Abstract
A dot-immunogold filtration assay (DIGFA) was described for simultaneous screening of three fluoroquinolones (FQs): enrofloxacin (ENR), ciprofloxacin (CIP) and norfloxacin (NOR). Colloidal gold particles were used as visible labels, and anti-ENR polyclonal antibodies from mice and rabbits were exploited for gold labelling and membrane coating, respectively. Several parameters affecting DIGFA were optimised, and the operation was validated with pure buffer matrix and spiked eel samples, respectively. The detection limits in eel samples were estimated to be about 20 µg kg−1 for ENR and CIP, and 50 µg kg−1 for NOR. With pretreated nitrocellulose membranes, the determination could be completed within 30 minutes, and the results could be read just by the naked eye without the help of any equipment.
Introduction
Fluoroquinolones (FQs) () are a group of synthetic antibacterial agents widely used for the treatment of bacterial infection. The FQs residues in animal edible tissues could cause serious public health problems for which many countries and regions have set strict maximal residue limits (MRLs) for such antibiotics in animal foods (Cerniglia & Kotarski, Citation1999; Hernández-Arteseros, Barbosa, Compaño, & Prat, Citation2002). Enrofloxacin (ENR) is one of most commonly used FQs in veterinary and aquaculture, and the MRL for it, is usually within the sum of its metabolite ciprofloxacin (CIP). Norfloxacin (NOR) is forbidden to treat animals in the EU and USA, but in some Asian countries such as China and Korea, it can still be used in the poultry industry or aquiculture (Agricultural Industry Criteria of People's Republic of China, Citation2001, Citation2005; Cho et al., Citation2008). In China ENR, CIP and NOR are the main FQs to be monitored in fishery products, and the MRLs for them are 50~100 µg kg−1(Agricultural Industry Criteria of People's Republic of China, Citation2001, Citation2005).
Today, high-performance liquid chromatography (HPLC) is the most commonly used methods for screening of FQs residues (Idowu & Peggins, Citation2004; Marazuela & Moreno-Bondi, Citation2004), and liquid chromatography–tandem mass spectrometry (LC–MS) has been reported as a confirmatory technique (Schneider & Donoghue, Citation2002; Turnipseed, Walker, & Roybal, Citation1998). But these methods require expensive and exquisite equipments and are time consuming therefore, not suitable for fast routine monitoring of FQs in real samples, especially in farms and factories where the analytical facilities are very limited. Microbiological testing is often used for routine screening of FQs and other antimicrobial agents, but it usually takes about 20 hours, and the sensitivity is much lower compared with HPLC-based methods (Cho et al., Citation2008). Enzyme-linked immunoassay (such as enzyme-linked immunosorbent assay (ELISA)) has been developed as an alternative approach for its high sensitivity and specificity (Bucknall, Silverlight, Coldham, Thorne, & Jackman, Citation2003; Duan & Yuan, Citation2001; Hiroo, Atsuko, Yasumasa, & Akio, Citation2002; Holtzapple, Buckley, & Stanker, Citation1997). But the problem linked with it is that such immunoassays usually take at least 3–5 hours, and the enzyme proves to be very sensitive to endogenous and exogenous interference which often leads to high variation of the analysis (Duan & Yuan, Citation2001; Zhan, Wang, Chen, Jing, & Hideo, Citation2004).
The dot-immunogold filtration assay (DIGFA) is a kind of solid phase immunoassay. It adopts the principle of filtration for the reaction between antigen and antibody, and exploits nitrocellulose membranes (NCMs) as support and colloidal gold as label. In previous studies, DIGFA has been successfully developed for analysis of biomacromolecules such as antibodies and other proteins, where it demonstrated plenty of advantages over other analytical techniques (Huang, Lan, & Tong, Citation1996; Spielberg, Kabeya, & Ryder, Citation1989; Wen et al., Citation2005): it could be simply performed with only a colloid gold kit instead of complex laboratory facilities; the results could be read qualitatively by the naked eye without the help of any equipment, and the operation proved to be more rapid and stable than enzyme-linked immunoassays. Although all these characteristics allow DIGFA very suitable for in situ fast screening of food contaminants, until now its application for screening of multiple antibacterial agents has not been mentioned according to our knowledge.
