Abstract
A sandwich enzyme-linked immunosorbent assay specific to beef myoglobin was developed using monoclonal antibodies to sodium dodecyl sulphate-denatured beef myoglobin and a peptide with an amino acid sequence unique to beef myoglobin. This method was very specific for beef myoglobin, and showed no cross-reactivity with not only pork and chicken myoglobins, but also with other food proteins such as egg, cow's milk, wheat and peanut. The limit of detection and that of quantification for the beef protein was 9.5 and 12.8 ng/ml, respectively. When the content of beef protein in pork- and chicken-based model processed foods containing varying amounts of beef were determined by this method, the recovery of beef protein agreed well with the actual ratio of added beef regardless of the processing conditions. Also, the amount of beef protein in several commercially processed foods could be reasonably determined and confirmed by this method.
Introduction
Beef is eaten around the world as a delicious and important source of protein, and various beef products, such as sausages, meat sauce and hamburger steak, are produced and consumed. However, there are cases in which the avoidance of beef ingestion is required due to religious beliefs, personal principles such as vegetarianism, and beef-specific diseases such as allergies and bovine spongiform encephalopathy (Hsieh, Ofori, Rao, & Bridgeman, Citation2007; Restani, Ballabio, Tripodi, & Fiocchi, Citation2009). Proper labelling is necessary to ensure the quality, safety and fair trade of beef-containing foods for consumers, and a simple and specific analytical method should be established for the detection of beef in food products and feeds. Enzyme-linked immunosorbent assay (ELISA) is well known (Taylor, Nordlee, Niemann, & Lambrecht, 2009) and is widely utilised as a method for determining the presence of specific proteins in processed foods (Hefle, Jeanniton, & Taylor, Citation2001; Kiening et al., Citation2005; Matsuda et al., Citation2006; Sakai et al., Citation2008). A few ELISA-based methods have been developed for the detection of meat proteins in food products (Andrews, Berger, Mageau, Schwab, & Johnston, 1992; Chen & Hsieh, Citation2000). In this study, we attempted to develop a sensitive sandwich ELISA using monoclonal antibody (mAb) to a peptide with an amino acid sequence unique to beef myoglobin (Mb).
Food proteins are usually denatured during processing, especially by heating, and this denaturation contributes to the shape and texture of the product. In these cases, potential target proteins for the ELISA are often irreversibly aggregated, insoluble and difficult to extract (Taylor et al., Citation2009). Watanabe et al. (Citation2005) reported that a solution containing sodium dodecyl sulphate (SDS) and 2-mercaptoethanol could efficiently extract food proteins, and this method was adopted as the official Japanese method for the detection of major allergenic ingredients in food products (Akiyama, Imai, & Ebisawa, Citation2011). Therefore, we also used SDS and 2-mercaptoethanol for the extraction and dilution of samples to be assayed. Consequently, the target proteins should be able to react with the mAbs under denatured condition.
In this study, we produced mAbs to SDS-denatured beef Mb and a synthetic peptide unique to beef Mb, and developed a sandwich ELISA specific and sensitive to beef Mb. This sandwich ELISA enabled us to accurately determine the beef content in model processed foods and some commercial foods.
Materials and methods
Food materials
Beef leg meat, pork heart and leg meat and chicken heart and breast meat were prepared separately in our meat department unit to prevent cross-contamination. Samples of commercially processed foods were purchased at supermarkets in Osaka.
Chemicals
The following materials were obtained from the indicated sources: Freund's complete and incomplete adjuvants (Difco Laboratories Inc., Detroit, MI, USA); bovine serum albumin (BSA), polyethylene glycol and pristine (Sigma, St. Louis, MO, USA); 3,3′5,5′-tetramethylbenzidine (TMB; Kirkegaard & Perry Laboratories Inc., Baltimore, MD, USA); horseradish peroxidase-conjugated goat anti-mouse IgG and alkaline phosphatase-conjugated goat anti-mouse IgG (Zymed Inc., Vienna, Austria); Imject Maleimide Activated Immunogen Conjugation Kit and EZ-Link Sulfo-NHS-Biotinylation Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA); Peroxidase Labeling Kit-SH (Dojindo Laboratories Inc., Kumamoto, Japan); 2-D Quant Protein Assay Kit and streptavidin-horseradish peroxidase (GE Healthcare UK Ltd., Buckinghamshire, UK); 5-bromo-4-chloro-3-indoxyl phosphate/nitro blue tetrazolium chloride (BCIP/NBT) Alkaline Phosphatase Substrate Kit V (Vector Laboratories Inc., Burlingame, CA, USA). All other chemicals used were of analytical grade.
