Abstract
Septic shock is a life-threatening disease worldwide often associated with thrombocytopenia. Platelets play a crucial role in bridging the gap between immunity, coagulation, and endothelial cell activation, potentially influencing the course of the disease. However, there are few studies specifically evaluating the impact of thrombocytopenia on the prognosis of pediatric patients. Therefore, the study investigates effects of early thrombocytopenia in the prognosis of children with septic shock. Pediatric patients with septic shock from 2015 to 2022 were included monocentrically. Thrombocytopenia was defined as a platelet count of <100 × 109/L during the first 24 hours of septic shock onset. The primary outcome was the 28-day mortality. Propensity score matching was used to pair patients with different platelet counts on admission but comparable disease severity. A total of 419 pediatric patients were included in the analysis. Patients with thrombocytopenia had higher 28-day mortality (55.5% vs. 38.7%, p = .005) compared to patients with no thrombocytopenia. Thrombocytopenia was associated with reduced 28-PICU free days (median value, 0 vs. 13 days, p = .003) and 28-ventilator-free (median value, 0 vs. 19 days, p = .001) days. Among thrombocytopenia patients, those with platelet count ≤50 × 109/L had a higher 28-day mortality rate (63.6% vs. 45%, p = .02). Multiple logistic regression showed that elevated lactate (adjusted odds ratio (OR) = 1.11; 95% confidence interval (CI): 1.04–1.17; P <0.001) and white blood cell (WBC) count (OR = 0.97; 95% CI: 0.95–0.99; p = .003) were independent risk factors for the development of thrombocytopenia. Thrombocytopenia group had increased bleeding events, blood product transfusions, and development of organ failure. In Kaplan-Meier survival estimates, survival probabilities at 28 days were greater in patients without thrombocytopenia (p value from the log-rank test, p = .004). There were no significant differences in the type of pathogenic microorganisms and the site of infection between patients with and without thrombocytopenia. In conclusion, thrombocytopenia within 24 hours of shock onset is associated with an increased risk of 28-day mortality in pediatric patients with septic shock.
Plain Language Summary
What is the context?
Septic shock is a life-threatening disease worldwide, leading to higher mortality.
Platelets play a crucial role in bridging the gap between immunity, coagulation, and endothelial cell activation.
Although it is known that platelets are associated with prognosis, most studies have focused on adult populations. Limited data are available on the incidence of thrombocytopenia and its correlation with clinical outcomes , specifically, in pediatric patients with sepsis and septic shock.
What is new?
The present study suggests that thrombocytopenia within 24 hours of septic shock onset reflects a reliable tool for predicting the prognosis of septic shock in pediatric patients.
Furthermore, elevated lactate and reduced white-blood-cell count were independent risk factors for the development of thrombocytopenia in pediatric patients with septic shock.
What is the impact?
This study suggests that thrombocytopenia within 24 hours of septic shock onset is associated with an increased risk of 28-day mortality and decreased ventilation-free, PICU-free days in pediatric patients with septic shock. In septic shock, thrombocytopenia is also associated with increased bleeding events, blood product transfusions, and organ dysfunction.
Introduction
Sepsis is characterized by a life-threatening organ dysfunction, the underlying cause of which is dysregulation of the host response to infection.Citation1 Notably, there were 41% of sepsis worldwide occurred in children under the age of 5, resulting in around 2.9 million children deaths, accounting for 26% of all sepsis-related mortality.Citation2 Septic shock is characterized by sepsis accompanied by underlying circulatory and metabolic abnormalities, leading to higher mortality. Platelets are derived from megakaryocytes in the bone marrow.Citation3 In septic shock, platelets can be activated through various pathways, participating in coagulation, antimicrobial, and immune responses.Citation3–5 Platelets also function as amplifiers in septic shock, contributing to a positive feedback loop that further intensifies the inflammatory response.Citation6
Thrombocytopenia is a commonly reported finding in patients admitted to the intensive care unit (ICU), which has been demonstrated to be associated with an increased mortality and longer ICU length of stay in adult patients with sepsis.Citation7–9 Nadiejda et al.Citation10 first reported that a platelet count <100 × 109/L within the first 24 hours of septic shock onset was independently associated with an increased risk of 28-day mortality in adult patients.