In this paper, a DIGFA was developed and tested for simultaneous determination of ENR, CIP and NOR in eel samples. According to our knowledge, this is the first report on fast screening of FQs using DIFFA. The established method demonstrated enough sensitivity to fit most MRLs of the three FQs in the world, and was much faster and simpler compared with ELISA, HPLC and microbiological assays.
Experimental
Reagents
(Fluoro)quinolones, chloramphenicol, oxytetracycline and sulphamethoxazole were purchased from Veterinary Medicine Supervisory Institute of China (Beijing, P.R. China). Stock solutions (200 µg ml−1) of (fluoro)quinolones were prepared in 0.01 M NaOH and diluted by phosphate buffers (0.01M, pH 7.4, PBS). Bovine serum albumin (BSA), ovalbumin (OVA), N-hydroxysuccinimide (NHS), N-(3-dimethylaminopropyl)-N′-carbodiimide hydrochloride (EDC), incomplete Freund's adjuvant (IFA) and complete Freund's adjuvant (CFA) were purchased from Sigma Chemical Co (St. Louis, MO, USA). 3, 3′, 5, 5′-tetramethylbenzidine (TMB) was purchased from Amresco (Solon, OH, USA). Goat anti-mouse IgG-HRP and goat anti-rabbit IgG-HRP were purchased from Zhongshan Biology Co (Beijing, P.R. China). N. N′-Dimethylformamide (DMF) was purchased from Shanghai Chemical Co. (Shanghai, P.R. China). Chloroauric acid hydrated (H[AuCl4]•4H2O) was purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, P.R. China). Acetonitrile (chromatographic grade) was purchased from Ruiming Biology Co. (Qingdao, P.R. China). All the other chemicals were analytical grade. Deionised water purified by a Milli-Q system (Millipore, Danvers, MA, USA) was used.
Female BALB/C mice were from Centre for New Drugs Evaluation of Shandong University (Jinan, Shandong, P.R. China). Female New Zealand rabbits were from Medicine Supervisory Institute of Qingdao (Qingdao, P.R. China). Eel (Anguilla japonica) samples were obtained from Shandong Import and Export Inspection and Quarantine Bureau and proved to contain no (fluoro)quinolones by HPLC determination. The muscle of eel samples was collected and minced, and stored at −20°C. Each sample was thawed before further use.
Apparatus
All centrifugations were performed at 4°C (BR-4i, Jouan, St-Herblain, France). The results of ELISA were read and recorded with a plate reader (Multiskan MK3, Lab systems, Shanghai, P.R. China). A protein purifier (AKTABox-900, Amersham Biosciences, Piscataway, NJ, USA) equipped with a superdexTM75 column was used for purification of antibodies. A transmission electron microscope (H-2600, Hitachi, Tokyo, Japan) was used for observation of colloidal gold particles and gold-labelled antibodies.
The immune filtration device was designed as . There is a test hole (6 mm) in the centre of the cover. Before a performance, the support box was filled with filtration paper as water-absorbing materials, and a NCM (Millipore, 0.22 µm) was put on the surface of the paper to fit the position of the hole, then the support box and the cover were fixed together to make a whole device.