Preparation of myoglobins and standard solutions from beef, pork and chicken
Beef Mb was purified from fresh leg meat, and pork and chicken Mbs were purified from fresh heart meat. Minced meat (100 g each) was homogenised with two-fold (w/v) of distilled water and mixed for 30 min at 4°C. After centrifugation at 4500 ×g for 15 min at 4°C, the supernatant was filtered through filter paper No. 1 (Whatman Japan Inc., Tokyo, Japan). The filtrate from beef leg meat was brought to 90% ammonium sulphate saturation and those from pork and chicken heart meat were brought to 70% ammonium sulphate saturation. After stirring for 1 h at 4°C, each solution was centrifuged at 20,000 ×g for 15 min at 4°C. After filtration, the supernatant was adjusted to pH 6.85 and brought to 95% ammonium sulphate saturation. After stirring for 1 h at 4°C, it was centrifuged at 20,000 ×g for 15 min at 4°C. The precipitate was dissolved in distilled water and dialysed against 20 mM Tris–HCl (pH 8.3). The solution was applied to an ion exchange column (Toyoscreen DEAE 650 M; Tosoh Inc., Tokyo, Japan) equilibrated with 20 mM Tris–HCl (pH 8.3). The column was eluted at a flow rate of 1.0 ml/min by a linear gradient of 0–0.5 M NaCl in 20 mM Tris–HCl (pH 8.3). The purity of each Mb was checked by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) under reduced conditions according to the method of Laemmli (Citation1970), and a sequence of 10 amino acids at the N-terminus was determined by Takara Bio Inc. (Shiga, Japan).
Minced meat (250 g each) of beef leg, pork leg or chicken breast meat was homogenised with an equal volume of distilled water and freeze-dried. The resulting lyophilised powder (20 mg each) was added to 20 ml of phosphate buffer saline (PBS: 0.02 M phosphate buffer, 0.15 M NaCl, pH 7.2) containing 0.5% SDS and 2% 2-mercaptoethanol and shaken for 12 h at room temperature for extraction (Watanabe et al., Citation2005). After centrifugation at 20,000 ×g for 15 min at room temperature, the supernatant was filtered to obtain a standard solution of which the protein content was determined by a 2-D Quant Protein Assay Kit.
Preparation of monoclonal antibodies
Because the beef Mb would be denatured during extraction, the purified beef Mb was denatured with 1% SDS and heated at 95°C for 10 min (denatured beef Mb: D-Mb) before being used as an immunogen for inducing the production of mAbs to D-Mb. 2-Mercaptoethanol was not used at this step because there are no disulphide bonds in beef Mb (Fuentes, Palacios, Garces, Caballero, & Moneo, 2004). Synthesis of Peptide A and Peptide B was entrusted to Toray Research Center Inc. (Tokyo, Japan). Peptide A (Cys-Ala84-Glu85-Val86-Lys87-His88-Leu89-Ala90-Glu91-Ser92-His93-Ala94-Asn95 of beef Mb) and Peptide B (Met142-Cys-Ala144-Gln145-Tyr146-Lys147-Val148-Leu149-Gly150-Phe151-His152-Gly153 of beef Mb) were conjugated with mariculture keyhole limpet haemocyanin from the Imject Maleimide Activated Immunogen Conjugation Kit for immunisation of mice. Five BALB/c mice (female, 8 weeks old) were immunised intraperitoneally with emulsion containing 50 µg of D-Mb or each peptide-conjugate per mouse in Freund's complete adjuvant. After two weeks, mice were boosted once with the same volume of immunogen emulsified in Freund's incomplete adjuvant. Ten days later, sera were collected from the tail vein and tested for specific antibody production using a direct ELISA with D-Mb, or Peptide A or B conjugated with ovalbumin of the Imject Maleimide Activated Immunogen Conjugation Kit on the solid phase of a 96-well microtiter plate (Corning Inc., Washington, DC, USA). The antibodies bound to D-Mb or Peptide A or B were detected with diluted horseradish peroxidase-conjugated goat anti-mouse IgG and TMB substrate solution. The reaction was stopped by the addition of 1.0 M sulphuric acid (50 µl/well), and then the absorbance was measured at 450 nm with a microplate reader (Spectra Fluor, Tecan, Salzburg, Austria). The mice showing higher titer of antibody were injected with 50 µg D-Mb or each peptide conjugate in PBS into the tail vein. Three days later, myeloma cells (P3X63. AG80. 