Abstract
In this study, two anti-peanut agglutinin (PNA) polyclonal antibodies were successfully prepared. Using mouse anti-PNA polyclonal antibody as the capture antibody and rabbit anti-PNA polyclonal antibody as the detection antibody, a novel sandwich enzyme-linked immunosorbent assay (ELISA) was developed to quantify PNA. The detection limit of the ELISA method was low (0.49 ng/mL), and the linear dynamic range was between 0.78 and 100 ng/mL. The recovery ranged from 93.86% to 139.3%, whereas the intra- and inter-assay coefficients of variation were less than 10.25% and 12.06%, respectively. Sample analysis verified this method as a reliable tool for the detection of PNA in processed foods.
Introduction
Food allergy is becoming a major health concern worldwide. In industrialised countries, up to 8% of children and 2% of adults are affected by food allergies (Ebisawa, Ikematsu, Imai, & Tachimoto, 2003; Woods et al., Citation2002). Peanut is one of the most frequent causes of food allergy. Due to the widespread use of peanut as an ingredient in manufactured foods to enhance flavour and nutritional quality, it is the food allergen most capable of causing severe, life-threatening, and even fatal, allergic reactions. Because avoidance is the only available treatment for food allergies at this time, extraordinary care to eliminate all peanut-containing foods from the diet is very important for patients with peanut allergy.
Peanut agglutinin (PNA) is one of the major allergens in peanut. During the past few years, several methods have been established for the determination of food allergens. The most commonly used analytical method for allergen detection is based on the enzyme-linked immunosorbent assay (ELISA) technique (Besler, Citation2001; Bonwick & Smith, Citation2004; Dodo, Marsic, Callender, Cebert, & Viquez, Citation2002; Fuller, Goodwin, & Morris, Citation2007; Morishita et al., Citation2008; Poms, Klein, & Anklam, 2004; Schneider, Weigel, Werkmeister, & Pischetsrieder, Citation2010). Some studies have verified that competitive ELISA and sandwich ELISA are useful for the quantification of allergens in potentially allergenic foods (Doi et al., Citation2008; Wei et al., Citation2003; Luis et al., Citation2008; Ma et al., Citation2010). Polymerase chain reaction (PCR) technology is also commonly used for the detection of allergens (Stephan & Vieths, Citation2004). Biosensor-based methods are increasingly used for allergen analysis in foods (Jonsson, Citation2002; Poms et al., Citation2004; Singh, Sharma, Baltus, & Suni, Citation2010). Furthermore, mass spectrometry (MS) has been applied to detect and identify food allergens (Monaci & Visconti, Citation2009). To develop a fast, sensitive and quantitative method for the detection of PNA in processed foods, we attempted to generate a rabbit/mouse anti-PNA polyclonal antibody, and designed a novel quantitative sandwich enzyme immunoassay for PNA. During the ELISA procedure, we attempted to use mouse anti-PNA polyclonal antibody to capture PNA. Previous studies have usually used glycoproteins to capture lectin (Güll, Wirth, & Gabor, Citation2007; Vincenzi et al., Citation2002).
Materials and methods
Reagents and materials
Peanut agglutinin (PNA), complete and incomplete Freund's adjuvant and enzyme immunoassay-grade HRP-labelled goat anti-rabbit/mouse immunoglobulin were obtained from Sigma (St. Louis, MO, USA). Gelatin and D-galactose were obtained from Beijing Biodee Biotechnology Co., Ltd., China. 3,3′,5,5′-tetra methyl benzidine (TMB) was purchased from Aladdin Chemistry Co., Ltd., Shanghai, China. All other reagents and chemicals were obtained from the National Pharmaceutical Group Chemical Reagent Co., Ltd., China.
Instruments
A high speed refrigerated bench-top centrifuge was purchased from Heal Force Development Ltd., China. Wellwash plus was obtained from Thermo Electron Corporation (Shanghai, China). An injection emulsifier machine was obtained from Lange Automation Technology Co., Ltd. China. A multiskan microplate reader was obtained from Thermo Labsystem (Helsinki, Finland).
