous proteins ON and OPN are indicative of mature osteoblasts and these proteins form a part of the organic bone matrix where they facilitate matrix mineralization by promoting the incorporation of hydroxyapatite crystals into the bone matrix by specifically coupling organic collagen and inorganic hydroxyapatite with their binding sites.Citation40,Citation41 In contrast to ON, OPN expression tended to be downregulated, when PRF was indirectly applied to pOBs. Since PRF apparently stimulates and accelerates the osteogenic differentiation process, it is possible that OPN expression might be beyond mRNA regulation at the time measured and is already present as a finished protein. The upregulation of osteogenic marker mRNA (ALP, OPN, col-1, BMP-2) has been also demonstrated in a study that co-cultivated neutrophils with osteoblasts and endothelial cells.Citation42 Since PRF has been shown to contain leukocytes, osteogenic differentiation could be also due to the action of these immune cells. Previous studies have shown that the white blood cell concentration is particularly higher in low RCF-PRF compared to high RCF-PRF, supporting our finding that low RCF-PRF upregulates gene expression of osteogenic differentiation factors.Citation17,Citation18 In addition, several other studies underlined the PRF mediated effect on the processes of osteogenic differentiation and mineralization, while the precise regulatory mechanisms of PRF remain to be elucidated.Citation33,Citation35,Citation43 In conclusion, it can be supposed that PRF activates the osteogenic differentiation process in pOBs towards more mature osteoblasts. Adequate tissue perfusion is another essential factor for bone regeneration since vascularization allows cell recruitment, growth factor delivery, removal of waste products and the supply of nutrients and oxygens.Citation44,Citation45 For analyzation of the proangiogenic potential of PRF in a more bone tissue like construct, our studies used an established co-culture model consisting of pOBs and HDMECs.Citation21 After a cultivation period of 7 and 14 days, co-cultures were stained by immunofluorescence for endothelial cell specific marker to visualize microvessel-like structure formation. It could be observed that PRF not only significantly stimulated the formation of microvessel-like structures, but also reduced the time of this formation. After 7 days of culture, co-cultures treated with low RCF PRF already revealed the existence of microvessel-like structures compared to the other experimental groups, where no microvessel-like structure formation could be assessed. After a 14-day incubation period, the amount of angiogenic structures increased significantly in co-cultures treated with PRF compared to the control co-cultures. Since there was a significant increase in vascular structure formation in PRF-treated samples, it can be concluded that vascularization was PRF-induced and not solely the result of the co-culture itself. The PRF-mediated effect on microvessel-like structure formation was more evident when low RCF PRF instead of high RCF PRF was applied to the co-culture. Other studies on the proangiogenic effect of PRF confirmed these results.Citation19,Citation20,Citation46 When PRF treated endothelial cells were co-cultured with fibroblast, angiogenesis was markedly enhanced and vessel formation was particularly more stimulated when co-cultures were treated with low RCF PRF. Another study confirmed the potency of liquid PRF in promoting the formation of microvessel-like structures in co-cultures composed of outgrowth endothelial cells and pOBs. PRF contains several factors that might have a positive impact on vessel formation like highly concentrated cells such as platelets, leukocytes and their growth factors all contributing to vessel formation.Citation17,Citation18 Using triple culture systems consisting of osteoblast, endothelial cells and neutrophils or macrophages, outgrowth endothelial cells and pOBs, neutrophils and macrophages were found to positively stimulate angiogenesis by releasing proangiogenic factors such as VEGF.Citation42,Citation47,Citation48 Other leukocyte and platelet derived growth factors include TGF-β and PDGF that affect endothelial cell survival, differentiation, proliferation and vessel stability, ultimately promoting vascular structure formation.Citation27,Citation49–51 Since platelets and leukocytes are more highly concentrated within low RCF PRF compared to high RCF PRF the higher induction of newly established microvessel-like structures may be due to their involvement. Proangiogenic growth factors TGF-β1 and PDGF-BB were found significantly higher in co-cultures treated with low RCF PRF compared to co-cultures treated with high RCF PRF confirming this assumption. However, since the co-culture is a dynamic process, VEGF may have been taken up by the HDMECs and thus be consumed at the time of measurement as revealed by another study explaining the