1; DS Pharma Biomedical Co., Ltd, Osaka, Japan) were fused with spleen cells from immunised mice according to the method of Köhler and Milstein (Citation1975). Ten days after fusion, the cell culture supernatants were screened for specific antibodies by a direct ELISA as described above. The positive cells were cloned twice by limiting dilution and then injected intraperitoneally into mice previously primed with pristine. Ascitic fluids were precipitated by saturated ammonium sulphate solution and dissolved in PBS. The mAbs were named after the code numbers of cells cloned and were purified with a Protein G column (GE Healthcare UK Ltd.). Peroxidase-conjugated and biotin-conjugated mAbs to D-Mb were prepared with a Peroxidase Labeling Kit-SH and EZ-Link Sulfo-NHS Biotinylation Kit, respectively. Mouse experiments in this study were carried out under the guidelines of the Animal Experiment Committee of the university following the bulletin (No.71, 2006) of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Competitive ELISA
Polystyrene 96-well microtiter plates were coated with 100 µl of D-Mb (1 µg/ml) in coating buffer (0.1 M phosphate buffer, pH 7.2) for 12 h at 4°C. After washing four times with a washing buffer (10 mM phosphate buffer, 0.15 M NaCl, 0.1% Tween20, pH 7.4), the plates were blocked with 1% BSA for 2 h at 37°C. Diluted mAbs to D-Mb, Peptide A or Peptide B (80 µl each) were added to an equal volume of various concentrations of Peptide A, Peptide B, D-Mb or native beef Mb (N-Mb) as a competitor, then the mixtures were incubated for 1 h at room temperature. D-Mb was diluted in washing buffer containing 0.1% BSA and 0.3% SDS, and Peptide A, Peptide B and N-Mb were diluted in washing buffer containing 0.1% BSA without SDS. Each mixture (100 µl) was transferred to the wells of the D-Mb-coated plate, and incubated for 1 h at room temperature. After removal of unbound materials by washing, 100 µl of diluted peroxidase-conjugated goat anti-mouse IgG was added to each well, and then incubated for 30 min at room temperature. Finally, the enzyme reaction was performed in the plate as mentioned above for the direct ELISA.
Sandwich ELISA
Polystyrene 96-well microtiter plates were coated with 100 µl of mAbs to Peptide B (5 µg/ml) in coating buffer for 12 h at 4°C. After washing four times with washing buffer, the plates were blocked with 1% BSA for 2 h at 37°C. D-Mb and the food sample solution were diluted with dilution buffer (the washing buffer with 0.1% BSA, 0.05% SDS and 0.2% 2-mercaptoethanol). Diluted D-Mb or food sample solution was added to an antibody-coated plate in triplicate, and incubated for 1 h at room temperature. After washing six times with the washing buffer, 100 µl of diluted peroxidase-conjugated mAb to D-Mb was added to each well, and the plate was then incubated for 30 min at room temperature. One hundred microlitres of the diluted biotin-conjugated mAb to D-Mb was then added to each well, and the plate was incubated for 30 min at room temperature. After washing, 100 µl of diluted streptavidin-horseradish peroxidase was added to each well, and the plate was incubated for 30 min at room temperature. Finally, the plate was applied to the enzyme reaction as described for the direct ELISA. Limit of detection (LOD) or limit of quantification (LOQ) for the sandwich ELISA were calculated by adding 3 times or 10 times the standard deviation of the buffer blank to the mean of the buffer blank, respectively (Seiki et al., Citation2007).
Western blotting
Western blotting was carried out according to the method of Towbin, Staehelin, and Gordon (Citation1979) with slight modifications. After SDS–PAGE on a 15% gel under reduced conditions, the resolved protein bands were transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories Inc., Alfred Nobel Drive Hercules, CA, USA) at 1 mA/cm2. After electroblotting, the membrane was blocked with phosphate buffer saline with Tween 20 (PBST: 0.137 M NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4·12H2O, 1.5 mM KH2PO4, 0.1% Tween20) for 12 h at 4°C. After washing three times with PBST, the membrane was incubated with diluted mAb to D-Mb or Peptide B for 1 h at room temperature. The excess antibodies were removed by washing three times with PBST, then the membrane was incubated with alkaline phosphatase-conjugated goat anti-mouse IgG diluted with PBST for 1 h at room temperature. After washing three times with PBST, the membrane was equilibrated with 0.1 M Tris buffer (pH 9.5), and then detected with BCIP/NBT Alkaline Phosphatase Substrate Kit V. The colour development was stopped by shaking the membrane in distilled water.