Regarding the risk factors for the development of thrombocytopenia in ICU patients, sepsis was found to be the primary risk factor for thrombocytopenia.Citation11,Citation12 Markers associated with disease severity, such as the requirement of mechanical ventilation, duration of vasopressor use, and increasing severity of illness scores have also been implicated as risk factors for thrombocytopenia.Citation13,Citation14 Meanwhile, several studies have shown that disseminated intravascular coagulation (DIC)Citation15,Citation16 and various pathogenic bacteriaCitation17,Citation18 may also related to the development of thrombocytopenia. However, these findings are not consistent among various studies.
To date, most studies have focused on adult populations. Limited data are available on the incidence of thrombocytopenia and its correlation with clinical outcomes in pediatric patients with sepsis and septic shock. Tarek et al.Citation19 reported that thrombocytopenia suggested a better prognosis in pediatric sepsis patients, in contrast to previous adult studies. Their conclusion also seems to be inconsistent with clinical practice. Reut et al.Citation20 argued that thrombocytopenia was associated with longer lengths of hospital stay. However, they only included children with positive blood cultures, rather than sepsis or septic shock.
In this context, we intended to compare the characteristics and outcomes between thrombocytopenia group and no thrombocytopenia group in pediatric patients with septic shock. The primary outcome measured was the 28-day mortality. We assume that the thrombocytopenia group has a higher risk of 28-day mortality.
Methods
This is a retrospective cohort study conducted at Pediatric Intensive Care Unit (PICU) of Children’s Hospital of Chongqing Medical University. This study was approved by the Institutional Review Board of Children’s Hospital of Chongqing Medical University. Considering the retrospective nature of this research, the need to obtain informed consent was waived.
We identified children with septic shock between January 2015 and December 2022 using discharge diagnosis data from the electronic medical databases. Septic shock was defined according to the criteria of the SCCM guidelines of 2005Citation21 and the pediatric guidelines of 2020,Citation22 namely severe infection leading to cardiovascular dysfunction. Only the first PICU admission was included for patients who had multiple PICU admissions. We excluded patients with comorbidities and certain interventions known to impact platelet counts, including platelet transfusion prior to admission to PICU, platelet disorders disease (including immunodeficiency diseases which can result in thrombocytopenia, such as Wiskott-Aldrich syndrome), hematologic malignancies, chemotherapy-induced myelosuppression, use of chemotherapy (in the last 30 days prior to admission), heparin-induced thrombocytopenia (HIT). In addition, children who underwent surgery and transferred to PICU just for postoperative ventilation support rather than anti-shock treatment, and subsequently transferred back to the surgical ward on the following day, were excluded to ensure data completeness, continuity, and to decrease the confounding factors associated with surgery. A total of 508 admissions were reviewed, of which 419 were included in the analysis.
All the data were individually obtained from electronic medical records and manually reviewed. The following data were collected: International Society of Pediatric Index of Mortality (PIM)-3, Pediatric Sequential Organ Failure Assessment (pSOFA) scores, Pediatric Sepsis-induced Coagulation Score (pSIC), International Society on Thrombosis and Hemostasis (ISTH) disseminated intravascular coagulation (DIC) score, age, gender, site of infection, microbial organism, respiratory failure, acute kidney injury (AKI), disseminated intravascular coagulation (DIC), bleeding events, blood product transfusion (within 1 week after shock onset), comorbidities, use of antibiotics, use of vasopressor. The following laboratory values were collected: white blood cell count (WBC), neutrophil percentage (N%), lymphocyte percentage (L%), hemoglobin (Hb), C-reactive protein (CRP), procalcitonin (PCT), serum creatinine, lactate, platelet count (PLT), alanine aminotransferase (ALT), albumin, glucose, total bilirubin, activated partial thromboplastin time (APTT), international normalized ratio (INR), fibrinogen, D-dimer. Furthermore, we recorded the requirement for continued renal replacement therapy (CRRT) and mechanical ventilation (MV), as well as the duration of CRRT and MV.
We defined thrombocytopenia as a platelet count <100 × 109/L. If more than one platelet value is available within the first 24 hours of septic shock onset, the lowest value was used. Patients with a platelet count of <100 × 109/L during 24 hours of septic shock onset were assigned to the thrombocytopenia group and those with a count of ≥100 × 109/L to the no thrombocytopenia group. Thrombocytopenia was confirmed by blood smear to rule out EDTA (Ethylenediaminetetraacetic acid)-dependent pseudothrombocytopenia.
