Abstract
Furaltadone is a banned drug for use in food producing animals and the marker residue of furaltadone in edible tissues is its metabolite, 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ). In this study, a novel polyclonal antibody of furaltadone was produced by using of the conjugate of furaltadone-bovine serum albumin as immunogen. The obtained antibody showed good specificity and sensitivity toward AMOZ with an IC50 value of 2.3 ng/mL. Then, an indirect competitive immunoassay (ELISA) with heterologous coating antigen was developed to directly determine AMOZ in animal meat with a simple sample preparation. The limit of detection for AMOZ in meat was 0.4 ng/g. At three fortification levels, the intra- and inter-assay recoveries from blank samples were in a range of 85.0%–103%. The ELISA method was further confirmed with a HPLC method in which AMOZ was determined after derivatization with 2-naphthaldehyde. The two methods showed good agreement from the analysis of incurred pork samples.
1. Introduction
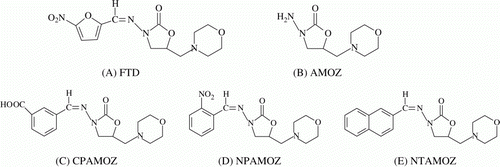
Furaltadone (FTD, A) is a nitrofuran drug that has been used for the treatment of certain bacterial infectious in animals for many years. However, FTD and its metabolite have been proven to show carcinogenic and mutagenic effects (Van Koten Vermeulen, Wouters, & Van Leeuwen, Citation1993). Therefore, the use of FTD in food producing animals has been banned in the United States, European Union (EU) and China, and EU has set a minimum residue performance limit of 1.0 µg/kg for FTD and/or its metabolite in edible tissues. However, nitrofurans can be illegally administrated to animals. During 2002–2003, nitrofuran residues were detected in poultry and aquaculture products imported to Europe from other countries and the residue of FTD metabolite was detected in poultry meat from Portugal and pork meat from Italy and Greece (Cooper, Caddell, Elliott, & Kennedy, Citation2004). Therefore, it is urgent to monitor the residues of nitrofurans in edible tissues.
Nitrofurans are metabolised rapidly in vivo and their parent drugs cannot be detected even within several hours after the cessation of administration, but their metabolites as tissue-bound residues are persistent for several weeks (McCracken, Blanchflower, Rowan, McCoy, & Kennedy, Citation1997; Nouws & Laurensen, Citation1990). These metabolites can be released by the hydrolysis of imine bond in the consumers’ stomach to show harm effect (Hoogenboom et al., Citation2002). Therefore, the nitrofuran metabolites are usually regarded as the marker residues of their respective parent drugs in edible tissues, such as the metabolite of FTD, 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ, B).
There have been many papers involving LC-MS or LC-MS/MS method for the determination of nitrofuran metabolites including AMOZ in various matrixes (Chu & Lopez, Citation2007; Diblikova, Cooper, Kennedy, & Franek, Citation2005; Finzi, Donato, Sucupira, & De Nucci, Citation2005; Leitner, Zllner, & Lindner, Citation2001; Lopez, Feldlaufer, Williams, & Chu, Citation2007; Mottier et al., Citation2005; Verdon, Couedor, & Sanders, Citation2007). These methods are sensitive and confirmatory, but the expensive instruments may not be available in every laboratory. Comparison with those instrumental methods, ELISA is a low cost and sensitive method capable of screening large amount of samples in a single test. Many papers involving ELISA method have been reported for detection of the metabolites of furazolidone, nitrofurantoin and nitrofurazone (Chang, Peng, Wu, Wang, & Yuan, Citation2008; Cooper et al., 2004; Cooper, Elliott, & Kennedy, Citation2004; Liu, Zhao, Zhang, Lu, Liu, & Xi, Citation2007; Vass et al., Citation2008; Vass, Diblikova, & Franek, Citation2008). However, there has been only one paper reporting ELISA method for the detection of AMOZ in shrimp (Pimpitak, Putong, Komolpis, Petsom, & Palaga, Citation2009).