Preparation of model processed foods and sample solutions
Minced beef leg meat was mixed with minced pork leg meat or chicken breast meat to be 100%, 10%, 1%, 0.1%, 0.01% and 0% (w/w). Then, potato starch, salt and water were added at the weight ratio of 0.2:0.2:0.6 to 9 of the mixed meat. The mixtures were incubated at room temperature, 80 or 120°C for 30 min, cooled in running water and then preserved in a freezer at–30°C until use. Eighteen millilitres of PBS (pH 7.2) containing 0.5% SDS and 2% 2-mercaptoethanol was added to 2 g of the mashed sample. After shaking for 12 h at room temperature for extraction, the mixture was centrifuged at 20,000 ×g for 15 min and the supernatant was filtered to obtain a solution for each sample to be assayed.
Results and discussion
Selection and preparation of antigens
To establish a mAbs ELISA specific to beef, the selection of an appropriate target protein is important. BSA (Fiocchi, Rastani, & Riva, Citation2000; Tanabe et al., Citation2002) and immunoglobulin (Ayuso et al, Citation2000; Han, Matsuno, Ito, Ikeuchi, & Suzuki, Citation2000) are reported to be the major allergen in beef. These allergenic proteins or myofibrillar abundant proteins may usually be selected as a target antigen for the detection of beef by a sandwich ELISA. However, the ELISA specific to beef should be capable of distinguishing beef at least from cow's milk and also from pork and chicken. Serum albumin and immunoglobulin are constitutive components in cow's milk and amino acid sequences of major myofibrillar proteins such as myosin (Chikuni, Tanabe, Muroya, & Nakajima, Citation2002) are highly conservative with over 95% homology among these animals. We selected beef myoglobin (Mb) as a target antigen, because it is a constitutive and relatively rich component in meat (4–10 mg/g) but not in milk, and its amino acid sequence is somewhat different among animals: 88% homology between beef Mb (Han, Dautrevaux, Chailax, & Biserte, Citation1970) and pork Mb (Rousseaux, Dautrevaux, & Han, Citation1976) and 74% between beef Mb and chicken Mb (Deconinck et al., Citation1975).
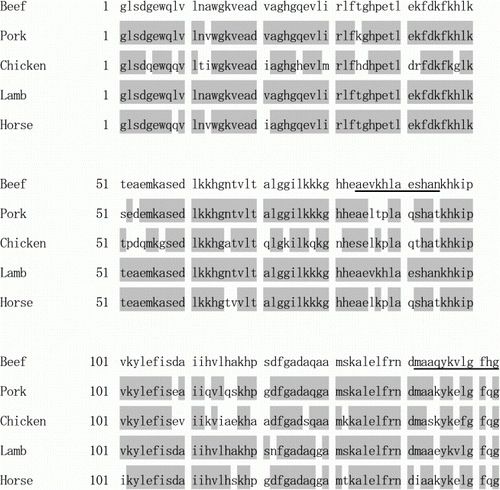
Beef Mb was purified from fresh leg meat (See A, lane 4) and was used as an immunogen after denaturation by heating with SDS because the actual samples are also denatured during extraction (Watanabe et al., 2005). If whole beef Mb is used as an immunogen, the obtained antibodies might cross-react with pork or chicken Mb (Levieux & Levieux, Citation1996). Therefore, we tried to obtain mAbs to peptides with amino acid sequences specific to beef Mb. Two sequences, Ala84-Asn95 and Met142-Gly153, which are relatively characteristic of beef Mb, were selected (). Amide bonding of the peptides to the carrier protein was avoided to simplify the structure of the conjugates because Glu and Lys were present in the sequences of both peptides. Then, two peptides, Peptide A (Cys-Ala84-Asn95) and Peptide B (Met142-Cys-Ala144-Gly153), were chemically synthesised. The N-terminal Cys in Peptide A was supplemented to Ala84-Asn95 and the second Cys in Peptide B was substituted for Ala143 in Met142-Gly153. They were conjugated to a carrier haemocyanin through disulphide bonds to enhance their immunogenicity, and also to ovalbumin to enable screening for cells producing antibodies reactive to the peptides.