Buffers and solutions
(1) Phosphate Buffered Saline (PBS): pH 7.4 consisted of 0.137 M NaCl, 2.7 mM KCl, 1.9 mM KH2PO4 and 8.1 mM Na2HPO4.12H2O; (2) Coating buffer: 0.05 M carbonate/bicarbonate buffer, pH 9.5; (3) washing buffer (PBST): PBS containing 0.05% (v/v) Tween 20; (4) blocking buffer: coating buffer containing 0.1% (w/v) gelatin; (5) antibody dilution buffer: PBS containing 0.1% (w/v) gelatin and 0.05% (v/v) Tween 20; (6) substrate buffer: 18 µL of 30% H2O2 were adding to 100 mL 0.1 M citrate phosphate buffer at pH 5.0; (7) TMB solution: 60 mg TMB dissolved in 100 mL glycol; (8) TMB substrate solution: 5:1 (v/v) mixture of substrate buffer and TMB solution; (9) stop solution: 2 M sulfuric acid.
Commercial food products
Commercial food products were purchased at a local supermarket and included 11 samples with a declaration that the food components contained traces of peanut, three samples without any declaration about the presence of peanut or peanut components in the list of ingredients, and one sample of peanut oil without peanut protein.
Preparation of polyantibody to PNA
Before immunisation, the sugar-binding sites of PNA were blocked by D-galactose. The immunogen was PNA-D-galactose.
Three female BALB/c mice (six weeks) were injected subcutaneously with 200 µL of 1:1 (v/v) mixture of Freund's complete adjuvant and NS (physiological saline) containing 0.5 mg/mL of immunising immunogen. After the first injection, a supplementary injection of 200 µL of 1:1 (v/v) mixture of Freund's incomplete adjuvant and NS containing 0.5 mg/mL of immunising antigen was administered at 21, 42 and 63 days. Seven days after the fourth immunisation, serum titres were detected by indirect ELISA. We then chose the mouse with the highest titre and boosted with 100 µL 0.5 mg/mL immunogen solution. Whole blood was collected one week after the final injection.
Two female New Zealand rabbits (1.5–2.0 kg) were injected intradermally on the back with the first injection. The immunogen was mixed 1:1 with Freund's complete adjuvant. After the first injection, an intramuscular injection of immunogen with Freund's incomplete adjuvant was performed four times at intervals of two weeks. The injection volume was 1 mL and contained 1 mg immunogen. Ten days after the fifth immunisation, serum titres were detected by indirect ELISA. The mouse with the highest titre was injected intravenously with 500 µL of 1 mg/mL immunising antigen solution. Whole blood was collected one week after the final injection.
All serum was isolated by centrifugation, aliquoted and stored at −20°C.
ELISA procedure
Indirect ELISA: Microplates were coated with 100 µL/well of PNA in coating buffer at 37°C for 2 h. After washing three times with washing buffer, carbonate buffer was added to the wells (100 µL/well) and incubated for 2 h at 37°C. After another washing step, the established polyclonal antibody was serially diluted from 1:1000 to 1:128,000 with antibody dilution buffer, added to the wells (100 µL/well) and incubated for 30 min at 37°C. After washing, HRP-labelled goat anti-rabbit/mouse immunoglobulin was diluted 1:3000, and 100 µL/well was added and incubated for 30 min at 37°C. After an other washing step, 100 µL/well of TMB substrate solution was added and incubated for 15 min at 37°C in the dark. The reaction was stopped by adding 50 µL/well of 2 mol/L sulfuric acid, and the absorbance was measured at 450 nm.