In those ELISA methods, the nitrofuran metabolites were usually derivatized with 3/4-carboxybenzaldehyde and the derivatives were used as the haptens to produce the antibodies (such as CPAMOZ, C), and the nitrofuran metabolites in various samples were derivatized to o-nitrobenzaldehyde type derivatives before ELISA analysis (such as NPAMOZ, D). In addition, the nitrofuran metabolites were also need to be transferred to NP-type derivatives before analysis by MS methods. Therefore, those ELISA methods are inconvenient due to the required long derivatization time and complicated sample preparation.
Then, the development of a simple and rapid ELISA for the detection of AMOZ is very desirable. The objective of this study was to develop an ELISA method based on the anti-FTD antibody to detect AMOZ in animal meats directly without the derivatization step. Furthermore, the ELISA method was confirmed with a novel HPLC method (Chumanee, Sutthivaiyakit, & Sutthivaiyakit, Citation2009) with AMOZ derivatized to the 2-naphthaldehyde (NTA) type derivative (E).
2. Materials and methods
2.1. Reagents and chemicals
Furaltadone (purity>99.5%), nitrofurantoin, nitrofurazone, furazolidone, NTA, bovine serum albumin, ovalbumin and Freund's adjuvant were all purchased from Sigma (St. Louis, MO, USA). AMOZ (purity>99.5%) was purchased from WITEGA (Berlin, Germany). All the other chemical reagents were analytical grade or better from Beijing chemical company (Beijing, China). High-purity water was obtained by a MilliQ plus water purification system (Bedford, MA, USA). Standard stock solutions of FTD and AMOZ (1 mg/mL) were prepared with methanol and stored in dark glass bottles at 4 °C to be stable for 3 months. The working solutions with series concentrations were prepared by diluting stock solutions with PBS for ELISA use and with methanol for HPLC use (stored at 4 °C to be stable for two weeks). PBS (pH 7.2) was prepared by dissolving 0.2 g KH2PO4, 0.2 g KCl, 1.15 g Na2HPO4 and 8.0 g NaCl in 1000 mL demineralised water.
2.2. Apparatus
Ultraviolet (UV)-Vis spectrophotometer was Model 751GW from Shanghai Analytical Instrument (Shanghai, China). ELISA plate reader was Model 550 from Bio-Rad (Hercules, CA, USA). The HPLC system consisted of Shimadzu LC-6AD liquid chromatography, SPD-20A UV detector (Shimadzu, Japan) and a reversed phase Diamonsil C18 column (150×4.6 mm, 5 µm) (DIKMA, USA).
2.3 Synthesis of FTD conjugates
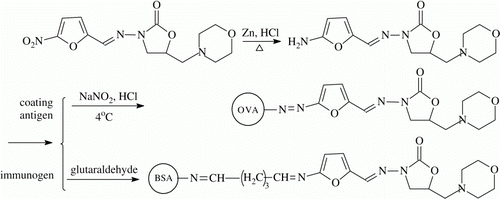
Furaltadone was coupled to bovine serum albumin (BSA) by using of glutaraldehyde reaction and to ovalbumin (OA) by diazotization reaction. The synthesis process is shown in . Eighty milligrams of zinc powder were added into acetonitrile/methanol solution containing 55 mg FTD, and pH value of the mixture was adjusted to about 5.0 with 0.5 M HCl. Then the mixture was stirred at 40 °C for 30 min. The solution was cooled down to 4 °C for preparation of the FTD conjugates (Solution A).
Furaltadone-bovine serum albumin (FTD-BSA): Solution A was dropped slowly into 10 mL of PBS solution containing 160 mg BSA, then 100 µL of 25% glutaraldehyde solution was added and the mixture was mixed round for 4 h at room temperature to prepare the immunogen FTD–BSA. The obtained conjugate was further purified by passing through a home-made Sephadex G25 cartridge. The eluate with orange-red colour was dialysed against three changes of PBS for three days and stored at –20 °C.