The primary outcome was 28-day mortality. The secondary outcomes included bleeding events, blood product transfusion, the need for MV and CRRT, the duration of CRRT, ventilator-free, and PICU-free days with a maximum of 28 days. We defined ventilator-free days as time alive and free of mechanical ventilation, and PICU-free days as time alive and not in PICU.
The statistical analysis was conducted using SPSS Statistics for Windows, Version 26 (IBM Corp, Armonk, NY, USA) and R for windows, Version 4.3.1 (R Core Team, 2021). The Shapiro-Wilk test was used to test normality. Continuous variables were presented as medians (interquartile range), and categorical variables as number (percentage). A Mann–Whitney U test was used to compare continuous variables, and Chi-squared or Fisher’s exact test to categorical variables. Multiple logistic regression analysis was used to identify risk factors for thrombocytopenia. We examined time to death using Kaplan-Meier survival curves and made comparisons using the log-rank test. P<0.05 was considered to represent a statistically significant difference. Considering that the prognosis of septic shock is related to the severity of the disease, we used propensity score matching to create groups with distinct platelet counts but with comparable disease severity to determine the association between thrombocytopenia and prognosis. Two groups were used 1:1 nearest neighbor matching with a caliper value of 0.02. The variables used in the derivation of the propensity score included: PIM-3, use of vasopressor, age, gender, tumor, rheumatism, primary immunodeficiency disease (PID). The pSOFA, pSIC, and ISTH-DIC scores were not included in the matching process as they are related to platelet counts. Blood-related markers were also not taken into account for propensity matching as they could be mediating variables in thrombocytopenia.
Results
Our analysis encompassed 419 patients admitted to the PICU with septic shock. In total, 144 (34.4%) had thrombocytopenia. Baseline characteristics were indicated in . Both unmatched cohort and propensity-matched cohort had no significant difference in age, gender, and the use of antibiotics. In the unmatched cohorts, thrombocytopenia group had significantly higher pSOFA (median value, 13 vs. 7, P<0.001), PIM-3 (median value, 0.08 vs. 0.06, P<0.001), pSIC (median value, 6 vs. 4, P<0.001) and ISTH-DIC (median value, 5 vs. 3, P<0.001) scores. Furthermore, thrombocytopenia group also had increased vasopressor use (86.1% vs. 71.3%, p = .001) and higher frequency of PID (9% vs. 2.9%, p = .006) compared to no thrombocytopenia group.
Table I. Baseline characteristics in the unmatched and propensity-matched study population.
Propensity-matching successfully eliminated the differences of severity between the two groups. In the propensity-matched cohort, PIM-3 scores, vasopressor use and the frequency of patients with PID were no longer significantly different between the two groups. Median platelet count was 200 × 109/L (IQR, 134–336) in no thrombocytopenia group, 40 × 109/L (IQR, 17–71) in thrombocytopenia group. Thrombocytopenia patients had lower WBC (median value, 6.8 × 109/L vs. 9.5 × 109/L, p = .002), Hb (median value, 93 g/L vs. 103 g/L, P<0.001), albumin (median value, 23.8 g/L vs. 27.7 g/L, P <0.001) and higher lactate (median value, 2.9 mmol/L vs. 2 mmol/L, p = .01), ALT (median value, 73 U/L vs. 41.5 U/L, p = .001), total bilirubin (median value, 9 μmol/L vs. 5.7 μmol/L, P<0.001) than no thrombocytopenia group . There were no statistically significant differences in N%, L%, CRP and glucose between the two groups .
Regarding coagulation parameters, after propensity matching, the thrombocytopenia group had significantly higher INR (median value, 1.6 vs. 1.3, P<0.001), aPTT (median value, 61.9 s vs. 44.8 s, P< 0.001) and D-dimer values (median value, 6.4 mg/L vs. 4.6 mg/L, p = .004) than no thrombocytopenia group, while fibrinogen values were significantly lower than those of the no thrombocytopenia group (median value, 1.7 g/L vs. 2.4 g/L, p = .001) . Thrombocytopenia was not associated with the type of pathogenic microorganisms or the results of microorganism cultures.
There was no significant difference in the site of infection between the two groups . Both unmatched cohort and propensity-matched cohort exhibited higher rates of respiratory failure, AKI, and DIC in thrombocytopenia group .
Table II. Site of infection.
Table III. Organ dysfunction for patients with and without thrombocytopenia.
