Abstract
Citrinin (CIT)-protein synthetic antigen was prepared by conjugating CIT with keyhole limpet hemocyanin by 1,4-butanediol diglycidyl ether. The ultraviolet and infrared spectrometry analyses indicated that CIT was conjugated with the carrier protein successfully. By fusion and cloning, a hybridoma cell line K2-F3 which stably secreted the highly specific monoclonal antibody (McAb) against CIT was obtained. The indirect competitive ELISA showed that the IC50 value was 0.3 ng/mL and the limit of detection, 0.01 ng/mL. Cross-reactivity was less than 0.01% with four mycotoxins, aflatoxin B1, ochratoxin A, zearalenone, deoxynilvalenol and three pigments, rubropunctatine, rubropunctamine, monascin. The mean recovery of citrinin from artificially contaminated red yeast rice was from 89 to 92%, with CVs from 6.8 to 9.0%. The ELISA developed in this study can accurately determine CIT in artificially contaminated red yeast rice.
Introduction
Citrinin (CIT, ) is a toxic fungal metabolite produced by genera Penicillium(Ei-Banna, Pitt, & Leistner, Citation1987), Aspergillus (Manabe, Citation2001) and Monascus (Blanc, Loret, & Goma, Citation1995). CIT often occurs in naturally contaminated commodities, such as in corn, barley, wheat, apple and other foods (Jackson & Ciegler, Citation1978; Hartl & Stenzel, Citation2007; Gimeno & Martins, Citation1983; Comerio, Fernandez Pinto, & Vamonde, Citation1998). CIT is hepato-nephrotoxic and implicated in disease outbreaks in animals and humans. Nowadays, food safety problems due to the contamination of CIT have been a growing concern among the government and public. Because CIT is a toxin, for the quality control and safety precaution of Monascus products, standard methods for the determination of CIT have been established in China and Japan.
Various analytical methods have been reported for the determination of CIT such as TLC (Damodaran, Ramadoss & Shanmugasundaram Citation1973), HPLC (Reinhard & Zimmerli, Citation1999),LC–MS (Tuomi, Hohnsson, Hintikka, & Reijula, Citation2001) , GC–MS (Shu & Lin, Citation2001) and ELISA techniques (Kononenko & Burkin, Citation2007). Xu, Jia, Gu & Sung (Citation2006) summarised qualitative and quantitative analytical methodology for CIT and evaluated their advantages and disadvantages. Among the reported methods, HPLC is the most widely used method for the determination of CIT, with ultraviolet (UV) or fluorescence detection. The UV detection produced relatively low sensitivities of 1–5 mg/mL (Reinhard & Zimmerli, Citation1999), while the detection limit of CIT reached 0.5 ng/mL with a fluorescence detection (Zheng, Xin, & Guo, Citation2009). Although some development in analysis of CIT has been achieved, a rapid and sensitive quantitative analysis method remains a challenge.
Immunochemical assay techniques can be used to examine the contaminated samples on a large scale. Enzyme immunoassay methods with high sensitivity and specificity have been widely used for the determination of mycotoxins including CIT (Goryacheva Rusanova, Burmistrova, & Saeger, Citation2009). Several studies used the polyclonal antibodies (PcAbs) for the determination of CIT by ELISA. PcAbs were poor in sensitivity and specificity, and systematic errors were high because of high cross-reactivities. Due to the lower recoveries and higher coefficients of variation, the sensitivity of ELISA using PcAb was probably not sufficient to analyse CIT at low concentration (Xu, Jia, Gu & Sung, Citation2006). However, there are a few reports about the preparation of McAb against CIT (Liu Yu, He & Xu, Citation2009; Wang, Li, & Guo, 2010). The sensitivity of the determination of CIT based on McAbs is not satisfactory because of the higher IC50 values (10–200 ng/mL). For more sensitive and accurate determination of CIT, a McAb against CIT with high sensitivity and specificity may be preferable.
In the present work, a new bifunctional coupling method was proposed for the preparation of CIT-protein synthetic antigen. Based on the acquired McAb against CIT, a rapid and sensitive analytical method for the determination of CIT was developed using indirect competitive ELISA.