Furaltadone-ovalbumin (FTD-OA): 1 mL of 0.5 M NaNO2 was added into solution A dropwise and the pH value of the mixture was maintained at 5.0 with 0.5 M HCl. The mixture was stirred for 2 h at 4 °C. The excessive NaNO2 was removed by adding several milligrams of ammonium sulfamate and pH value of the mixture was readjusted to 7.5 with 2 M NaOH. Finally, the mixture was added into 10 mL of PBS solution containing 80 mg OA dropwise, and the mixture was allowed to react for 12 h at 4 °C to prepare the coating antigen (FTD-OA). Then the conjugate was treated same as that of for FTD-BSA.
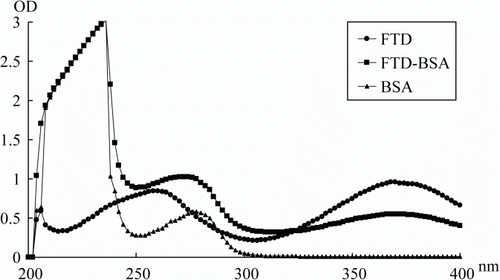
Furaltadone, BSA and OA, and all of the conjugates were scanned respectively on a UV spectrophotometer to identify the conjugation, and the coupling ratios of hapten to protein (hapten density) were determined by using of the previously reported method (Sashidhar, Capoor, & Ramana, Citation1994).
2.4. Production of the polyclonal antibody
Six New Zealand white rabbits numbered R1 to R6 were immunised with the emulsion of FTD-BSA (0.5 mg protein per animal) in Freund's complete adjuvant on the dorsal region subcutaneously, and were boosted with FTD-BSA in Freund's incomplete adjuvant at a three week-interval. After eight boosters, the rabbits were exsanguinated and the serum was collected. Finally, the IgG was isolated using the saturated ammonium sulfate precipitation method (Wengatz, Stoutamire, Gee, & Hammock, Citation1998) for development of the ELISA.
2.5. Indirect competitive ELISA
The checkerboard procedure was used to determine the optimal dilutions of coating antigen and antibody, in which the well with an absorbance of 1.0 are defined as the optimum coating antigen concentrations and antibody dilutions, respectively. After that, each well of a microtitre plate was coated with 100 µL of FTD-OA and incubated overnight at 4 °C, then blocked with 1% foetal calf serum. The plate was washed three times with PBS, then 50 µL of the optimal antibody dilution and 50 µL of AMOZ standard with concentrations of 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, 20.0 and 40.0 ng/mL (or sample extract) were added to the wells (in triplicate) for incubation of 1 h at 37 °C. The plate was washed as above. One hundred microlitres of horseradish peroxidase-labelled goat anti-rabbit IgG was added before incubation for 30 min at 37 °C. After washes, 100 µL of terramethylbenzidine (TMB) substrate system was added for 15 min incubation at 37 °C. Finally, the reaction was stopped by addition of 50 µL of 2 M H2SO4 to each well, and the plate was read on an ELISA plate reader at 450 nm to obtain the OD values.
The competitive inhibitory curve was developed by plotting the concentration (Log C) verse the B/B 0 (mean OD value of AMOZ standards divided by that of the zero standards) in OriginPro 7.5 software. The IC50 value and the limit of detection (LOD) for AMOZ were defined as the concentration of showing 50% and 10% of inhibition, respectively. FTD, three nitrofuran drugs (furazolidone, nitrofurantoin and nitrofurazone) and their metabolites were all determined as described above. Crossreactivity (CR) of the antibody to these competitors were calculated as follows: CR (%) = 100×IC50 AMOZ/IC50 competitor.