Thrombocytopenia group showed higher 28-day mortality (55.5% vs. 38.7%, p = .005), increased need for CRRT (37.2% vs. 25.5%, p = .03) and longer duration of CRRT (p = .04) . Additionally, thrombocytopenia group had less 28-PICU free (median value, 0 vs. 13 days, p = .003) and 28-ventilator free (median value, 0 vs. 19 days, p = .001) days compared to no thrombocytopenia group. Thrombocytopenia group had a higher incidence of bleeding events and required more blood product transfusions . Furthermore, among thrombocytopenic patients, those with platelet count ≤50 × 109/L had a higher 28-day mortality rate (63.6% vs. 45%, p = .02) .
Table IV. Clinical outcome in the unmatched and propensity-matched study population.
Table V. Clinical outcomes in thrombocytopenia patients.
We conducted a subgroup analysis for patients with thrombocytopenia. There was no statistical difference in 28-day mortality between patients with and without bleeding events (62.3% vs. 51.8%, p = .21, Supplementary Table S1), and between those with and without DIC (75% vs. 53.2%, p = .06, Supplementary Table S2). Multiple regression analysis was used to explore risk factors for the development of thrombocytopenia . Variables with significant p-values in univariate analyses were considered as candidate variables. We found that elevated lactate (adjusted odds ratio (OR) = 1.11; 95% confidence interval (CI): 1.04–1.17; P< 0.001) and WBC counts (OR = 0.97; 95% CI: 0.95–0.99; p = .003) were independent risk factors for the development of thrombocytopenia.
Table VI. Multivariate logistic regression model to identify risk factors for development of thrombocytopenia.
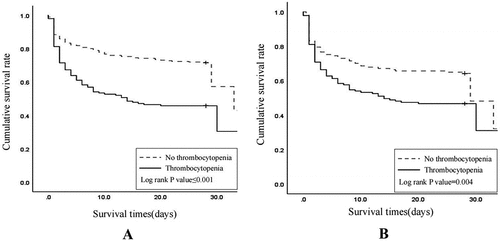
As shown by Kaplan-Meier survival estimates, survival probabilities at 28 days were greater in patients without thrombocytopenia in both the unmatched cohorts (p value from the log-rank test, <0.001) and propensity-matched cohorts (p value from the log-rank test, 0.004) .
Discussion
Platelets play a crucial role in bridging the gap between immunity, coagulation, and endothelial cell activation. Although multiple studies in the adult populations have indicated that thrombocytopenia can lead to a poor prognosis,Citation7–9,Citation23 the prognostic significance of thrombocytopenia in pediatric patients lacks studies with large sample sizes. We divided 419 children with septic shock into thrombocytopenia and no thrombocytopenia groups on basis of the lowest platelet count within 24 hours of the onset of shock. Subsequently, we performed propensity matching to analyze patients with different platelet counts but similar severity of illness. Our major finding was that thrombocytopenia associated with higher 28-day mortality rate. To our knowledge, this study may be the first to specifically evaluating early thrombocytopenia as a major prognostic factor in pediatric patients with septic shock.
The incidence of thrombocytopenia differs in several studies, likely explained by variations in subject characteristics and the definition of thrombocytopenia. In our study, thrombocytopenia occurred in 34.4% of pediatrics patients with septic shock, which is consistent with a previous study.Citation24 When thrombocytopenia was defined as a platelet count of <150 × 109/L, one study reported an incidence of 47.6%, with 94% of these were septic shock patients.Citation14 Another study reported 55% patients with septic shock developed thrombocytopenia.Citation7 There are limited data about the incidence of thrombocytopenia in pediatric patients. One study involving children with positive blood cultures reported 63.4% incidence of thrombocytopenia.Citation20 We exclusively enrolled patients diagnosed with septic shock and defined thrombocytopenia as a platelet count <100 × 109/L, which might explain our lower incidence of thrombocytopenia.
Studies have shown that thrombocytopenia are often associated with more severe conditions and treatment needs, including use of mechanical ventilation, duration of vasopressor, and development of organ dysfunction.Citation13,Citation14 We found that elevated lactate was an independent risk factor for thrombocytopenia. Notably, we also indicated a correlation between reduced WBC count and an increased development of thrombocytopenia within the initial 24 hours following shock. This could be explained by myelosuppression attributed to pathogen toxins and inflammatory mediators.Citation4,Citation25 Moreover, certain drugs also have been shown to inhibit bone marrow megakaryocytes during the treatment of septic patients.Citation25 Because our study population focused on children within 24 hours of shock, especially those newly admitted to the hospital, we did not consider drugs as a major factor.