Methods
Preparation, purification and characterisation of polyclonal antibodies (PAbs)
BSA and OVA were used as carriers for immunogens and coating antigens, respectively. The synthesis of the hapten-carrier complex was according to methods described by Liu, Lin, Cao, and Jiang (Citation2005a). Mice and rabbits were immunised with ENR–BSA conjugates to prepare anti-ENR serum. The obtained antiserum was mixed with phosphate-buffered saline (PBS, 0.01M, pH7.4) at a ratio of 1:1, and then ammonium sulphate was added to reach 40% saturation and react in an ice bath under stirring for 30 mins. The solution was centrifuged at 10,000 rpm for 15 min; the precipitation was collected and dissolved in PBS, followed by dialysis against PBS for 48 h. Then the antibody solution was centrifuged again at 10,000 rpm for 60 min; the supernatant was carefully removed and purified with AKTA purifier (flow rate 1.5 mL min−1). Different components were collected according to their absorbance at 280 nm, and characterised by indirect ELISA (Liu, Lin, Cao, & Jiang 2005a). The one that showed the highest antibody activity was selected for further use.
A 50% binding inhibition (IC50) values of antibodies were determined with competitive indirect ELISA (Liu et al., Citation2005b). The cross-reactivity was calculated according to the following equation:
Selection of antibodies for colloidal gold labelling and membrane coating was carried out according to a general sandwich ELISA protocol (Huang et al., Citation1998).
Preparation of colloidal gold probe
The gold-labelled anti-ENR polyclonal antibodies (PAbs) was prepared according to the method described by Oliver (Citation1999) with minor modifications: the dispersion of colloidal gold particles was obtained by heating a solution containing 0.01% chloroauric acid hydrated (H[AuCl4]•4H2O)and 0.1% sodium citrate for 15 mins. The solution was cooled and then adjusted to pH 8.0 with 0.1 M K2CO3. PAbs (50 µg mL−1) was added to react with gold particles for 10 min at room temperature, and then 5% of BSA was added to a final concentration of 1% and reacted for another 10 min. The final mixture was centrifuged at 4000 rpm for 20 min at 4°C, and the supernatant was again centrifuged at 10,000 rpm for 60 min at 4°C. The precipitation was dissolved in 1ml PBS (0.01M, pH 8.0, 1% BSA, 0.02% NaN3), and stored at 4°C for further use.
Sample preparation
The extraction solution was a 1:1 (v/v) mixture of methanol and PBS. 5 g homogenised eel muscle was weighed and spiked with FQs. Then 5 mL of extraction solution was added and centrifuged twice at 4000 rpm for 10 mins. The supernatants were combined, centrifuged at 10,000 rpm for 10 min, and evaporated under vacuum in a rotary evaporator at 45°C for about 15 min to remove methanol. The residue was transferred and diluted to 5 mL with PBS. The solution was filtrated through a micro-membrane ( 0.22 µm) before analysis.
The procedure of dot-immunogold filtration assay (DIGFA)
The NCM was coated with anti-ENR antibodies and then blocked before analysis. A 5 µL of sample solution was added into the test hole to react for 15 min at room temperature. Then 5 µL of colloidal gold probe was added and followed by addition of 50 µL lotion (PBS, 0.01M, pH 7.4, containing 0.1% Tween-20). When a reddish dot appeared, the sample was judged as positive; otherwise it was negative. Samples without FQs were used to set negative controls.
High-performance liquid chromatography (HPLC) determination
HPLC analysis was performed using Agilent 1100 instrument (Agilent, Santa Clara, CA, USA) with an automatic injector and a fluorescence detector. The analytical column was a SB-C18 (4.6 mm×250 mm) column (Agilent, Santa Clara, CA, USA) with a precolumn. Mobile phase: acetonitrile − 0.01 M tetrabutyl ammonium bromide (5: 95) adjusted to pH 3.1, flow rate: 1.5 mL min−1. Fluorescence detector: excitation wavelength: 280 nm, emission wavelength: 450 nm. Column temperature: 40°C. The volume of each sample injected: 20 µL.
The concentration of fluoroquinones in spiked eel samples were calculated from the calibration plots, which were prepared at concentrations ranging from 5.0 to 100 ng mL−1 for each FQ in triplicates. Working solutions were prepared by serious dilution of stock solutions with PBS (pH 7.4, 0.01 M).