2.6. Sample preparation
Two grams of fully homogenised meat sample (pork, chicken and fish) was accurately weighed into a polypropylene centrifuge tube and 1.0 mL of 1 M HCl was added. The mixture was stirred violently on a variable speed reciprocal shaker in a water bath at 37 °C for 30 min. Then 10 mL of methanol were added and the mixture was vertexed for 3 min. After the tube was centrifuged at 5000 r/min for 10 min, the supernatant was decanted into a clean test tube and evaporated to dryness under a stream of nitrogen at 45 °C on a water bath. Finally, the dry residue was redissolved in 2 mL of methanol/PBS (2/8, v/v) and filtrated through a Millipore filter for ELISA analysis. Some blank meat samples (chicken, pork and fish) were obtained from the controlled slaughterhouses or fisheries. The extracts from the blank samples were used to prepare the matrix-matched AMOZ standard with concentration range between 0.1 and 40.0 ng/mL for development of the matrix matched inhibitory curve. Some blank samples were fortified AMOZ standard at level of 0.5, 1.0 and 2.0 ng/g to carry out the recovery test.
2.7. HPLC-UV confirmation
HPLC procedure was according to a recent report (Chumanee et al., 2009). Mobile phase was a mixture of acetonitrile (A) and 5 mM ammonium acetate (pH 7.5) with gradient elution. The gradient programme was started from 10% A, increased linearly to 40% A in 1 min, then increased linearly to 48% A in 4 min and held for 10 min, then increased linearly to 100% A and held for 10 min. The flow rate was 0.8 mL/min and equilibration time was 15 min. The detector wavelength was at 308 nm and the injection volume was 20 µL. Meat sample (20 g) was hydrolysed by using of 0.2 M HCl and derivatized with NTA at 37 °C for 12 h in the dark. Then the pH of the mixture was adjusted to 7.5 with NaOH/K2HPO4 and the mixture was extracted with ethyl acetate (20 mL). After the obtained ethyl acetate solvent was evaporated to dryness, the dry residue was reconstituted in acetonitrile/H2O (0.4 mL) to be defatted with n-hexane. Finally, the acetonitrile/H2O layer was filtrated with a Millipore filter before analysis by HPLC-UV. This sample preparation implies the AMOZ level in the sample is concentrated for 50 times.
2.8. Incurred samples
Some incurred pork samples were from FTD-administrated pigs. Briefly, four pigs were fed with FTD-contained feeds for seven days and the dosage was 15 mg/kg bodyweight per day. One hour after last feeding two pigs were slaughtered and the left two were slaughtered three days later. The collected pork samples were determined with a LC-ESI/MS/MS method and the results have been published elsewhere (Xia et al., Citation2008). These samples were analysed by the proposed ELISA procedure and HPLC method.
3. Results and discussion
3.1. FTD conjugate
For development of an immunoassay to determine an analyte, the first step is to synthesise its artificial immunogen. In the previous reports, the CP-type derivatives of the nitrofuran metabolites were used as the haptens to produce the antibodies and the obtained antibodies can recognise their NP-type derivatives and their respective parent drugs (Chang et al., 2008; Cooper et al., 2004; Liu et al., 2007; Pimpitak et al., 2009; Vass et al., Citation2008; Vass, Diblikova, & Franek, Citation2008). Now that the antibodies of the metabolite derivatives can recognise their parent drugs, could the antibodies of parent nitrofuran drugs recognise their metabolites? However, there has been no article reporting such research so far. After careful observation of the molecules of FTD and its related compounds, we found the general chemical structure of FTD is very similar to that of the CPAMOZ (). Then we assumed the antibody of FTD is supposed to recognise AMOZ. Therefore, FTD was used as a hapten to produce the antibody.
There is no chemical group available in the molecule of FTD to be utilised to link directly with protein carrier, but the nitryl group can be deoxidised to an amidogen as the active chemical group to link with carrier. In our previous paper, this method has been used to prepare the immunogen of furazolidone (Li, Liu, & Wang, Citation2009). Therefore, FTD was reduced to the active FTD hapten under the conditions of pH 5.0 and temperature of 40 °C with the assistance of zinc. The reaction solution turned orange-red from yellow, revealing the nitryl group was deoxidised to amidogen. The UV scan diagram of the hapten solution was almost same as that of FTD, indicating the whole molecule is unbroken (data not shown). During the experiments, different pH and temperature were evaluated. The reaction solution turned dark with temperature rising from 40 °C to 80 °C and pH decrease from 5.0 to 1.0, and the UV scan diagram of the dark solution was very different from that of FTD. This indicates the molecule of FTD maybe is damaged at high temperature and low pH whereas the mild reaction conditions have no influence on its general chemical structure.