Materials and methods
Chemicals, reagents and instruments
Citrinin (CIT), keyhole limpet hemocyanin (KLH), bovine serum albumin (BSA), PEG with average molecular mass 2000 (PEG2000) and horseradish peroxidase labelled goat anti-mouse IgG were purchased from Sigma (St. Louis, MO, USA). Ovalbumin (OVA) was purchased from Worthington (Lakewood, NJ, USA). 1,4-Butanediol diglycidyl ether was obtained from Alfa Aesar (Ward Hill, MA, USA). The isotyping test kit was obtained from Southern Biotech (Birmingham, AL, USA). rProtein A Sepharose Fast Flow was obtained from Amersham Biosciences (Uppsala, Sweden). High-binding polystyrene plates were obtained from Costar (Lowell, MA, USA). Bicinchoninic acid (BCA) protein assay kit was purchased from Pierce (Rockford, IL, USA). Other solvents and reagents used were of analytical grade and obtained from commercial sources.
UV spectra were recorded on a UV-1800 instrument (Shimadzu, Kyoto, Japan). IR spectra were recorded on a SP2000 instrument (Perkin-Elmer, Waltham, MA, USA). ELISA was performed by using SH-1000 instrument (Hitachi, Kyoto, Japan).
Preparation and identification of CIT-protein synthetic antigen
The 1,4-butanediol diglycidyl ether was used for the synthesis of CIT conjugates with proteins. CIT (0.5 mg) was dissolved in 0.5 mL NaOH (0.6 mol/L), then 0.5 mL NaBH4 (2 mg/mL) and 0.5 µL 1,4-butanediol diglycidyl ether were added. After 4 h reaction at 25°C , 31.8 mg Na2CO3 and 2.1 mg KLH were added. The mixture was incubated at 37°C for 24 h. After the reaction, 100 mg Glycine (free base) was added to products. The products incubated at 37°C for further 48 h. The final conjugates were dialysed at 4°C for 48 h against phosphate-buffer saline (PBS, 0.01 mol/L phosphate, 0.1 mol/L NaCl, pH 7.4). The CIT–KLH conjugate was lyophilised and stored until usage. The coating antigen CIT–OVA was prepared with the same method. 0.5 mg KLH and 1.0 mg CIT–KLH were dissolved in 1.0 mL PBS buffer (0.01 mol/L, pH 7.4) and scanned by UV-1800 at 200–450 nm. 1.0 mg KLH and 1.0 mg CIT–KLH were scanned, respectively, by SP2000 with a pressing potassium bromide troche.
Coupling ratios (moles of a hapten per mole of a protein carrier) were determined as follows. KLH concentration of the conjugate was determinated at 570 nm via the BCA protein assay kit. CIT concentration of the conjugate was calculated from the UV absorbance spectrum (Alarcón, Palleschi, Compagnone, Pascale, Visconti & Barna-Vetró, Citation2006).
Preparation of the McAb against CIT
Eight BALB/c mice were immunised with CIT–KLH. Each mouse received 50 µg CIT–KLH conjugate in Freund's complete adjuvant in the first intraperitoneal (i.p) injection. After 2 weeks, each mouse received injections of the same conjugates in Freund's incomplete adjuvant i.p injection three times, every 2 weeks. Antisera were obtained from the mice every 2 weeks after the primary injection and tested for the titer by ELISA. Each mouse received the same conjugates without adjuvant intra peritoneally 3 days before fusion. The spleen cells of the mouse immunised by CIT–KLH conjugate were hybridised with SP2/0 myeloma cells by PEG2000. A 50% PEG2000 solution was added to the cell mixture for fusion. Hybridoma lines producing antibodies were cloned at least three times by limiting dilution and tested by ELISA. Antibody-containing ascites from BALB/c mice were prepared according to the method described by Groopman, Trudelt, Donaheut, Marshak-Rothstein & Wagan (Citation1984). The McAbs against CIT from the mouse ascites were purified by rProtein A affinity chromatography (Chou, Citation2004). SDS–PAGE was used to identify the purified McAbs (Duan et al., Citation2009). Isotyping was carried out with the test kit.