Activated platelets are involved in hemostasis and coagulation, leading to the imbalance between platelet production and consumption during sepsis. Hemophagocytosis,Citation26 pyroptosisCitation6 and the formation of neutrophil extracellular traps (NETs)Citation27,Citation28 can also contribute to sepsis-induced thrombocytopenia. Septic shock is closely associated with DIC which is characterized as a life-threatening condition affecting the coagulation system,Citation29 leading to secondary thrombocytopenia.Citation15,Citation16 In our study, the incidence of DIC in thrombocytopenia group before propensity matching was 13.9%. One study involving critically ill adult patients with sepsis revealed an incidence of DIC at 25%.Citation14
In addition, platelets are important in several DIC diagnostic scores. The monitoring of platelet levels serves as a valuable tool for assessing a patient’s coagulation status and determine the severity of the condition. However, there has been a lack of widely applicable diagnostic scores for DIC in pediatric patients. One study proposed pSIC score, which can detect non-overt DIC in children with sepsis earlier.Citation30 We showed that thrombocytopenia patients had higher pSIC and ISTH-DIC score, and these patients also had a higher incidence of bleeding events or DIC. There is still a need for further large-scale multicenter studies to assess the role of thrombocytopenia in DIC and explore diagnostic scoring systems for pediatric patients.
Several studies have reported that bacteria can interact with platelets either directly or indirectly, thereby promoting platelet adhesion or inducing platelet aggregation.Citation31–33 This interaction subsequently leads to thrombosis and platelet depletion.Citation34,Citation35 Regarding the relationship between types of bacteria and thrombocytopenia, studies have shown conflicting results. Fungal and gram-negative infections have been found to be associated with a higher incidence and longer duration of thrombocytopenia,Citation17,Citation18 while other studies have reported no difference in the incidence of thrombocytopenia between gram-positive germ, gram-negative germ, and fungal infections.Citation20,Citation36,Citation37 Our study did not find any significant difference in terms of pathogenic organisms between the thrombocytopenia and no thrombocytopenia groups. Considering that different species of bacteria interact with platelets through different mechanisms, future studies are needed to accurately explain this phenomenon.
In septic shock, platelets can bind to the endothelium, ensuring blood vessel homeostasis, preventing microbleeds, and playing a crucial role in maintaining vascular integrity.Citation38 We found that patients with thrombocytopenia were more prone to developing bleeding events and required more blood product transfusions. This is similar to previous studies.Citation39,Citation40 Studies have shown that thrombocytopenic patients were also more likely to have a major bleeding event (defined as a drop in Hb ≥20 g/L within 24 hours).Citation9,Citation14 However, in septic shock, considering the resuscitation and fluid shifts, there are possible limitations to these results.
Thrombocytopenia has been known to be associated with a poor prognosis in adults with sepsis and septic shock.Citation9,Citation41 However, there has been limited research on this relationship in pediatric critically ill patients. In a retrospective study involving 206 children with severe sepsis and septic shock, thrombocytopenia suggested a better prognosis.Citation19 This finding has limited utility as they set the age range of the included population from 0 to 14 years, ignoring the specificity of neonatal sepsis and how it differs from pediatric sepsis.Citation42,Citation43 Another study reported that thrombocytopenia was associated with longer lengths of hospital stay in children with positive blood cultures.Citation20 In the present study, we demonstrated that thrombocytopenia during the first 24 hours of septic shock onset is predictive of increased 28-day mortality and fewer 28-PICU free and 28-ventilator-free days. We also show that among thrombocytopenia patients, those with platelet count ≤50 × 109/L had a higher 28-day mortality rate. One previous study also reported that the initial platelet count ≤50 × 109/L can independently predict ICU mortality.Citation44
Studies in the adult field have reported that the development of bleeding events may explain the increased mortality in thrombocytopenia patients with septic shock.Citation10 Whether bleeding caused by thrombocytopenia will increase the mortality rate of children with septic shock is still not completely clear. A subgroup analysis of septic shock patients with thrombocytopenia was conducted. Compared with those without concomitant bleeding events or DIC, the mortality rate of children with concomitant bleeding events or DIC is higher, but neither is statistically significant. However, it is worth noting that the statistical results may be limited by the relatively small sample size of patients with septic shock and thrombocytopenia in this study. Therefore, prospective multicenter large sample studies in the future to explore how thrombocytopenia affects the prognosis of children with septic shock are needed.