Monoclonal antibodies
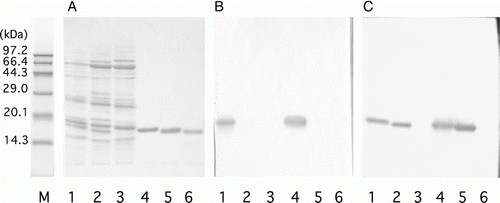
First, three mAbs reactive to Peptide A conjugated to ovalbumin were obtained. They reacted with D-Mb coated on an ELISA plate. The binding was inhibited at higher concentrations of free Peptide A (IC50 was about 10–100 µg/ml) but not with free N-Mb nor with free D-Mb on a competitive ELISA (data not shown). Next, mAbs 11H, 1D and 10B reactive to Peptide B conjugated to ovalbumin were obtained. They also reacted with D-Mb coated on an ELISA plate, but the binding was inhibited on a competitive ELISA at lower concentrations of free peptide B (IC50 was about 0.01 µg/ml) and with free N-Mb or D-Mb. The typical data obtained with mAb 11H, shown in A, suggest that these mAbs recognise beef Mb exactly at the Met142-Gly153 sequence close to the C-terminus. The C-terminus of beef Mb seems to be pushed to the outsides of the molecule and is available for antibody binding in its native state. This is consistent with a report by Abaza and Atassi (Citation1992) which showed that the region of 145–151 in any animal Mb is an antigenic site that is exposed on the outside. On western blotting analysis, however, mAbs 1D and 10B weakly cross-reacted with pork Mb but not with chicken Mb, and only mAb 11H was specific to beef Mb (B). The difference in the specificity among obtained mAbs to a particular peptide is most likely due to the differences in the amino acid sequence of their antigen-binding sites. To construct a sandwich ELISA with this beef-specific mAb 11H, five mAbs were obtained from mice immunised with D-Mb. Among the selected mAb, mAb 11E reacted with D-Mb more strongly than with N-Mb, and the reaction was stronger than the mAb 11H reaction in the competitive ELISA (). The mAb 11E reacted with beef and pork Mb, but not with chicken Mb or other beef proteins on western blotting analysis (C).
Figure 2. Reactivity of antibodies against beef myoglobin (Mb) on a competitive ELISA. A competitive ELISA for beef Mb (see Materials and methods) in the presence of various amounts of D-Mb (•) or N-Mb (○) as competitors. Absorbance obtained with no competitor was set as 100%. Antibody against Peptide B (mAb 11H) was used in panel (A) and that against D-Mb (mAb 11E) in panel (B).
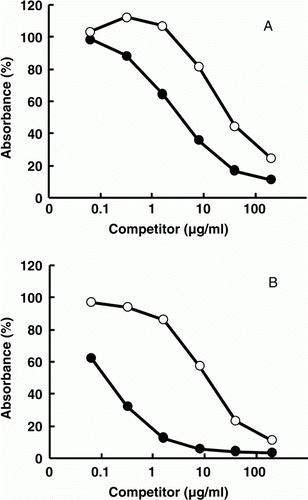
Figure 3. Specificity of mAbs against beef myoglobin (Mb). (A) Protein staining with coomassie brilliant blue; (B) immunostaining with antibody against Peptide B (mAb 11H); (C) immunostaining with antibody against denatured Mb (mAb 11E). M: molecular weight markers. Ten micrograms each of protein extracted from beef (lane 1), pork (lane 2) and chicken (lane 3), and 1 µg each of Mbs from beef (lane 4), pork (lane 5) and chicken (lane 6) were applied to 15% agarose gels.
Sandwich ELISA
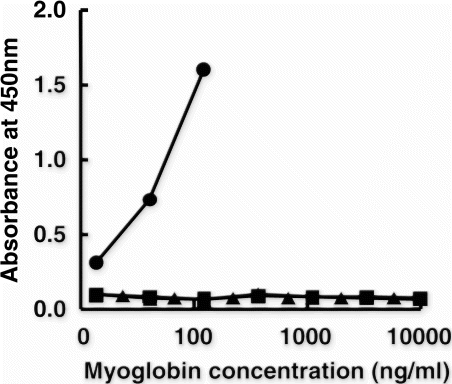
As expected from the results above suggesting that epitopes of mAbs 11E to D-Mb and 11H to peptide B are different, a sandwich ELISA was constructed using both mAbs. Monoclonal Ab 11H was selected as the capture antibody because of its strict specificity to beef Mb (B) and mAb 11E was used as the peroxidase-conjugated antibody. The ELISA thus constructed was specific to D-Mb without cross-reactivity to pork and chicken D-Mbs even at high concentrations ().