Determination of titer of antibodies by indirect non-competitive ELISA
ELISA Plates were coated with CIT–OVA conjugate. The CIT–OVA conjugate was diluted to 10 µg/mL with sodium carbonate buffer (0.1 mol/L, pH 9.5) and 100 µL was added per well to plates. Plates were incubated overnight at 4°C. Then, the plates were washed three times with 200 µL PBS buffer containing 0.05% Tween 20 for removing the excess of the unbound antigen. The plates were blocked with casein sodium salt (2% in PBS) and incubated for 2 h at 37°C. The plates were washed three times with 200 µL PBS buffer containing 0.05% Tween 20. The antibody was diluted (1:10 to 1:200,000) and added to each well. Plates were incubated for 2 h at 37°C and were then washed three times with 200 µL PBS buffer containing 0.05% Tween 20. Horseradish peroxidase-labelled goat anti-mouse IgG was diluted 1:2000 in PBS buffer and 100 µL was added to each well. After 1 h incubation at 37°C, the plates were washed again and 100 µL of 3,3′,5,5′-tetramethylbenzidine (TMB) chromogen-substrate was added to each well. After 20 min incubation at room temperature, the reaction was stopped with 100 µL of H2SO4 (1 mol/L). The optical density was measured at 450 nm with a SH-1000 instrument. The titer was defined as the dilution giving an optical density greater than 2.1 times the negative control (P/N ≥ 2.1).
Determination of CIT by indirect competitive ELISA
Plates coated with CIT–OVA conjugate were prepared as above. McAbs diluted 1:2000 were added in a volume of 50 µL per well. The CIT standard was accurately weighed (1.0 mg) and added into 1.0 mL of ethanol solution in a tube and diluted with 10% methanol. Following that, 50 µL of the CIT standard (0 ng/mL, 0.001 ng/mL, 0.005 ng/mL, 0.01 ng/mL, 0.05 ng/mL, 0.1 ng/mL, 0.5 ng/mL, 1 ng/mL, 10 ng/mL, 100 ng/mL) was added. These reagents were incubated simultaneously for 2 h at 37°C. The plates were washed three times with 200 µL PBS buffer containing 0.05% Tween 20. Horseradish peroxidase-labelled goat anti-mouse IgG was diluted 1:2000 in PBS buffer and 100 µL was added to each well. After 1 h incubation at 37°C, the assay was developed as described above. The limit of detection (LOD) was calculated as three times the standard deviation from the mean signal measured from the blank wells. Inhibition was calculated as follows:
Cross-reactivity
The specificity of the new McAb was studied with four mycotoxins and three pigments as competitive antigens in the indirect competitive ELISA test. Four mycotoxins were AFB1, OTA, ZEA and DON. Three pigments were rubropunctatine, rubropunctamine and monascin. Plates coated with CIT–OVA conjugate were prepared with a concentration of 10 µg/mL. Fifty microlitre of the each standard (0 ng/mL, 0.01 ng/mL, 0.1 ng/mL, 1.0 ng/mL, 10 ng/mL, 100 ng/mL, 1000 ng/mL) was added to each well. The determination was performed by the indirect competitive ELISA. IC50 represents the concentration at which binding of the antibody to the coating antigen is inhibited by 50%.
Evaluation of the precision in the determination of CIT in artificially contaminated red yeast rice samples by ELISA
Red yeast rice was selected as a matrix for testing ELISA performance. Samples (1.000 g) were artificially contaminated with methanol solutions of citrinin to give final concentrations of 1.0, 10, 100 ng/g. Each sample was added to 10 mL of 70% ethanol solution in a tube. The sample was treated in an ultrasonicator at 300 W for 10 min. The CIT in the extract was diluted with 10% methanol and determined by indirect competitive ELISA.