The strength of our study is the inclusion of a large group of pediatric patients with septic shock. From this we drew meaningful clinical inferences. For the study, we defined thrombocytopenia as a platelet count lower than 100 × 109/L, a widely accepted threshold. We believe that this definition maximizes the clinical relevance of the obtained findings. We used propensity matching to reduce confounding factors and excluded patients with thrombocytopenia that might be associated with causes unrelated to sepsis and septic shock.
There are several limitations to this study. Firstly, bias may remain after propensity matching due to unmeasured confounders. Secondly, this was a single-center retrospective study, which may limit some causal inferences and the generalizability of this study. However, it may be the first clinical study specifically evaluating early thrombocytopenia as a major prognostic factor in pediatric patients with septic shock. Thirdly, this study only collected platelet data within 24 hours after the onset of shock and was unable to assess the impact of dynamic changes of platelets on the prognosis of children with septic shock. Nevertheless, assessing platelet levels within 24 hours after shock is inexpensive to measure and easy to perform in routine practice, providing valuable information for clinician decision-making. Akca et al.Citation45 concluded that changes in platelet counts during ICU stays are more predictive of outcome than a single platelet count value. The predictive value of platelet count changes in children with septic shock is still unclear and further studies are needed. Finally, we excluded hematological malignancies, chemotherapy, and post-chemotherapy myelosuppression while children with these diseases are at high risk of septic shock. Therefore, the actual prevalence and incidence of thrombocytopenia in children with septic shock may be underestimated. Furthermore, because this is a retrospective, single-center study with a limited number of patients, thrombocytopenia as an adverse prognostic marker in children with sepsis should be used as complementary to the clinical course rather than as a independent indicator or a treatment goal.
Conclusion
Thrombocytopenia is common in children with septic shock and has important prognostic implications. Our study suggests that thrombocytopenia within 24 hours of septic shock onset is associated with an increased risk of 28-day mortality and decreased ventilation-free, PICU-free days in pediatric patients with septic shock. In septic shock, thrombocytopenia is also associated with increased bleeding events, blood product transfusions, and organ dysfunction.
Supplemental Material
Download PDF (112.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09537104.2024.2363242
Additional information
Funding
References
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM. et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–9. doi:10.1001/jama.2016.0287.
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet (London, England). 2020;395(10219):200–11. doi:10.1016/S0140-6736(19)32989-7.
- Semple JW, Italiano JE Jr., Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–74. doi:10.1038/nri2956.
- Shannon O. The role of platelets in sepsis. Res Pract Thromb Haemost. 2021;5(1):27–37. doi:10.1002/rth2.12465.
- Italiano JE Jr., Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet α granules and differentially released. Blood. 2008;111(3):1227–33. doi:10.1182/blood-2007-09-113837.
- Su M, Chen C, Li S, Li M, Zeng Z, Zhang Y, Xia L, Li X, Zheng D, Lin Q. et al. Gasdermin D-dependent platelet pyroptosis exacerbates NET formation and inflammation in severe sepsis. Nat Cardiovasc Res. 2022;1(8):732–47. doi:10.1038/s44161-022-00108-7.
- Sharma B, Sharma M, Majumder M, Steier W, Sangal A, Kalawar M. Thrombocytopenia in septic shock patients–a prospective observational study of incidence, risk factors and correlation with clinical outcome. Anaesth Intensive Care. 2007;35(6):874–80. doi:10.1177/0310057X0703500604.
- Martin CM, Priestap F, Fisher H, Fowler RA, Heyland DK, Keenan SP, Longo CJ, Morrison T, Bentley D, Antman N. et al. A prospective, observational registry of patients with severe sepsis: the Canadian sepsis treatment and response registry. Crit Care Med. 2009;37(1):81–8. doi:10.1097/CCM.0b013e31819285f0.
- Menard CE, Kumar A, Houston DS, Turgeon AF, Rimmer E, Houston BL, Doucette S, Zarychanski R. Evolution and impact of thrombocytopenia in septic shock: a retrospective cohort study. Crit Care Med. 2019;47(4):558–65. doi:10.1097/CCM.0000000000003644.