Figure 4. Specificity of the sandwich ELISA. The antibody against Peptide B (mAb 11H) was used as the capture antibody, and the antibody against denatured Mb (D-Mb) (mAb 11E) was used as the peroxidase-conjugated antibody. (•): beef D-Mb; (▪): pork D-Mb and (▴): chicken D-Mb.
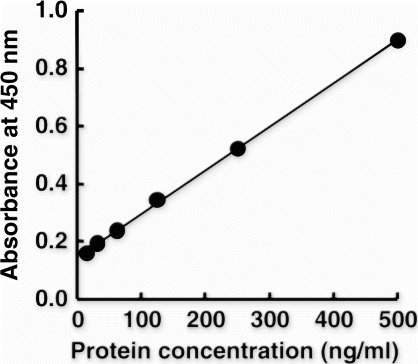
For the practical usage of this ELISA, it is best to show the data in terms of beef protein content and not as beef Mb content. Thus, the beef standard solution (see Materials and methods) was introduced as the standard material instead of D-Mb. In addition, the biotin–avidin system was incorporated to improve assay sensitivity. As shown in , a linear standard curve was obtained at concentrations below 500 ng/ml of beef standard solution. The LOD and LOQ of the beef protein were calculated to be 9.5 and 12.8 ng/ml, respectively (See Materials and methods). This improved system did not cross-react with pork, chicken and horse standard solutions, but it did react with lamb at approximately 10% of the reactivity to beef (data not shown). This difference is consistent with the differences in amino acid sequence of the Peptide B region between Val147 in beef and lamb Mb and Glu147 in pork, chicken and horse Mb (). Except for lamb, this system exhibited no cross-reactivity to other food protein, including egg, milk, wheat, buckwheat, peanut, shrimp and crab (data not shown).
Figure 5. Standard curve of the sandwich ELISA for the beef protein standard. Protein standard from beef was applied to the sandwich ELISA, the sensitivity of which was enhanced with the biotin–avidin system. A linear regression line was obtained for the beef standard at 0–500 ng/ml. The equation was statistically expressed as Y=0.0015 X + 0.1456, R 2=0.9994, where Y is the absorbance and X is concentration of the beef protein standard.
Determination of beef protein content in processed foods
To evaluate the applicability of this beef-specific sandwich ELISA, beef protein content was determined in model processed foods produced with varying amount of beef mixed with pork or chicken under different processing temperatures. When 2 g of model processed foods prepared with 100% beef were extracted with 18 ml of PBS containing 0.5% SDS and 2% 2-mercaptoethanol, this sandwich ELISA determined the beef protein concentrations in the extracts of non-heated samples, samples heated at 80°C and samples heated at 120°C to be 8.4, 7.8 and 6.9 mg/ml, respectively (). Its coefficient of variation, calculated from nine replicates of the same sample solution, was below 5%. On the other hand, those determined by 2-D Quant Kit were 8.0, 3.0 and 2.2 mg/ml for the non-heated samples, samples heated at 80°C and samples heated at 120°C, respectively. This discrepancy of the values in heated samples might be explained by that the extraction efficiency of whole proteins was decreased by heating but that of beef Mb was not decrease of its high extractability (Fuentes et al., Citation2004). Consequently, a good agreement was obtained between 0.01% and 10% of the actual mixing ratio of beef and the recoveries determined with this sandwich ELISA, regardless of difference of basic meats and heating conditions (). This high specificity was accomplished by using mAb 11H, which is specific to beef Mb, as the capture antibody (B). An ELISA constructed with mAbs 11H and 11E reversely showed inferior specificity and sensitivity (data not shown). Beef is generally added into processed foods at concentrations of 1% to 50%; therefore, this sandwich ELISA is suitable for use in the detection of beef in commercially processed foods.