Results
Preparation of CIT-protein synthetic antigen
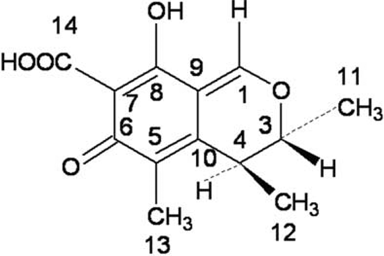
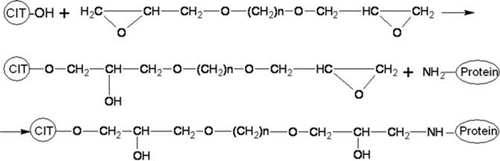
Synthetic scheme for CIT-protein conjugate by the bifunctional coupling method is shown in . The bifunctional coupling reagent is (C2H3O)–CH2–O– (CH2)n-O–CH2–(C2H3O), n = 2–10, in which (C2H3O) was epoxy group. By way of ring-opening polymerisation of epoxy group, CIT-protein synthetic antigen would be synthesised by first reacting with the hydroxyl group in CIT and then with the NH2 group in protein. In the present work, both the immunising antigen CIT–KLH and coating antigen CIT–OVA were synthesised using 1,4-butanediol diglycidyl ether (n=4).
Identification of CIT-protein synthetic antigen
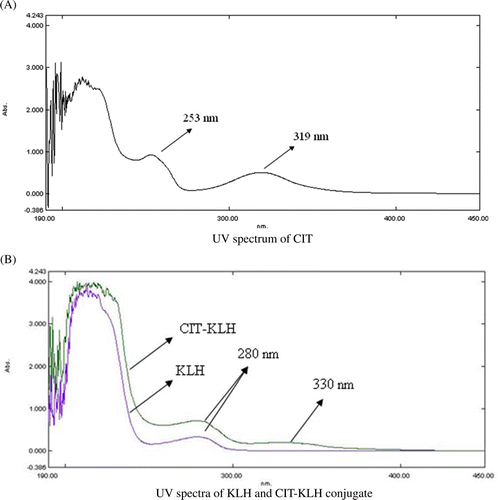
UV and IR absorption spectra were used to identify the CIT-protein synthetic antigen. The UV absorption spectra of CIT, KLH and CIT–KLH are shown in . The characteristic peaks of KLH and CIT were at 280 and 319 nm, respectively, while two characteristic peaks of both 280 and 330 nm were demonstrated in the UV spectrum of CIT–KLH conjugate.
Figure 3. UV spectra of CIT, KLH and CIT–KLH conjugate. (A) UV spectrum of CIT. (B) UV spectra of KLH and CIT–KLH conjugate
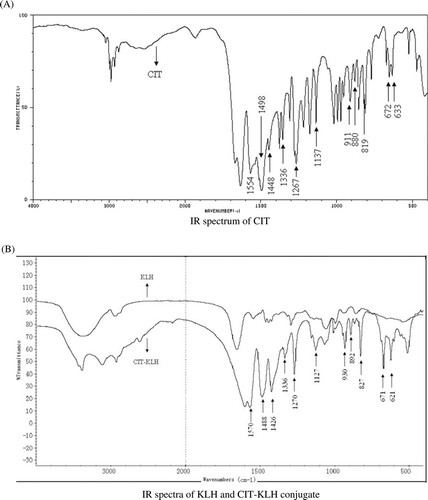
The IR absorption spectra of CIT, KLH and CTI–KLH are shown in . The IR absorption spectrum of CIT from spectral database system for organic compounds (SDBS, Japan) showed characteristic bands in the region of 600–1600 cm−1, especially at 633, 672, 819, 880, 1137, 1267, 1448, 1498 and 1554 cm−1. As for KLH, CIT–KLH displayed a characteristic broad stretching band nearby 3000 cm−1 for N–H bond. The intense band around 1500 cm−1 was due to C = O stretching vibration. Furthermore, the characteristic bands in the region of 600–1600 cm−1 were also found in CIT–KLH, indicating the presence of CIT structure. Based on the UV and IR absorption spectra, it could be concluded that the CIT had been conjugated successfully with KLH by 1,4-butanediol diglycidyl ether.