Sandwich ELISA
Flat-bottom polystyrene 96-well microplates were coated with 100 µL of mouse anti-PNA polyclonal antibody diluted in coating buffer at 37°C for 2 h. After washing three times with washing buffer, 200 µL carbonate buffer was added to the wells and incubated for 2 h at 37°C. After the wells were washed three times with washing buffer, 100 µL/well of a 2-fold serial dilution of PNA standard solution or sample extract was added. After incubation for 1 h at 37°C and washing three times, 100 µL/well antibody (rabbit anti-PNA polyclonal antibody) solution (1:8000 in dilution buffer) was added to each well and incubated for 1 h at 37°C. After further washing, HRP-labelled goat anti-rabbit immunoglobulin was diluted 1:3000, and 100 µL/well was added and incubated for 1 h at 37°C. After extensive washing, 100 µL/well of TMB substrate solution was added and incubated for 15 min at 37°C in the dark. The reaction was stopped by adding 50 µL/well of 2 mol/L sulfuric acid, and the absorbance was measured at 450 nm.
Preparation of samples
Solution samples were diluted 5-fold in 0.01 M PBS at pH 7.4. Solid samples were pestled first, then 0.01 M PBS at pH 7.4 was added to 0.2 mg sample per milliliter of homogenate. All samples were maintained at 4°C overnight and centrifuged at 5000×g for 15 min. The supernatant was centrifuged at 5000×g for a further 15 min. The supernatant was collected as the food sample extract and examined immediately.
Recovery analysis of spiked sample
Four concentration levels of PNA (1, 5, 30 and 60 ng/mL) were used to spike the PNA-free milk samples. The solution samples were treated the same as those in Section ‘Preparation of samples’, but were not diluted.
Results and discussion
Identification of polyantibody to PNA
Two polyclonal antibodies to PNA were measured in serum samples by indirect ELISA. The titres of mouse anti-PNA polyclonal antibody and rabbit anti-PNA polyclonal antibody reached 1:64,000 and 1:128,000, respectively. Therefore, preparation of the established polyclonal antibodies to PNA was successful.
Establishment of sandwich ELISA for determination of PNA
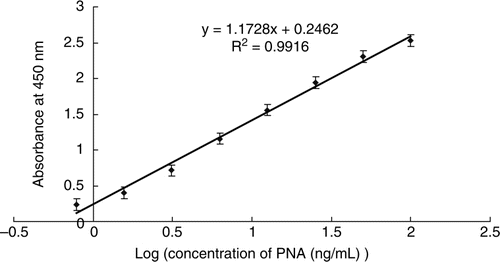
Using mouse anti-PNA polyclonal antibody as the capture antibody, rabbit anti-PNA polyclonal antibody as the detection antibody and HRP-labelled goat anti-rabbit immunoglobulin as the colour reaction antibody, we established a sandwich ELISA for PNA. Commercially purified PNA was used as the standard. shows the calibration curve for the determination of PNA using the developed ELISA. The detection limit of the ELISA method was low (0.49 ng/mL) and the linear dynamic range was between 0.78 and 100 ng/mL.
Recovery studies
Assay precision, accuracy and repeatability were determined by inter- and intra-assay reproducibility. shows the results of inter- and intra-assay laboratory validation for the developed ELISA. Four concentration levels of PNA (1, 5, 30 and 60 ng/mL) were used to spike the PNA-free milk samples. The intra-assay reproducibility with variation coefficients between 4.75% and 10.25% was determined. For the inter-assay reproducibility, spiking was performed in five independent experiments on five different days. The inter-assay variation ranged between 7.61% and 12.06%. Recovery studies showed that the recoveries ranged from 93.86% to 139.3% in the intra-assay. In the inter-assay, the recoveries varied from 98.43% to 129.5%.
Table 1. Recovery of PNA by quantitative sandwich ELISA.
Application to food products
To evaluate the developed ELISA, 11 types of commercially processed foods including peanut protein and four types of foods not including peanut protein were analysed using the optimised ELISA (). The OD450 of the food samples (S) were compared with the blank value (N). The result was positive when the S/N≥2.1. All 11 commercially processed foods including peanut protein showed positive results. The other four types of foods which were peanut protein-free gave negative results.
Table 2. Detection of PNA in the commercial foods.