Results and discussion
Selection of antibody for colloidal gold labelling
The developed DIGFA was performed with a sandwich model: the analyte was at first captured by antibodies coated on the NCM, and then colloidal gold-labelled antibodies were added to react with remained free binding sites of antigens and form a labelled antibody–antigen-coated antibody complex. A red dot will come into being due to the colloidal gold label. Although hapten was usually considered as a single epitope, we assumed that on some complex molecules such as FQs, the favourite binding areas might be different for antibodies of different sources (mice or rabbits). Therefore, through careful selection of coating antibodies and labelling antibodies, the overlapping of bound sites on antigens might be prevented and DIGFA could be applied for FQs as for polyvalent antigens. This assumption was supported by the results of a sandwich ELISA which was carried out for selection of antibodies. As shown in , when rabbit anti-ENR antibodies (R-PAbs) were selected as coating antibodies, very low concentrations of ENR (0.01 µg mL−1) could be detected with mice anti-ENR antibodies (M-PAbs). But when M-PAbs were used as coating antibodies, no significant binding between ENR and R-PAbs were observed even at an ENR concentration of 100,000 µg mL−1. So M-PAbs were chosen for colloidal gold labelling and R-PAbs for coating of NCMs.
Table 1. Absorbance values (450 nm) after sandwich ELISA for enrofloxacin. Each value represents the average of three measurements.
The preparation of colloidal gold-labelled antibody
The prepared colloidal gold solution was investigated under a transmission electron microscope, where circular particles with uniform size were observed. The diameter of these gold particles was about 15 nm (A). After the reaction between anti-ENR M-PAbs and colloidal gold, large areas of dark spots were encircled by colloidal gold particles (B). This demonstrated that gold particles were successfully linked to antibodies.
Pretreatment of nitrocellulose membranes (NCMs)
Main parameters for pretreatment of NCMs were studied, and the optimised conditions were listed in and used for further experiments. For coating of NCMs, as many as possible immobilised antibodies are desired for high sensitivity. More importantly, the immobilisation should be strong enough to prevent the washing off of antibodies during detection. Here, buffer systems and incubation conditions were evaluated for their influence on DIGFA. When the antibody concentration was fixed to 10 mg mL−1, the selected optimal condition was to coat NCMs at 37°C for 2 h in carbonate buffers (pH 9.6, 0.01 M); such a condition allowed the appearance of reddish dots with the deepest colour and best clarity.
Table 2. Parameters studied and the optimum selected for pretreatment of nitrocellulose membranes.
Another problem often faced in DIGFA is the non-specific binding of gold-labelled probes to membranes, which will lead to “false positive” results. In analogy with ELISA, one can use some blocking agents to suppress this effect. Here, this blocking was realised by using 0.5% BSA (in PBS) to treat coated membranes at 37°C for 2 h. After such treatment, the spots of controls showed no visible reddish colour.
Specificity, sensitivity and repeatability of dot-immunogold filtration assay (DIGFA) in pure buffer matrix
The specificity, sensitivity and repeatability of developed DIGFA were at first evaluated with in PBS (0.01 M, pH 7.4). The cross-reactivity of antibodies exploited in the DIGFA to some potential interference were determined and listed in . Except ENR, CIP, NOR and ofloxacin, the antibodies demonstrated no significant ability to bind with other (fluoro)quinolones and other three antimicrobial agents (chloramphenicol, oxytetracycline and sulphamethoxazole) most commonly used in aquiculture. Then DIGFA were performed for above 11 drugs to confirm the specificity of developed analysis. At a concentration of 1 µg g−1 (about 10 − 20 times of MRLs of ENR, CIP and NOR), significant positive results were only observed for ENR, CIP and NOR. After the determination of other eight drugs, the colour of spots were the same as that of negative controls; such results allowed us to exclude the interference of these commonly used antimicrobial agents to the analysis of ENR, CIP and NOR.
Table 3. The specificity of prepared anti-ENR antibodies. Each value of cross-reactivity represents the average of three measurements. The DIGFA were performed with the standards of 11 competitors which dissolved in phosphate buffers (pH 7.4, 0.01 M) at a concentration of 1 µg g−1.