- Thiery-Antier N, Binquet C, Vinault S, Meziani F, Boisramé-Helms J, Quenot JP. Is thrombocytopenia an early prognostic marker in septic shock? Crit Care Med. 2016;44(4):764–72. doi:10.1097/CCM.0000000000001520.
- Shalansky SJ, Verma AK, Levine M, Spinelli JJ, Dodek PM. Risk markers for thrombocytopenia in critically ill patients: a prospective analysis. Pharmacotherapy. 2002;22(7):803–13. doi:10.1592/phco.22.11.803.33634.
- Thiolliere F, Serre-Sapin AF, Reignier J, Benedit M, Constantin JM, Lebert C, Guélon D, Timsit JF, Souweine B. Epidemiology and outcome of thrombocytopenic patients in the intensive care unit: results of a prospective multicenter study. Intensive Care Med. 2013;39(8):1460–8. doi:10.1007/s00134-013-2963-3.
- Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271–8. doi:10.1378/chest.10-2243.
- Venkata C, Kashyap R, Farmer JC, Afessa B. Thrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcome. J Intensive Care. 2013;1(1):9. doi:10.1186/2052-0492-1-9.
- Hunt BJ. Bleeding and coagulopathies in critical care. N Engl J Med. 2014;370(22):2153. doi:10.1056/NEJMra1208626.
- Adelborg K, Larsen JB, Hvas AM. Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br J Haematol. 2021;192(5):803–18. doi:10.1111/bjh.17172.
- Bhat MA, Bhat JI, Kawoosa MS, Ahmad SM, Ali SW. Organism-specific platelet response and factors affecting survival in thrombocytopenic very low birth weight babies with sepsis. J Perinatol. 2009;29(10):702–8. doi:10.1038/jp.2009.72.
- Aydemir H, Piskin N, Akduman D, Kokturk F, Aktas E. Platelet and mean platelet volume kinetics in adult patients with sepsis. Platelets. 2015;26(4):331–5. doi:10.3109/09537104.2012.701027.
- Hazwani TR, Bin Obaid W, Alowirdi F, Alsomali R, Alali H, Alsadoon A, Alhamwah M, Alsubaiel S, Alomar B, Vishwakarma R. et al. Association between platelet count and multiorgan dysfunction and outcomes in patients with sepsis in the pediatric intensive care unit in Saudi Arabia. J Infect Public Health. 2021;14(11):1585–9. doi:10.1016/j.jiph.2021.09.012.
- Kassif Lerner R, Levinkopf D, Zaslavsky Paltiel I, Sadeh T, Rubinstein M, Pessach IM, Keller N, Lerner-Geva L, Paret G. Thrombocytopenia and bloodstream infection: incidence and implication on length of stay in the pediatric intensive care unit. J Pediatr Intensive Care. 2022;11(3):209–14. doi:10.1055/s-0040-1722338.
- Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi:10.1097/01.PCC.0000149131.72248.E6.
- Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, Nadel S, Schlapbach LJ, Tasker RC, Argent AC. et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46(Suppl 1):10–67. doi:10.1007/s00134-019-05878-6.
- Azkárate I, Choperena G, Salas E, Sebastián R, Lara G, Elósegui I, Barrutia L, Eguibar I, Salaberria R. Epidemiología y factores pronósticos de la sepsis grave/shock séptico. Seis años de evolución. Medicina intensiva. 2016;40(1):18–25. doi:10.1016/j.medin.2015.01.006.
- Vandijck DM, Blot SI, De Waele JJ, Hoste EA, Vandewoude KH, Decruyenaere JM. Thrombocytopenia and outcome in critically ill patients with bloodstream infection. Heart Lung. 2010;39(1):21–6. doi:10.1016/j.hrtlng.2009.07.005.
- Dewitte A, Lepreux S, Villeneuve J, Rigothier C, Combe C, Ouattara A, Ripoche J. Blood platelets and sepsis pathophysiology: a new therapeutic prospect in critically [corrected] ill patients? Ann Intensive Care. 2017;7(1):115. doi:10.1186/s13613-017-0337-7.
- François B, Trimoreau F, Vignon P, Fixe P, Praloran V, Gastinne H. Thrombocytopenia in the sepsis syndrome: role of hemophagocytosis and macrophage colony-stimulating factor. Am J Med. 1997;103(2):114–20. doi:10.1016/S0002-9343(97)00136-8.
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–82. doi:10.1038/nri1785.
- Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107(36):15880–5. doi:10.1073/pnas.1005743107.
- Levi M, van der Poll T. A short contemporary history of disseminated intravascular coagulation. Semin Thromb Hemost. 2014;40(8):874–80. doi:10.1055/s-0034-1395155.
- Xiang L, Ren H, Wang Y, Zhang J, Qian J, Li B, An K, Fu L. Clinical value of pediatric sepsis-induced coagulopathy score in diagnosis of sepsis-induced coagulopathy and prognosis in children. J Thromb Haemost. 2021;19(12):2930–7. doi:10.1111/jth.15500.
- Loughman A, Fitzgerald JR, Brennan MP, Higgins J, Downer R, Cox D, Foster TJ. Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by Staphylococcus aureus clumping factor a. Mol Microbiol. 2005;57(3):804–18. doi:10.1111/j.1365-2958.2005.04731.x.
- Siauw C, Kobsar A, Dornieden C, Beyrich C, Schinke B, Schubert-Unkmeir A, Abele-Horn M, Speer C, Eigenthaler M. Group B streptococcus isolates from septic patients and healthy carriers differentially activate platelet signaling cascades. Thromb Haemostasis. 2006;95(5):836–49. doi:10.1160/TH05-08-0534.
- Kerrigan SW, Cox D. Platelet-bacterial interactions. Cellular and molecular life sciences: CMLS. Cell Mol Life Sci. 2010;67(4):513–23. doi:10.1007/s00018-009-0207-z.
- Claessens YE, Dhainaut JF. Diagnosis and treatment of severe sepsis. Crit Care (London, England). 2007;11(Suppl 5):S2. doi:10.1186/cc6153.
- Vincent JL, Yagushi A, Pradier O. Platelet function in sepsis. Crit Care Med. 2002;30(5 Suppl):S313–7. doi:10.1097/00003246-200205001-00022.
- Johansson D, Rasmussen M, Inghammar M. Thrombocytopenia in bacteraemia and association with bacterial species. Epidemiol Infect. 2018;146(10):1312–17. doi:10.1017/S0950268818001206.
- Manzoni P, Mostert M, Galletto P, Gastaldo L, Gallo E, Agriesti G, Farina D. Is thrombocytopenia suggestive of organism-specific response in neonatal sepsis? Pediatr Int. 2009;51(2):206–10. doi:10.1111/j.1442-200X.2008.02689.x.
- Kaiser R, Escaig R, Nicolai L. Hemostasis without clot formation: how platelets guard the vasculature in inflammation, infection, and malignancy. Blood. 2023;142(17):1413–25. doi:10.1182/blood.2023020535.
- Ben Hamida C, Lauzet JY, Rézaiguia-Delclaux S, Duvoux C, Cherqui D, Duvaldestin P, Stéphan F. Effect of severe thrombocytopenia on patient outcome after liver transplantation. Intensive Care Med. 2003;29(5):756–62. doi:10.1007/s00134-003-1727-x.
- Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30(8):1765–71. doi:10.1097/00003246-200208000-00015.
- Claushuis TA, van Vught LA, Scicluna BP, Wiewel MA, Klein Klouwenberg PMC, Hoogendijk AJ, Ong DSY, Cremer OL, Horn J, Franitza M. et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127(24):3062–72. doi:10.1182/blood-2015-11-680744.
- Molloy EJ, Bearer CF. Paediatric and neonatal sepsis and inflammation. Pediatr Res. 2022;91(2):267–9. doi:10.1038/s41390-021-01918-4.
- Hayes R, Hartnett J, Semova G, Murray C, Murphy K, Carroll L, Plapp H, Hession L, O’Toole J, McCollum D. et al. Neonatal sepsis definitions from randomised clinical trials. Pediatr Res. 2023;93(5):1141–8. doi:10.1038/s41390-021-01749-3.
- Brogly N, Devos P, Boussekey N, Georges H, Chiche A, Leroy O. Impact of thrombocytopenia on outcome of patients admitted to ICU for severe community-acquired pneumonia. J Infect. 2007;55(2):136–40. doi:10.1016/j.jinf.2007.01.011.
- Akca S, Haji-Michael P, de Mendonça A, Suter P, Levi M, Vincent JL. Time course of platelet counts in critically ill patients. Crit Care Med. 2002;30(4):753–6. doi:10.1097/00003246-200204000-00005.