Discussion
Nowadays, many countries demand mandatory labelling of major allergenic foods in food products (Ebisawa et al., Citation2003; European Commission, Citation2007; FDA, Citation2001). In order to ensure compliance with food labelling, the establishment of reliable detection and quantification methods for food allergens is necessary. However, the detection of allergens in food products can be very difficult. Most foods are processed in varied conditions. For example, heating can change the structure, solubility and bioactivity of allergen proteins. Furthermore, allergens are often present only in trace amounts or are masked by the food matrix. Some studies have examined processing conditions and the matrix effect on the quantitative detection of allergen residues using commercial ELISA test kits (Fu, Maks, & Banaszewski, Citation2010; Monaci, Brohée, Tregoat, & Hengel, Citation2011). Processing conditions and the food matrix can affect the quantitative analysis of allergens using these ELISA kits. The question yet to be answered is how sensitive do the detection methods need to be? In this research, a highly sensitive sandwich ELISA was developed to quantify PNA. In the ELISA procedure, we attempted to use mouse anti-PNA polyclonal antibody to capture PNA. Previous studies have usually used glycoproteins to capture lectin. Additionally, in order to avoid the generation of antibodies to the sugar-binding sites of PNA, those sites were blocked by D-galactose before immunisation. The detection limit of the ELISA method was low (0.49 ng/mL), and the linear dynamic range was between 0.78 and 100 ng/mL. Using milk as a matrix, four concentration levels of PNA were used to spike the milk samples. The intra-assay reproducibility with variation coefficients was determined to be between 4.75% and 10.25%. The inter-assay variation ranged between 7.61% and 12.06%. Recovery studies showed that the recoveries ranged from 93.86% to 139.3% in the intra-assay. In the inter-assay, the recoveries varied from 98.43% to 129.5%. When the developed ELISA technique was applied to food products, all 11 samples with a declaration that the food components contained traces of peanut gave positive results. Therefore, the established ELISA method is a reliable and sensitive method for determining PNA in processed foods.
Acknowledgements
This work is financially supported by the National Natural Foundation of China (21071066, 20835006, 91027038, 21101079, 21175034), the Key Programs from MOST (2010AA06Z302, 2010DFB3047, 2008BAK41B03, 2009BAK61B04, 2008ZX08012-001, 2010GB2C100167), and grants from Natural Science Foundation of Jiangsu Province, MOF and MOE (BK2010001, BK2010141, JUSRP10921, JUSRP11019, 201110060, 201110016, 201110061, 201010078, 201010216).
References
- Besler , M. 2001 . Determination of allergens in foods . Trends in Analytical Chemistry , 20 ( 11 ) : 662 – 671 .
- Bonwick , G.A. and Smith , C.J. 2004 . Immunoassays: Their history, development and current place in food science and technology . International Journal of Food Science and Technology , 39 : 817 – 827 .
- Dodo , H. , Marsic , D. , Callender , M. , Cebert , E. and Viquez , O. 2002 . Screening 34 peanut introduction for allergen content using ELISA . Food and Agricultural Immunology , 14 ( 2 ) : 147 – 154 .
- Doi , H. , Touhata , Y. , Shibata , H. , Sakai , S. , Urisu , A. and Akiyama , H. 2008 . Reliable enzyme-linked immunosorbent assay for the determination of walnut proteins in processed foods . Journal of Agricultural and Food Chemistry , 56 : 7625 – 7630 .
- Ebisawa , M. , Ikematsu , K. , Imai , T. and Tachimoto , H. 2003 . Food allergy in Japan . Journal of the World Allergy Organization , 15 : 214 – 217 .
- European Commission . 2007 . Commission Directive 2007/68/EC of 27 November 2007. Amending Annex IIIa to Directive 2000/13/EC of the European Parliament and of the Council as regards certain food ingredients . Official Journal , L 310 .
- FDA (Food and Drug Administration) . 2001 . Compliance policy guide, Sec. 555. 250 Statement of policy for labeling and preventing cross-contact of common food allergens . Washington , DC : Author .
- Fu , T.J. , Maks , N. and Banaszewski , K. 2010 . Effect of heat treatment on the quantitative detection of egg protein residues by commercial enzyme-linked immunosorbent assay test kits . Journal of Agricultural and Food Chemistry , 58 : 4831 – 4838 .