Table 1. The precision of the sandwich ELISA using beef-based model processed foods.a
Table 2. Recovery of beef protein in pork- or chicken-based model processed foods in relation to those in beef-based model processed foods.a
As shown in , this method could qualitatively confirm that the labelled beef content in several commercial foods was reasonable. It is notable that beef Mb was detectable by western analysis using mAb 11H in even demi-glace sauce, which contained beef extracts (data not shown). Beef extract is generally prepared from the liquid obtained from boiling beef meats, tendons or bones in water, and sometimes the liquid is treated with proteinases to add taste and flavour. Shimakura, Tonomura, Hamada, Nagashima, and Shiomi (Citation2005) reported that tropomyosin, the major crustacean allergen, was degraded to peptide fragments during manufacturing of crustacean extractives. However, beef Mb seems to tolerate heating and proteinase treatment during manufacturing. Thus, beef Mb is an excellent target for the detection of beef protein because of its low homology in amino acid sequence with other Mbs, easy-extractability and tolerance to heating and proteinase treatment.
Table 3. Determination of beef protein content in selected commercial foods.
A detection kit utilising methods developed by theUnited States Department of Agriculture is commercially available for the detection of cooked beef meat in foods (Andrews et al., Citation1992). However, the kit is qualitative and can only detect the presence of cooked beef meat at concentrations of 1% or more. Previously, we reported the establishment of a sandwich ELISA using polyclonal Abs specific to beef Mb (Kotoura et al., Citation2009); however, its sensitivity was low (LOD: 29.4 ng/ml, LOQ: 36.4 ng/ml), the determination of beef protein content in foods was affected by heating, and it cross-reacted with lamb at the same reactivity as with beef (data not shown). We have largely succeeded in improving these problems in the current sandwich ELISA: (1) the sensitivity was higher with a LOD and LOQ of 9.5 and 12.8 ng/ml, respectively; (2) the determination of beef protein content was hardly affected by heating and (3) cross-reactivity with lamb was reduced, at about 10% of the reactivity to beef. Moreover, the new ELISA was composed of mAbs, which have better reliability in terms of quality and quantity than polyclonal antibodies.
In conclusion, we have developed a sandwich ELISA specific to beef protein by using two mAbs. This method is useful in evaluating the accuracy of the food labelling of beef products, and is suitable for use in the determination of beef protein content in processed foods and feeds, regardless of processing conditions such as temperature and enzyme treatment.
Acknowledgements
This work was performed as a part of the project on the development of determination methods for allergenic materials in foods organised by the Ministry of Health, Labour and Welfare of Japan.
References
- Abaza , M-S.I. and Atassi , M. Z. 1992 . Effects of amino acid substitutions outside an antigenic site on protein binding to monoclonal antibodies of predetermined specificity obtained by peptide immunization: Demonstration with region 145–151 (antigenic site 5) of myoglobin . Journal of Protein Chemistry , 11 : 687 – 689 .
- Akiyama , H. , Imai , T. and Ebisawa , M. 2011 . Japan food allergen labeling regulation–History and Evaluation . Advances in Food and Nutrition Research , 62 : 139 – 171 .
- Andrews , C.D. , Berger , R.G. , Mageau , R.P. , Schwab , B. and Johnston , R.W. 1992 . Detection of beef, sheep, deer, and horse in cooked meat products by enzyme-linked immunosorbent assay . Journal of AOAC International , 75 : 572 – 576 .
- Ayuso , R. , Lehrer , M. , Reese , M.L. , Ibañez , M.D. , Esteban , M.M. Ownby , D.R. 2000 . Identification of bovine IgG as a major cross-reactive vertebrate meat allergen . Allergy , 55 : 348 – 354 .
- Chen , F.C. and Hsieh , Y.-H.P. 2000 . Detection of pork in heat processed meat products by monoclonal antibody-based ELISA . Journal of AOAC International , 83 : 79 – 85 .
- Chikuni , K. , Tanabe , R. , Muroya , S. and Nakajima , I. 2002 . Comparative sequence analysis of four myosin heavy chain isoforms expressed in porcine skeletal muscles: sequencing and characterization of porcine myosin heavy chain slow isoform . Animal Science Journal , 73 : 257 – 262 .
- Deconinck , M. , Peiffer , S. , Depreter , J. , Paul , C. , Schnek , A.G. and Leonis , J. 1975 . The primary sequence of chicken myoglobin (Gallus gallus) . Biochimica et Biophysica Acta , 386 : 567 – 575 .
- Fiocchi , A. , Rastani , P. and Riva , E. 2000 . Beef allergy in children . Nutrition , 16 : 454 – 457 .
- Fuentes , M.M. , Palacios , R. , Garces , M.M. , Caballero , M.L. and Moneo , I. 2004 . Isolation and characterization of a heat-resistant beef allergen: myoglobin . Allergy , 59 : 327 – 331 .