Then, the hapten was coupled to BSA by using of glutaraldehyde reaction. During the conjugation, the resulting colour was always present throughout the process of coupling, purification and dialysis, revealing the hapten is coupling to the protein. Furthermore, the UV scan diagram of FTD-BSA contained the characteristic peaks of FTD and BSA (). All these things suggested the successful conjugation. The hapten density is important for an immunoassay. Extreme high level of hapten density cannot stimulate the lymphocyte to produce the sensitive antibody toward the hapten whereas the coating antigen with high level of hapten density can decrease the sensitivity of an immunoassay (Franek, Diblikova, Cernoch, Vass, & Hruska, Citation2006). In this study, the coupling ratio of FTD/protein was 18 in immunogen and was 12 in coating antigen, which are appropriate.
3.2. ELISA development
An immunogen with a long-space chain can produce the antibody of showing high binding ability for the hapten (Franek et al., Citation2001). In addition, heterology in the coating antigen was commonly used to improve the sensitivity of an ELISA method (Franek et al., Citation2001, 2006; Li, Liu, Zhang, Li, & Wang, Citation2010). In this study, the coating antigen FTD-OA with a short spacer and the antibody from a long-spacer immunogen FTD-BSA were used to develop a heterologous indirect competitive ELISA for direct determination of AMOZ. Antibody R4 showing the highest titer was selected for the subsequent experiments. Optimum dilutions of coating antigen and antibody R4 were determined as 1:10,000 and 1:25,600, respectively.
In a previous paper (Diblikova et al., 2005), the authors employed a homobifunctional agent 1, 4-butenediol diglycidyl ether to prepare the immunogen AMOZ-protein in order to produce the antibody against free AMOZ. The polyclonal antibody exhibited high binding activity toward the hapten-protein conjugate in ELISA but no binding inhibition in the presence of free AMOZ. The authors speculated that the low chemical stability of AMOZ during conjugation procedure, spacer binding effect or molecular size of AMOZ might be the possible reasons.
In this study, the hapten was furaltadone and its molecule has higher molecular size and chemical stability than AMOZ. Therefore, the hapten could be used to produce the specific antibody toward AMOZ, similar to the CP-type derivatives of nitrofuran metabolites. Furthermore, the short-spacer coating antigen FTD-OA could eliminate the antibody's spacer binding effect for the space arm in FTD-BSA. These things indicate the possible reasons the previous researchers suggested are avoided. Therefore, the obtained anti-FTD antibody showed high specificity and sensitivity toward free AMOZ with IC50 of 2.3 ng/mL and LOD of 0.4 ng/mL. The competitive calibration curve for AMOZ is shown in with concentration range of 0.1–40 ng/mL. Therefore, the use of parent FTD as hapten can produce the specific antibody for AMOZ and our original assumption was correct. Furthermore, these results also indicate the reduction of FTD and the immunogen preparation were successful. The production of anti-AMOZ antibody in this study is simpler than the previous paper. This is the first paper reporting the production of an antibody of parent nitrofuran drug to determine its metabolite. That if the antibodies of other parent nitrofurans could recognise their metabolites is remained to be studied.
As can be expected, the antibody showed high sensitivity and crossreactivity to FTD with an IC50 of 0.8 ng/mL. Therefore, the antibody could also be used to detect parent FTD. The antibody showed negligible crossreactivity to other parent nitrofurans and their metabolites (<1%). The IC50 values and CRs of the present antibody and the reported monoclonal antibody (Pimpitak et al., 2009) are shown in . It can be said from these results that our antibody is comparable to the reported monoclonal antibody and it can meet the requirement of residue detection for AMOZ.
Table 1. IC50 and CRs of the antibody and the reported antibody for nitrofuran competitors.