Preparation of the McAbs against CIT
After a two-month series of immunisations of mice with the CIT–KLH conjugate, mouse sera had a mean titer of 96,000. The isolated spleen cells from the immunised mouse were fused with SP2/0 myeloma cells by PEG2000. A 50% PEG2000 solution was added to the cell mixture for fusion. Limiting-dilution cloning was used to isolate the positive clone. Through screening, hybridoma K2-F3 was obtained which stably secreted McAb against CIT. Mouse ascites fluids were elicited with hybridoma, and the titer of McAbs purified by rProtein A affinity chromatography was 1.38 × 105. Isotyping revealed IgM isotype for the McAb. The molecular weight of heavy chain and light chain of IgM were approximately 65 and 25 KD, respectively from the analysis with SDS–PAGE (data not shown).
The determination of CIT by indirect competitive ELISA
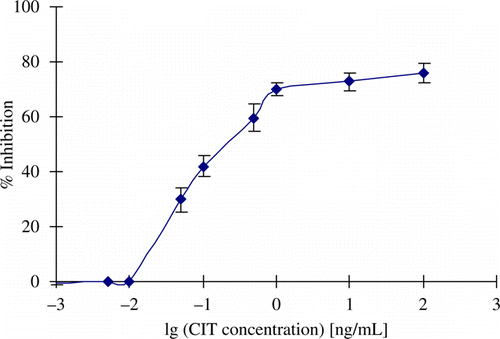
The standard curve for the determination of CIT by indirect competitive ELISA with McAb is shown in . CIT was used as a positive control to compete with the CIT–OVA antigen for the anti-CIT McAb. There was a dose-dependent increase in inhibition. The IC50 value was 0.3 ng/mL. The LOD was 0.01 ng/mL, with good linearity in the range 0.01–1.0 ng/mL.
Cross-reactivity of McAb with structurally similar molecules
Four mycotoxins and three Monascus pigments were used as competitive antigens in the indirect competitive ELISA for testing the specificity of the McAb. Their structures are shown in . As demonstrated in , the cross-reactivity was less than 0.01% for all compounds. None of four mycotoxins or three pigments inhibited antibody binding to coating antigen in the solid phase.
Table 1. Cross-reactivity of McAb.
Evaluation of the precision in the determination of CIT in artificially contaminated red yeast rice samples by ELISA
Citrinin often occurs in naturally contaminated Monascus products such as red yeast rice which was produced by Monascus fermentation. Red yeast rice was therefore selected as a matrix for testing ELISA performance. Samples were artificially contaminated with methanol solutions of citrinin to give final concentrations of 1, 10, 100 ng/g. The recovery of citrinin from artificially contaminated red yeast rice is shown in . The ELISA produced higher levels of recovery of citrinin added to red yeast rice. Mean recoveries were 92%, 92% and 89% for citrinin added at 1, 10, 100 ng/g, respectively. The systematic error was low, with the coefficient of variation between 6.8% and 9.0%. The ELISA developed in this study can accurately determine CIT in the artificially contaminated red yeast rice.
Table 2. Determination of CIT in artificially contaminated red yeast rice samples.
Discussion
To prepare the desired antibody, a CIT-protein synthetic antigen with a proper structure is necessary. There are four active functional groups in the CIT molecule, each of which can be used to conjugate protein molecule (). The reaction of the carbonyl group with carboxymethoxylamine or aminobenzaldehyde hydrazine and the reduction of the carbonyl group at C6 were found unsuccessful to prepare haptens (Kononenko & Burkin, Citation2007). The active ester method was reported for the preparation of CIT–KLH by conjugating the carboxyl group at C7 with the amino group of protein molecule (Liu et al., Citation2009). As shown in , although the determined antibody titer was as high as 40,000, CIT could not inhibit the antibody binding to the coating antigen (CIT–OVA) in the indirect competitive ELISA. This result indicated that this method did not elicit antibody with high specificity (Wang et al., 2010). Similar results were also reported by Liu et al. (Citation2009). The carbonyl group at C6 and the carboxyl group at C7 in CIT are probably essential parts of CIT epitope. They should be protected during the preparation of the hapten conjugate.