- Fuller , H.R. , Goodwin , P.R. and Morris , G.E. 2007 . An enzyme-linked immunosorbent assay (ELISA) for the major crustacean allergen, tropomyosin, in food . Food and Agricultural Immunology , 17 ( 1 ) : 43 – 52 .
- Güll , G. , Wirth , M. and Gabor , F. 2007 . Development of a sensitive and reliable ELISA for quantification of wheat germ agglutinin . Journal of Immunological Methods , 318 : 20 – 29 .
- Jonsson , H. 2002 . Sensitive and specific biosensor detection of hazelnut proteins and other allergens in food . Proceedings TNO International Food Allergy Forum . The Netherlands : Noordwijkerhout . 61 .
- Luis , R. , Mata , L. , Estopanan , G. , Lavilla , M. , Sanchez , L. and Perez , M.D. 2008 . Evaluation of indirect competitive and double antibody sandwich ELISA tests to determine β-lactoglobulin and ovomucoid in model processed foods . Food and Agricultural Immunology , 19 ( 4 ) : 339 – 350 .
- Ma , X. , Sun , P. , He , P.L. , Han , P.F. , Wang , J.J. Qiao , S.Y. 2010 . Development of monoclonal antibodies and a competitive ELISA detection method for glycinin, an allergen in soybean . Food Chemistry , 121 : 546 – 551 .
- Monaci , L. , Brohée , M. , Tregoat , V. and Hengel , A.V. 2011 . Influence of baking time and matrix effects on the detection of milk allergens in cookie model food system by ELISA . Food Chemistry , 127 : 669 – 675 .
- Monaci , L. and Visconti , A. 2009 . Mass spectrometry-based proteomics methods for analysis of food allergens . Trends in Analytical Chemistry , 28 ( 5 ) : 581 – 591 .
- Morishita , N. , Kamiya , K. , Matsumoto , T. , Sakai , S. , Teshima , R. Urisu , A. 2008 . Reliable enzyme-linked immunosorbent assay for the determination of soybean proteins in processed foods . Journal of Agricultural and Food Chemistry , 56 : 6818 – 6824 .
- Poms , R.E. , Klein , C.L. and Anklam , E. 2004 . Methods for allergen analysis in food: A review . Food Additives and Contaminants , 21 ( 1 ) : 1 – 31 .
- Schneider , N. , Weigel , I. , Werkmeister , K. and Pischetsrieder , M. 2010 . Development and validation of an enzyme-linked immunosorbent assay (ELISA) for quantification of lysozyme in cheese . Journal of Agricultural and Food Chemistry , 58 : 76 – 81 .
- Singh , R. , Sharma , P.P. , Baltus , R.E. and Suni , I.I. 2010 . Nanopore immunosensor for peanut protein Ara h1 . Sensors and Actuators B , 145 : 98 – 103 .
- Stephan , O. and Vieths , S. 2004 . Development of a real-time PCR and a sandwich ELISA for detection of potentially allergenic trace amounts of peanut (Arachis hypogaea) in processed foods . Journal of Agricultural and Food Chemistry , 52 : 3754 – 3760 .
- Vincenzi , S. , Zoccatelli , G. , Perbellini , F. , Rizzi , C. , Chignola , R. Curioni , A. 2002 . Quantitative determination of dietary lectin activities by enzyme-linked immunosorbent assay using specific glycoproteins immobilized on microtiter plates . Journal of Agricultural and Food Chemistry , 50 : 6266 – 6270 .
- Wei , Y. , Sathe , S.K. , Teuber , S.S. and Roux , K.H. 2003 . A sensitive sandwich ELISA for the detection of trace amounts of cashew (Anacardium occidentale L.) nut in foods . Journal of Agricultural and Food Chemistry , 51 : 3215 – 3221 .
- Woods , R.K. , Stoney , R.M. , Raven , J. , Walters , E.H. , Abramson , M. and Thien , F.C. 2002 . Reported adverse food reactions overestimate true food allergy in the community . European Journal of Clinical Nutrition , 56 ( 1 ) : 31 – 36 .