3.3 Sample preparation and ELISA determination
Nitrofuran metabolites are in the formation of protein-bound residues. They were usually released by using of HCl or protease, derivatized to NP-type derivatives (incubation at 37 °C overnight or at 55 °C for 2 h) before ELISA analysis (Chang et al., 2008; Cooper et al., 2004; Diblikova et al., 2005; Liu et al., 2007; Pimpitak et al., 2009; Vass et al., Citation2008; Vass, Diblikova, & Franek, Citation2008). In this study, AMOZ was released with 0.5 M HCl at 37 °C, extracted with methanol, purified with Millipore filter and directly analysed by ELISA. Therefore, this sample preparation was simpler than the reported method.
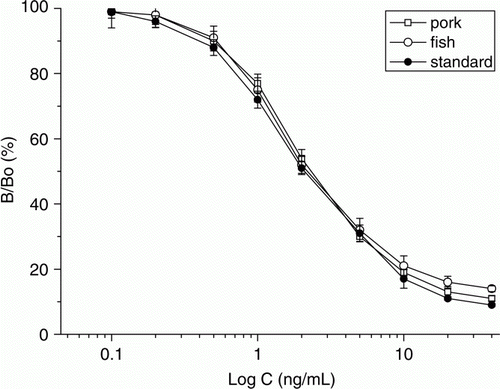
For evaluation of the sample extraction, AMOZ standards prepared with the extracts of blank pork and blank fish at series concentrations were used for development of the matrix matched calibration curves. As shown in , the matrix-matched curves were similar to that of AMOZ standard, revealing the extraction method is satisfactory. Therefore, the LOD for AMOZ in meat was calculated as the AMOZ standard, that is, 0.4 ng/g. This result was a little higher than the reported ELISA method (Pimpitak et al., 2009). At three fortification levels, the intra-recoveries (five duplicates in a day) and inter-recoveries (consecutive six days) from the blank meat samples were in a range of 85.0%–102% and 87.0%–103%, respectively, with coefficients of variation (CV) lower than 12% ().
Table 2. Recoveries of AMOZ from the blank meats determined by ELISA and HPLC.
3.4. HPLC confirmation
For confirmatory determination of nitrofuran metabolites, MS technique is usually used. However, Chumanee et al. (2009) have validated a HPLC method for determination of 4 nitrofuran metabolites including AMOZ in shrimp by using of NTA as the derivatizing reagent. They reported the NTA-type derivatives showed twofold higher absorptivity than the NP-type derivatives with satisfactory sensitivity (LOD < 0.3 ng/g), and the method showed good agreement with a LC-MS/MS method. Therefore, a HPLC method was used in the present study to confirm the ELISA results. Retention time was 10.325 min for NTAMOZ and the LOD for AMOZ in meats, defined as signal/noise (S/N = 3), was 0.6 ng/g. Recovery results from the blank fortified samples are listed in . Both of the methods gave satisfactory recoveries and the HPLC method was more consistent and stable than ELISA with CV lower than 8%. Therefore, the ELISA could be used as a rapid and simple routine screening method for large number of samples with results verified by the HPLC method or the reported MS method.
3.5. Incurred samples
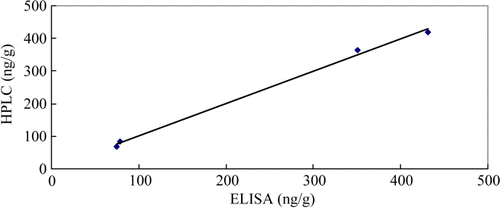
The incurred pork samples were determined by the ELISA and the HPLC method with results shown in . A correlation between the concentrations obtained by the two methods was found with a correlation coefficient (r 2) of 0.996 (). The AMOZ concentrations detected by the two methods were also comparable to that of LC-MS/MS results (Xia et al., Citation2008).
Table 3. AMOZ levels in the incurred pork determined by ELISA, HPLC and the reported LC-MS/MS.
4. Conclusion
This paper first reported the production of the antibody of parent furaltadone and the development of an ELISA procedure for direct determination of its metabolite AMOZ in meats. The sample preparation was simple without the time-waste derivatization step. In general, this method was better than the reported ELISA method. Therefore, the proposed ELISA could be a practical screen tool for routine monitoring the residue of furaltadone metabolite in large amount of samples and the ELISA positive results maybe need an instrumental method for confirmation.