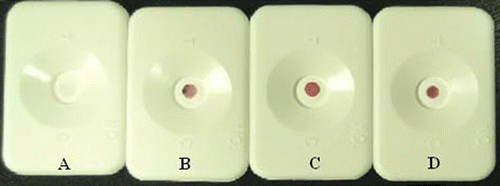
For serious ENR dilutions, when the concentration was higher than 10 ng mL−1, positive results could be clearly observed by the naked eye (, ), and the reddish colour of spots became deeper and deeper with the increment of ENR concentration from 10 ng mL−1 to 50 ng mL−1. The same experiments were also carried out for CIP and NOR (), and the lowest concentrations to distinguish significant positive results were observed to be 10 ng mL−1 and 50 ng mL−1, respectively.
Figure 4. DIGFA for different concentration of enrofloxacin in phosphate buffers (pH 7.4, 0.01 M). A: blank; B: 10 ng mL−1; C: 20 ng mL−1; D: 50 ng mL−1.
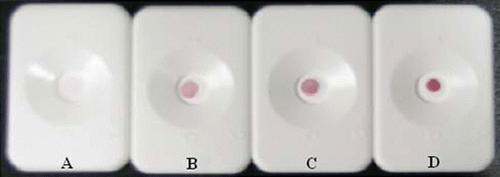
Table 4. The sensitivity and repeatability of DIGFA for ENR, CIP and NOR standards in phosphate buffers (pH 7.4, 0.01M).
The repeatability of DIGFA for ENR, CIP and NOR was evaluated with 3~6 parallel determinations at the concentration of 10~50 ng mL−1. In replicate experiments, the observed reddish spots were approximately homogeneous in visibility (), which proved a good repeatability of the analysis.
Analysis of fluoroquinolones (FQs) in spiked eel samples
The eel samples were spiked with ENR, CIP and NOR at concentrations from 10 µg kg−1 to 50 µg kg −1, and then extracted by a mix of water and methanol. The extracts were analysed with HPLC and DIGFA, respectively. The average recovery of HPLC determinations was from 66.9 to 86.9%, and the relative standard deviation was from 2.02 to 4.57%, which demonstrated a good efficiency of extraction ().
Table 5. Determination of EF, CIP and NOR in eel muscles.
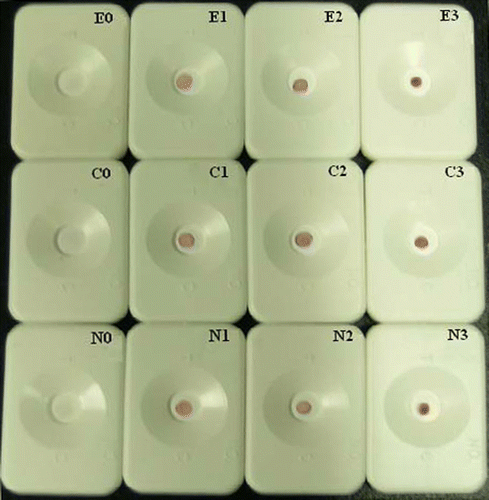
There were no significant difference in the dot colour of DIGFA among replicate samples, and the results could fit well with those of HPLC determinations. When the detection limit was defined as the lowest concentration of the analyte in spiked samples to result in significant positive results, it was estimated to be 20 µg kg−1 or a little lower for ENR and CIP, and 50 µg kg−1 or a little lower for NOR (, ). The sensitivity was much higher than that observed in our previous works, in which DNA was exploited as a broad-selective receptor for FQs and the detection limit of a developed DNA-based biosensor was estimated to be about 3 µg mL−1 for ENR in milk samples (Cao, Lin, & Mirsky, Citation2007). Given the average extracting recovery of 66.9~86.9%, the observed sensitivity of DIGFA for spiked samples consisted with that for pure buffer matrix. According to regulations of most countries and areas, the MRL for ENR, CIP and NOR in fishery products or other foodstuffs have been settled above 50 µg kg−1, usually at a range of 100 µg kg−1−200 µg kg−1 (Agricultural Industry Criteria of People's Republic of China, Citation2001, Citation2005; Hernández-Arteseros et al., Citation2002; Lin et al., Citation2002). So in most cases, the developed DIGFA is sensitive enough for screening of ENR, CIP and NOR residues.