low concentration of VEGF within PRF/co-culture supernatants.Citation19 During bone formation, osteoblasts release VEGF, which binds to receptors on the surface of adjacent endothelial cells via paracrine signaling.Citation52 In turn, endothelial cells release osteogenic growth factors such as BMP-2.Citation53 This close communication of endothelial and osteoblasts becomes clear by immunoflourenscence staining as pOB occupy a position adjacent to the sprouting endothelial cells a.Citation54,Citation55 This underscores the importance of the interplay of osteogenic differentiation and angiogenesis in the context of bone regeneration. PRF stimulates these processes by providing important immune cells as wells as proangiogenic and proosteogenic growth factors. Consequently, PRF, with its stimulating effect on osteogenic differentiation and vascularization, combines a variety of properties that are particularly relevant for improving regeneration processes. Since increased vascular density can also contribute to tumorigenesis, it is important to consider this ulterior motive in the clinical application of PRF.Citation56 However, there is no evidence in the literature that PRF promotes tumorigenesis or tumor flareup in clinical applications. Additionally, this is currently the subject of further studies. To the best of our knowledge, no other pathological side effects have been identified, rather PRF has positive properties such as pain relief and scar prevention.Citation7,Citation9,Citation10 Since PRF treatment of monocultures and co-cultures is characterized by a dynamic interplay of different cell types and growth factors, it is difficult to exactly define the precise molecular processes and influences exerted by PRF. Although the present results provide insights into the potential effects of PRF on the regeneration process, more detailed investigations with regard to PRF properties should be the subject of future investigations. In conclusion, Platelet rich fibrin represents a promising autologous tool for regenerative purposes as it provides important cells and growth factors associated with wound healing. Based on the results, PRF seems to unites proosteogenic, potentially osteoconductive and proangiogenic properties that increased the processes of both osteogenic differentiation as well as angiogenic structure formation. Consequently, PRF may be utilized for applications in versatile fields of medicine in the context of improving bone tissue regeneration.
Acknowledgments
The authors would like to thank Mrs. Verena Hoffmann for excellent technical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Percoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994;2(3):165–11. doi:10.1046/j.1524-475X.1994.20305.x.
- Lindholm C, Searle R. Wound management for the 21st century: combining effectiveness and efficiency. Int Wound J. 2016;13(Suppl 2):5–15. doi:10.1111/iwj.12623.
- Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. Vol. 9. BMC Med; 2011. p. 66.
- Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3(3):192–5. doi:10.1097/00005131-198909000-00002.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):37–44. doi:10.1016/j.tripleo.2005.07.008.
- Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118(6):147e–159e. doi:10.1097/01.prs.0000239606.92676.cf.
- Ghanaati S, Herrera-Vizcaino C, Al-Maawi S, Lorenz J, Miron RJ, Nelson K, Schwarz F, Choukroun, J, Sader, R. Fifteen years of platelet rich fibrin in dentistry and oromaxillofacial surgery: how high is the level of scientific evidence? J Oral Implantol. 2018;44(6):471–92. doi:10.1563/aaid-joi-D-17-00179.
- Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang HL. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 2017;21(6):1913–27. doi:10.1007/s00784-017-2133-z.
- Al-Maawi S, Becker K, Schwarz F, Sader R, Ghanaati S. Efficacy of platelet-rich fibrin in promoting the healing of extraction sockets: a systematic review. Int J Implant Dent. 2021;7(1):117. doi:10.1186/s40729-021-00393-0.
- Choukroun J, Diss A, Simonpieri A, Girard M-O, Schoeffler C, Dohan SL, Dohan AJJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):56–60. doi:10.1016/j.tripleo.2005.07.011.
- Al-Maawi S, Dohle E, Lim J, Weigl P, Teoh SH, Sader R, Ghanaati S. Biologization of pcl-mesh using platelet rich fibrin (prf) enhances its regenerative potential in vitro. Int J Mol Sci. 2021;22(4). doi:10.3390/ijms22042159.
- Al-Maawi S, Herrera-Vizcaíno C, Orlowska A, Willershausen I, Sader R, Miron RJ, Choukroun J, Ghanaati S. Biologization of Collagen-Based biomaterials using Liquid-Platelet-Rich fibrin: new insights into clinically applicable tissue engineering. Materials (Basel). 2019;12(23). doi:10.3390/ma12233993.
- Lorenz J, Al-Maawi S, Sader, R, Ghanaati, S. Individualized titanium mesh combined with platelet-rich fibrin and deproteinized bovine bone: a new approach for challenging augmentation. J Oral Implantol. 2018;44(5):345–51. doi:10.1563/aaid-joi-D-18-00049.