Table 3. Comparison of immunogenicity of CIT conjugates prepared by different methods.
The formaldehyde condensation method is the most widely used method for the synthesis of CIT-protein conjugate with the active H at C1 (Abramson, Usleber, & Martlbauer, Citation1995). The CIT–BSA conjugate was synthesised and successfully used to elicit the production of CIT-specific antibodies. In the previous work, we immunised the mice with CIT–BSA and the titer of PcAb in the antiserum reached 65,000 (Wang et al., Citation2010). Our experimental data showed that the detection limit for the acquired PcAb and McAb were 10 and 1 ng/mL, respectively, which were similar to the reported data as shown in . However, the formaldehyde condensation method had two disadvantages: (1) the resulting antibody resulted in an insensitive assay; (2) it was difficult to regulate the coupling ratio of CIT/protein of the conjugate. A high reactant ratio of CIT/protein, in a 286-fold molar excess and formaldehyde/CIT, in a 52,700-fold molar excess, were necessary for the preparation of CIT-protein synthetic antigen (Kononenko & Burkin, Citation2007).
The bifunctional coupling method was used to covalently couple protein and CIT based on the hydroxyl group at C8 in CIT (Liu et al., Citation2009; Xiao et al., Citation1995). Different experimental results were reported with the synthetic antigen prepared by 1,1-carbonyldiimidazole (Liu et al., Citation2009; Xiao et al., Citation1995). In this study, the CIT–KLH conjugate was synthesised by 1,4-butanediol diglycidyl ether with the hydroxyl group at C8. The CIT–KLH antigen elicited the desired immune response in mice, resulting in a high titer of 96,000 in the antiserum, approximately 2–8 times higher than those acquired by other methods. This favourable result may have been achieved because of the flexible chain with a suitable length provided by 1,4-butanediol diglycidyl ether [(CH2)n,n = 4] between CIT and KLH. The McAb against CIT with high specificity and sensitivity was obtained in the present work. In this work, a new bifunctional coupling method was developed for the preparation of a synthetic antigen, which had the advantages of mild conditions, no toxicity and easy regulation of the coupling ratio of CIT/protein. It was perhaps most important that the antigen had the high immunogenicity to elicit a stable and specific immune response.
Based on the acquired McAb against CIT, a rapid and sensitive analysis method for the determination of CIT was developed by an indirect competitive ELISA. The ELISA developed in this study can accurately determine CIT in the artificially contaminated red yeast rice, with good precision.
Acknowledgements
This work was supported by a grant from fund of Fujian Key Laboratory of Medical Devices and Pharmaceutical Technology (No. 09003).
References
- Abramson , D. , Usleber , E. and Martlbauer , E. 1995 . An indirect enzyme immunoassay for the Mycotoxin Citrinin . Applied and Environmental Microbiology , 61 ( 5 ) : 2007 – 2009 .
- Alarcón , S.H. , Palleschir , G. , Compagnone , D. , Visconti , A. and Barna-Vertró , I. 2006 . Monoclonal antibody based electrochemical immunosensor for the determination of ochratoxin A in wheat . Talanta , 69 ( 5 ) : 1031 – 1037 .
- Blanc , P.J. , Loret , M. and Goma , G. 1995 . Production of citrinin by various species of Monascus . Biotechnology Letters , 17 : 291
- Chou , S.F. 2004 . Production and purification of monoclonal and polyclonal antibodies against cholera toxin . Hybrid Hybridomics , 23 ( 4 ) : 258 – 261 .
- Comerio , R. , Fernandez Pinto , V.E. and Vamonde , G. 1998 . Influence of water activity on penicillium citrinum growth and kinetics of citrinin accumulation in wheat . International Journal of Food Microbiology , 42 : 219 – 223 .
- Damodaran , C. , Ramadoss , C.S. and Shanmugasundaram , E.R.B. 1973 . A rapid procedure for the isolation, identification and estimation of citrinin . Analytical Biochemistry , 52 : 482 – 488 .