Figure 6. The DIGFA for three fluoroquinolones residues in spiked eel muscles. E0, E1, E2 and E3 referred to blank and samples spiked with enrofloxacin of 20 µg kg−1, 50 µg kg−1 and 100 µg kg−1, respectively; C0, C1, C2 and C3 referred to blank and samples spiked with ciprofloxacin of 20 µg kg−1, 50 µg kg−1 and 100 µg kg−1, respectively; N0, N1, N2 and N3 referred to blank and samples spiked with norfloxacin of 50 µg kg−1, 100 µg kg−1 and 150 µg kg−1, respectively.
It is to note that the analysis (except pretreatments of samples and NCMs) could be completed in 30 minutes; it was much faster than traditional ELISA, HPLC and microbiological assays, which usually cost at least several hours. The greatly simplified procedure (only two steps) as well as non-enzyme labelled probes both help to increase the stability and precision of the determination. The test result was visible and could be read by the naked eye instead of any instrument, which made it very suitable for field analysis.
Conclusions
According to our knowledge, this is the first description of DIGFA for the detection of (fluoro)quinolones. The operation was optimised and tested with a fishery food sample, and proved to be much faster and simpler than present analytical methods such as ELISA and HPLC. In most cases, the proposed method is also sensitive and precise enough for qualitative detection of ENR, CIP and NOR in animal products. This allows us to suggest its applications for rapid simultaneous screening of these three FQs in foodstuffs or other biological matrix, for example, the routine monitoring of a large number of food samples in factories, farms and markets where the laboratory facilities are usually limited.
Acknowledgements
This work was supported by the National Natural Science Funding of China (No. 30400336) and National Agricultural Funding of China (NYHYZX 07-046).
References
- Agricultural Industry Criteria of People's Republic of China . 2001 . Non-polluted food Anguilla japonica (NY5068-2001) . Beijing : Standards Press of China .
- Agricultural Industry Criteria of People's Republic of China . 2005 . Non-polluted food Sciaenidae (NY5060-2005) . Beijing : Standards Press of China .
- Bucknall , S. , Silverlight , J. , Coldham , N. , Thorne , L. and Jackman , R. 2003 . Antibodies to the quinolones and fluoroquinolones for the development of generic and specific immunoassays for detection of the seresidues in animal products . Food Additives and Contaminants , 20 : 221 – 228 .
- Cao , L.M. , Lin , H. and Mirsky , V.M. 2007 . Surface plasmon resonance biosensor for enrofloxacin based on deoxyribonucleic acid . Analytica Chimica Acta , 589 : 1 – 5 .
- Cerniglia , C.E. and Kotarski , S. 1999 . Evaluation of veterinary drug residues in food for their potential to affect human intestinal microflora . Regulatory Toxicology and Pharmacology , 29 ( 3 ) : 238 – 261 .
- Cho , H.J. , Abd El-Aty , M. , Goudah , A. , Sung , G.M. , Yi , H. Seo , D.C. 2008 . Monitoring of fluoroquinolone residual levels in chicken eggs by microbiological assay and confirmation by liquid chromatography . Biomedical Chromatography , 22 : 92 – 99 .
- Duan , J.H. and Yuan , Z.H. 2001 . Development of an indirect competitive ELISA for ciprofloxacin residues in food animal edible tissues . Journal of Agricultural and Food Chemistry , 49 : 1696 – 1700 .
- Hernández-Arteseros , J.A. , Barbosa , J. , Compaño , R. and Prat , M.D. 2002 . Analysis of quinolone residues in edible animal products . Journal of Chromatography A , 945 : 1 – 24 .