- Albilia JB, Weisleder H, Wolford LM. Treatment of posterior dislocation of the mandibular condyle with the double mitek mini anchor technique: a case report. J Oral Maxillofac Surg. 2018;76(2):396 e1–e9. doi:10.1016/j.joms.2017.09.017.
- Ghanaati S, Al-Maawi S, Conrad T, Lorenz J, Rössler R, Sader R. Biomaterial-based bone regeneration and soft tissue management of the individualized 3D-titanium mesh: an alternative concept to autologous transplantation and flap mobilization. J Craniomaxillofac Surg. 2019;47(10):1633–44. doi:10.1016/j.jcms.2019.07.020.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):51–5. doi:10.1016/j.tripleo.2005.07.010.
- Wend S, Kubesch A, Orlowska A, Al-Maawi S, Zender N, Dias A, Miron RJ, Sader R, Booms P, Kirkpatrick CJ. et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J Mater Sci Mater Med. 2017;28(12):188. doi:10.1007/s10856-017-5992-6.
- Choukroun J, Ghanaati S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg. 2018;44(1):87–95. doi:10.1007/s00068-017-0767-9.
- Dohle E, El Bagdadi K, Sader R, Choukroun J, James Kirkpatrick C, Ghanaati S. Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J Tissue Eng Regen Med. 2018;12(3):598–610. doi:10.1002/term.2475.
- Herrera-Vizcaino C, Dohle E, Al-Maawi S, Booms P, Sader R. Kirkpatrick CJ, Choukroun J, Ghanaati S. Platelet-rich fibrin secretome induces three dimensional angiogenic activation in vitro. Eur Cell Mater. 2019;37:250–64. doi:10.22203/eCM.v037a15.
- Dohle E, Fecht T, Wolfram T, Reinauer F, Wunder A, Heppe K, Sader R, Kirkpatrick CJ, Ghanaati S. In vitro coculture of primary human cells to analyze angiogenesis, osteogenesis, and the inflammatory response to newly developed osteosynthesis material for pediatric maxillofacial traumatology: a potential pretesting Model before in vivo experiments. J Tissue Eng Regen Med. 2023;2023:1–13. doi:10.1155/2023/4040504.
- Hofmann A, Konrad L, Gotzen L, Printz H, Ramaswamy A, Hofmann C. Bioengineered human bone tissue using autogenous osteoblasts cultured on different biomatrices. J Biomed Mater Res A. 2003;67(1):191–9. doi:10.1002/jbm.a.10594.
- Unger RE, Krump-Konvalinkova V, Peters K, Kirkpatrick CJ. In vitro expression of the endothelial phenotype: comparative study of primary isolated cells and cell lines, including the novel cell line HPMEC-ST1.6R. Microvasc Res. 2002;64(3):384–97. doi:10.1006/mvre.2002.2434.
- Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, Landes C, Sader R, Kirkpatrick CJ, Choukroun J. et al. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40(6):679–89. doi:10.1563/aaid-joi-D-14-00138.
- Wang X, Zhang Y, Choukroun J, Ghanaati S Miron RJ. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets. 2018;29(1):48–55. doi:10.1080/09537104.2017.1293807.
- Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–88. doi:10.7150/ijbs.2929.
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9(1–3):283–9. doi:10.2741/1184.
- Gruber R, Varga F, Fischer MB, Watzek G. Platelets stimulate proliferation of bone cells: involvement of platelet-derived growth factor, microparticles and membranes. Clin Oral Implants Res. 2002;13(5):529–35. doi:10.1034/j.1600-0501.2002.130513.x.
- Gurevich O, Vexler A, Marx G, Prigozhina T, Levdansky L, Slavin S, Shimeliovich I, Gorodetsky R. Fibrin microbeads for isolating and growing bone marrow–derived progenitor cells capable of forming bone tissue. Tissue Eng. 2002;8(4):661–72. doi:10.1089/107632702760240571.
- Ng AM, Saim AB, Tan KK, Tan GH, Mokhtar SA, Rose IM, Othman F, Idrus RB. Comparison of bioengineered human bone construct from four sources of osteogenic cells. J Orthop Sci. 2005;10(2):192–9. doi:10.1007/s00776-004-0884-2.
- Fiedler J, Etzel N, Brenner RE. To go or not to go: migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J Cell Biochem. 2004;93(5):990–8. doi:10.1002/jcb.20219.