- Duan , Z.H. , Lin , Z.S. , Yao , H.R. , Gao , Y.H. , Zang , K. Zhao , S.Q. 2009 . Preparation of artificial antigen and egg yolk-derived immunoglobulin(IgY) of citrinin for enzyme-linked immunosorbent assay . Biomedical and Environmental Sciences , 22 : 237 – 243 .
- Ei-Banna , A.A. , Pitt , J.I. and Leistner , L. 1987 . Production of mycotoxins by Penicillium species . Systematic and Applied Microbiology , 10 : 42 – 46 .
- Gimeno , A. and Martins , M.L. 1983 . Rapid thin layer chromatographic determination of patulin, citrinin, and aflatoxin in apples and pears, and their juices and jams . Journal of AOAC (Association of Official Analytical Chemists) , 66 ( 1 ) : 85 – 91 .
- Goryacheva , I.Y. , Rusanova , T.Y. Burmistrova , N.A. 2009 . Immunochemical methods for the determination of Mycotoxins . Analytical Chemistry , 64 : 768 – 785 .
- Groopman , J.D. , Trudelt , L.J. , Donahuet , P.R. , Marshak-Rothstein , A. and Wagan , G.N. 1984 . High-affinity monoclonal antibodies for aflatoxins and their application to solid-phase immunoassays . Proceedings of the National Academy of Sciences USA , 81 ( 29 ) : 7728 – 7731 .
- Hartl , A. and Stenzel , W.-R. 2007 . Development of a method for the determination of citrinin in barley, rye and wheat by solid phase extraction on aminopropyl columns and HPLC-FLD . Mycotoxin Research , 23 ( 3 ) : 127 – 131 .
- Jackson , L.K. and Ciegler , A. 1978 . Production and analysis of citrinin in corn . Applied and Environmental Microbiology , 36 ( 3 ) : 408 – 411 .
- Kononenko , G.P. and Burkin , A.A. 2007 . Immunoenzyme method for the determination of citrinin . Analytical Chemistry , 62 ( 7 ) : 769 – 774 .
- Liu , R.R. , Yu , Z. , He , Q.H. and Xu , Y. 2009 . Preparation and identification of a monoclonal antibody against citrinin . Journal of Hygiene Research , 36 ( 2 ) : 190 – 193 .
- Manabe , M. 2001 . Fermented foods and mycotoxins . Mycotoxins , 51 : 25 – 28 .
- Reinhard , H. and Zimmerli , B. 1999 . Reversed-phase liquid chromatographic behavior of the mycotoxins citrinin and ochratoxin A . Journal of Chromatography A , 862 : 147 – 159 .
- Shu , P.Y. and Lin , C.H. 2001 . Simple and sensitive determination of citrinin in monascus by GC-Selected ion moritoring mass spectrometry . Analytical Sciences , 18 : 283 – 287 .
- Tuomi , T. , Hohnsson , T. , Hintikka , E.L. and Reijula , K. 2001 . Detection of aflatoxins(G(1-2),B(1-2)), sterigmatocystin, citrinine and ochratoxin A in samples contaminated by microbes . The Analyst , 126 ( 9 ) : 1545 – 1550 .
- Wang , Y.Y. , Li , Y.N. and Guo , Y.H. 2010 . Preparation of CIT-protein antigen and antibody with the bicoupling method . Progress in Biochemistry and Biophysics , 37 ( 3 ) : 337 – 341 .
- Xiao , H. , Clarke , J.R. , Marquardt , R.R. and Frohlich , A.A. 1995 . Improved methods for conjugating selected Mycotoxins to carrier proteins and dextran for immunoassays . Journal of Agricultural and Food Chemistry , 43 : 2092 – 2097 .
- Xu , B.J. , Jia , X.Q. , Gu , L.J. and Sung , C.K. 2006 . Review on the qualitative and quantitative analysis of mycotoxin citrinin . Food Control , 17 : 271 – 285 .
- Zheng , Y.Q. , Xin , Y.W. and Guo , Y.H. 2009 . Study on the fingerprint profile of monascus products with HPLC-FD, PAD and MS . Food Chemistry , 113 : 705 – 711 .