- Hiroo , W. , Atsuko , S. , Yasumasa , K. and Akio , T. 2002 . Monoclonal-based enzyme-linked immunosorbent assay and immunochromatographic assay for enrofloxacin in biological matrices . Analyst , 127 : 98 – 103 .
- Holtzapple , K. , Buckley , A. and Stanker , H. 1997 . Production and characterization of monoclonal antibodies against Sarafloxacin and cross-reactivity studies of related fluoroquinolones . Journal of Agricultural and Food Chemistry , 45 : 1984 – 1900 .
- Huang , Q. , Lan , X. and Tong , T. 1996 . Dot-immuneogold fitration assay as a screening test for syphilis . Clincal Microbiology , 34 : 2011 – 2013 .
- Huang , Q.J. , Tang , Y. , Liu , W.P. , Yu , S. , Lan , X.P. Xu , B. 1998 . Qualitative bedside assay of increased human serum myoglobin by sandwich dot-immunogold filtration for the diagnosis of acute myocardial infarction . Clinica Chimica Acta , 273 : 119 – 130 .
- Idowu , O.R. and Peggins , J.O. 2004 . Simple rapid determination of enrofloxacin and ciprofloxacin in bovine milk and plasma by high-performance liquid chromatography with fluorescence detection. Journal of Pharmaceutical and Biomedical Analysis , 35 : 143 – 153 .
- Lin , W.X. 2002 . The compilation of residue limits stands for pesticides and veterinary drugs in foodstuffs in the world , Dalian : Dalian Maritime University Press .
- Liu , C.E. , Lin , H. , Cao , L.M. and Jiang , J. 2005a . Studies on synthesis and characteristics of complete antigens of enrofloxacin . Journal of Fisheries of China , 29 : 534 – 539 .
- Liu , C.E. , Lin , H. , Cao , L.M. and Jiang , J. 2005b . Anti-enrofloxacin antibody production by using enrofloxacin-coated HSA as immunogen . Journal of Ocean University of China , 14 ( 3 ) : 262 – 266 .
- Marazuela , M.D. and Moreno-Bondi , M.C. 2004 . Multiresidue determination of fluoroquinolones in milk by column liquid chromatography with fluorescence and ultraviolet absorbance detection . Journal of Chromatography A , 1034 : 25 – 32 .
- Oliver , C. 1999 . Conjugation of colloidal gold to proteins . Method in Molecular Biology , 115 : 331 – 334 .
- Schneider , M.J. and Donoghue , D.J. 2002 . Multiresidue analysis of fluoroquinolone antibiotics in chicken tissue using liquid chromatography-fluorescence-multiple mass spectrometry . Journal of Chromatography B , 780 : 83 – 92 .
- Spielberg , F. , Kabeya , C.M. and Ryder , R.W. 1989 . Field testing and comparative evulation of rapid visually read screening for antibody to human immunodeficiency virus . Lancet , 1 : 580 – 585 .
- Turnipseed , S.B. , Walker , C.C. and Roybal , J.E. 1998 . Confirmation of fluoroquinolones in catfish muscle by electrospray liquid chromatography/mass spectrometry . Journal of AOAC International , 81 : 554 – 562 .
- Wen , L.Y. , Chen , J.H. , Ding , J.Z. , Zhang , J.F. , Lu , S.H. Yu , L.L. 2005 . Evaluation on the applied value of the dot-immunogold filtration assay (DIGFA) for rapid detection of anti-Schistosoma japonicum antibody . Acta Tropica , 99 ( 2–3 ) : 142 – 147 .
- Zhan , W.B. , Wang , X.J. , Chen , J. , Jing , X. and Hideo , F. 2004 . Elimination of shrimp endogenous alkaline phosphatase background and development of enzyme immunoassays for the detection of white spot syndrome virus (WSSV) . Aquaculture , 239 : 15 – 21 .