- Aubin JE. Advances in the osteoblast lineage. Biochem Cell Biol. 1998;76(6):899–910. doi:10.1139/o99-005.
- Dohan Ehrenfest DM, Diss, A., Odin, G., Doglioli, P., Hippolyte, M-P., Charrier, J-B. In vitro effects of Choukroun’s PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2009;108(3):341–52. doi:10.1016/j.tripleo.2009.04.020.
- Oosterlaken BM, Vena MP, de with G. In vitro mineralization of collagen. Adv Mater. 2021;33(16):e2004418. doi:10.1002/adma.202004418.
- Wu CL, Lee S-S, Tsai C-H, Lu K-H, Zhao J-H, Chang Y-C. Platelet-rich fibrin increases cell attachment, proliferation and collagen-related protein expression of human osteoblasts. Aust Dent J. 2012;57(2):207–12. doi:10.1111/j.1834-7819.2012.01686.x.
- Centrella M, McCarthy TL, Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987;262(6):2869–74. doi:10.1016/S0021-9258(18)61587-X.
- Asharani PV, Keupp K, Semler O, Wang W, Li Y, Thiele H, Yigit G, Pohl E, Becker J, Frommolt P. et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet. 2012;90(4):661–74. doi:10.1016/j.ajhg.2012.02.026.
- Grgurevic L, Macek B, Mercep M, Jelic M, Smoljanovic T, Erjavec I, Dumic-Cule I, Prgomet S, Durdevic D, Vnuk D. et al. Bone morphogenetic protein (BMP)1-3 enhances bone repair. Biochem Biophys Res Commun. 2011;408(1):25–31. doi:10.1016/j.bbrc.2011.03.109.
- Mbalaviele G, Sheikh S, Stains JP, Salazar VS, Cheng S-L, Chen D, Civitelli R. Beta-catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation. J Cell Biochem. 2005;94(2):403–18. doi:10.1002/jcb.20253.
- Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11(3):279–303. 10.1177/10454411000110030101.
- Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26(1):99–105. doi:10.1016/0092-8674(81)90037-4.
- Herath TDK, Larbi A, Teoh SH, Kirkpatrick CJ, Goh BT. Neutrophil-mediated enhancement of angiogenesis and osteogenesis in a novel triple cell co-culture model with endothelial cells and osteoblasts. J Tissue Eng Regen Med. 2018;12(2):1221–36. doi:10.1002/term.2521.
- He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):707–13. doi:10.1016/j.tripleo.2009.06.044.
- Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–14. doi:10.22203/eCM.v015a08.
- Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5(1):40–6. doi:10.1046/j.1087-0024.2000.00014.x.
- Kubesch A, Barbeck M, Al-Maawi S, Orlowska A, Booms PF, Sader RA, Miron RJ, Kirkpatrick CJ, Choukroun J, Ghanaati S. et al. A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: an experimental study in vivo. Platelets. 2019;30(3):329–340. doi:10.1080/09537104.2018.1445835.
- Dohle E, Bischoff I, Böse T, Marsano A, Banfi A, Unger RE, Kirkpatrick CJ. Macrophage-mediated angiogenic activation of outgrowth endothelial cells in co-culture with primary osteoblasts. Eur Cell Mater. 2014;27:149–64. doi:10.22203/eCM.v027a12. discussion 164-5.
- McCourt M, Wang JH, Sookhai S, Redmond HP. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg. 1999;134(12):1325–31. doi:10.1001/archsurg.134.12.1325. discussion 1331-2.
- Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153(2):347–58. doi:10.1016/j.jss.2008.04.023.
- Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8(1):21–43. doi:10.1016/S1359-6101(96)00048-2.
- Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974;71(4):1207–10. doi:10.1073/pnas.71.4.1207.
- Clarkin CE, Gerstenfeld LC. VEGF and bone cell signalling: an essential vessel for communication? Cell Biochem Funct. 2013;31(1):1–11. doi:10.1002/cbf.2911.
- Grosso A, Burger MG, Lunger A, Schaefer DJ, Banfi A, Di Maggio N. It takes two to tango: coupling of angiogenesis and Osteogenesis for bone regeneration. Front Bioeng Biotechnol. 2017;5:68. doi:10.3389/fbioe.2017.00068.
- Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29(12):1795–803. doi:10.1002/jor.21462.
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19(2):329–44. doi:10.1016/j.devcel.2010.07.010.
- Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71.