Additional information
Notes on contributors
Xiu Zhi Hu
Xiao Dong Yan and Xiu Zhi Hu have contributed equally to this studyReferences
- Chang , C. , Peng , D.P. , Wu , J.E. , Wang , Y.L. and Yuan , Z.H. 2008 . Development of an indirect competitive ELISA for the detection of furazolidone marker residue in animal edible tissues . Journal of Agricultural and Food Chemistry , 56 : 1525 – 1531 .
- Chu , P.S. and Lopez , M.I. 2007 . Determination of nitrofuran residues in milk of dairy cows using liquid chromatography-tandem mass spectrometry . Journal of Agricultural and Food Chemistry , 55 : 2129 – 2135 .
- Chumanee , S. , Sutthivaiyakit , S. and Sutthivaiyakit , P. 2009 . New reagent for trace determination of protein-bound metabolites of nitrofurans in shrimp using liquid chromatography with diode array detector . Journal of Agricultural and Food Chemistry , 57 : 1752 – 1759 .
- Cooper , K.M. , Caddell , A. , Elliott , C.T. and Kennedy , D.G. 2004 . Production and characterization of polyclonal antibodies to a derivative of 3-amino-2-oxazolidinone, a metabolite of the nitrofuran furazolidone . Analytica Chimica Acta , 520 : 79 – 86 .
- Cooper , K.M. , Elliott , C.T. and Kennedy , D.G. 2004 . Detection of 3-amino-2-oxa-zolidinone (AOZ), a tissue-bound metabolite of the nitrofuran furazolidone, in prawn tissue by enzyme immunoassay . Food Additive and Contaminants , 21 : 841 – 848 .
- Diblikova , I. , Cooper , K.M. , Kennedy , D.G. and Franek , M. 2005 . Monoclonal antibody-based ELISA for the quanti?cation of nitrofuran metabolite 3-amino-2-oxazolidinone in tissues using a simpli?ed sample preparation . Analytica Chimica Acta , 540 : 285 – 292 .
- Finzi , J.K. , Donato , J.L. , Sucupira , M. and De Nucci , G. 2005 . Determination of nitrofuran metabolites in poultry muscle and eggs by liquid chromatography-tandem mass spectrometry . Journal of Chromatography B , 824 : 30 – 35 .
- Franek , M. , Diblikova , I. , Cernoch , I. , Vass , M. and Hruska , K. 2006 . Broad-specificity immunoassays for sulfonamide detection: Immunochemical strategy of generic antibodies and competitors . Analytical Chemistry , 78 : 1559 – 1567 .
- Franek , M. , Zeravik , J. , Eremin , S.A. , Yakovleva , J. , Badea , M. Danet , A. 2001 . Antibody-based methods for surfactant screening . Fresenius' Journal of Analytical Chemistry , 371 : 456 – 466 .
- Hoogenboom , L.A.P. , van Bruchem , G.D. , Sonne , K. , Enninga , I.C. , van Rhijn , J.A. Heskamp , H. 2002 . Absorption of a mutagenic metabolite released from protein-bound residues of furazolidone . Environmental Toxicology and Pharmacology , 11 : 273 – 287 .
- Leitner , A. , Zllner , P. and Lindner , W. 2001 . Determination of the metabolites of nitrofuran antibiotics in animal tissue by high performance liquid chromatography-tandem mass spectrometry . Journal of Chromatography A , 939 : 49 – 58 .
- Li , J. , Liu , J. and Wang , J. 2009 . Multidetermination of four nitrofurans in animal feeds by a sensitive and simple enzyme-linked immunosorbent assay . Journal of Agricultural and Food Chemistry , 57 : 2181 – 2185 .
- Li , J. , Liu , J. , Zhang , H.C. , Li , H. and Wang , J.P. 2010 . Broad specificity indirect competitive immunoassay for determination of nitrofurans in animal feeds . Analytica Chemica ACTA , 678 : 1 – 6 .
- Liu , W. , Zhao , C. , Zhang , Y. , Lu , S. , Liu , J. and Xi , R. 2007 . Preparation of polyclonal antibodies to a derivative of 1-aminohydantoin (AHD) and development of an indirect competitive ELISA for the detection of nitrofurantoin residue in water . Journal of Agricultural and Food Chemistry , 55 : 6829 – 6834 .
- Lopez , M.I. , Feldlaufer , M.F. , Williams , A.D. and Chu , P.S. 2007 . Determination and confirmation of nitrofuran residues in honey using LC-MS/MS . Journal of Agricultural and Food Chemistry , 55 : 1103 – 1108 .
- McCracken , R.J. , Blanchflower , W.J. , Rowan , C. , McCoy , M.A. and Kennedy , D.G. 1997 . The prevalence and possible causes of bound and extractable residues of the furazolidone metabolite 3-amino-oxaxolidinone in porcine tissues . Food Additive and Contaminants , 14 : 287 – 294 .
- Mottier , P. , Khong , S. , Gremaud , E. , Richoz , J. , Delatour , T. Goldmann , T. 2005 . Quantitative determination of four nitrofuran metabolites in meat by isotope dilution liquid chromatography-electrospray ionization-tandem mass spectrometry . Journal of Chromatography A , 1067 : 85 – 91 .
- Nouws , J.F. and Laurensen , J.H. 1990 . Postmortal degradation of furazolidone and furaltadone in edible tissues of calves . Veterinary Quarterly , 12 : 56 – 59 .
- Pimpitak , U. , Putong , S. , Komolpis , K. , Petsom , A. and Palaga , T. 2009 . Development of a monoclonal antibody-based enzyme linked immunosorbent assay for detection of the furaltadone metabolite, AMOZ, in fortified shrimp samples . Food Chemistry , 116 : 785 – 791 .
- Sashidhar , R.B. , Capoor , A.K. and Ramana , D. 1994 . Quantitation of amino groups using amino acids as reference standards by trinitrobenzene sulfonic acid: A simple spectrophotometric method for the estimation of hapten to carrier protein ratio . Journal of Immunological Methods , 167 : 121 – 127 .
- Van Koten Vermeulen , J.E.M. , Wouters , M.F.A. , & Van Leeuwen , F.X.R . 1993 . Evaluation of certain veterinary drug residues in food (TRS no. 832) . Report on the 40th meeting of the Joint FAO/WHO Expert Committee on Food Additives , World Health Organization , Geneva .
- Vass , M. , Diblikova , I. and Franek , M. 2008 . ELISA for semicarbazide and its application for screening in food contamination . Analytica Chimica Acta , 608 : 86 – 94 .
- Vass , M. , Diblikova , I. , Kok , E. , Stastny , K. , Frgalova , K. Hruska , K. 2008 . In-house validation of an ELISA method for screening of semicarbazide in eggs . Food Additive and Contaminants , 25 : 930 – 936 .
- Verdon , E. , Couedor , P. and Sanders , P. 2007 . Multi-residue monitoring for the simultaneous determination of five nitrofurans (furazolidone, furaltadone, nitrofurazone, nitrofurantoine, nifursol) in poultry muscle tissue through the detection of their five major metabolites (AOZ, AMOZ, SEM,AHD, DNSAH) by liquid chromatography coupled to electrospray tandem mass spectrometry . Analytica Chimica Acta , 586 : 336 – 347 .
- Wengatz , I. , Stoutamire , D.W. , Gee , S.J. and Hammock , B.D. 1998 . Development of an enzyme-linked immunosorbent assay for the detection of the pyrethroid insecticide fenpropathrin . Joural of Agricultural and Food Chemistry , 46 : 2211 – 2221 .
- Xia , X. , Li , X. , Zhang , S. , Ding , S. , Jiang , H. Li , J. 2008 . Simultaneous determination of 5-nitroimidazoles and nitrofurans in pork by high performance liquid chromatography tandem mass spectrometry . Journal of Chromatography A , 1208 : 101 – 108 .