- Han , G.D. , Matsuno , M. , Ito , G. , Ikeuchi , Y. and Suzuki , A. 2000 . Meat allergy: investigation of potential allergenic proteins in beef . Bioscience, Biotechnology, and Biochemistry , 64 : 1887 – 1895 .
- Han , K.K. , Dautrevaux , M. , Chailax , X. and Biserte , G. 1970 . The covalent structure of beef heart myoglobin . European Journal of Biochemistry , 16 : 465 – 471 .
- Hefle , S.L. , Jeanniton , E. and Taylor , S.L. 2001 . Development of a sandwich enzyme-linked immunosorbent assay for the detection of egg residues in processed foods . Journal of Food Protection , 64 : 1812 – 1816 .
- Hsieh , Y.-H.P. , Ofori , J.A. , Rao , Q. and Bridgeman , C.R. 2007 . Monoclonal antibodies specific to thermostable proteins in animal blood . Journal of Agricultural and Food Chemistry , 55 : 6720 – 6725 .
- Kiening , M. , Niessner , R. , Drs , E. , Baumgartner , S. , Krska , R. Bremer , M. 2005 . Sandwich immunoassays for the determination of peanut and hazelnut traces in foods . Journal of Agricultural and Food Chemistry , 53 : 3321 – 3327 .
- Köhler , G. and Milstein , C. 1975 . Continuous cultures of fused cells secreting antibody of predefined specificity . Nature , 256 : 495 – 497 .
- Kotoura , S. , Murakami-Yamaguchi , Y. , Nakamura , Y. , Miake , K. , Sugiyama , M. Tanabe , S. 2009 . A sandwich ELISA for the determination of beef meat content in processed foods . Food Science and Technology Research , 15 : 613 – 618 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Levieux , D. and Levieux , A. 1996 . Antigenic specificity of monoclonal antibodies to beef myoglobin determined by cross-reactivity studies against myoglobins from domestic species . Meat Science , 42 : 239 – 249 .
- Matsuda , R. , Yoshioka , Y. , Akiyama , H. , Aburatani , K. , Watanabe , Y. Matsumoto , T. 2006 . Interlaboratory evaluation of two enzyme-linked immunosorbent assay kits for the detection of egg, milk, wheat, buckwheat, and peanut in foods . Journal of AOAC International , 89 : 1600 – 1608 .
- Restani , P. , Ballabio , C. , Tripodi , S. and Fiocchi , A. 2009 . Meat allergy . Current Opinion in Allergy and Clinical Immunology , 9 : 265 – 269 .
- Rousseaux , J. , Dautrevaux , M. and Han , K. 1976 . Comparison of the amino acid sequence of pig heart myoglobin with other ungulate myoglobins . Biochimica et Biophysica Acta , 439 : 55 – 62 .
- Sakai , S. , Matsuda , R. , Adachi , R. , Akiyama , H. , Maitani , T. Ohno , Y. 2008 . Interlaboratory evaluation of two enzyme-linked immunosorbent assay kits for the determination of crustacean protein in processed foods . Journal of AOAC International , 91 : 123 – 129 .
- Seiki , K. , Oda , H. , Yoshioka , H. , Sakai , S. , Urisu , A. Akiyama , H. 2007 . A reliable and sensitive immunoassay for the determination of crustacean protein in processed foods . Journal of Agricultural and Food Chemistry , 55 : 9345 – 9350 .
- Shimakura , K. , Tonomura , Y. , Hamada , Y. , Nagashima , Y. and Shiomi , K. 2005 . Allergenicity of crustacean extractives and its reduction by protease digestion . Food Chemistry , 91 : 247 – 253 .
- Tanabe , S. , Kobayashi , Y. , Takahata , Y. , Morimatsu , F. , Shibata , R. and Nishimura , T. 2002 . Some human B and T cell epitopes of bovine serum albumin, the major beef allergen . Biochemical and Biophysical Research Communications , 293 : 1348 – 1353 .
- Taylor , S.L. , Nordlee , J.A. , Niemann , L.M. and Lambrecht , D.M. 2009 . Allergen immunoassays – Considerations for use of naturally incurred standards . Analytical and Bioanalytical Chemistry , 395 : 83 – 92 .
- Towbin , H. , Staehelin , T. and Gordon , J. 1979 . Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets – Procedure and some applications . Proceedings of the National Academy of Sciences of the United States of America , 76 : 4350 – 4354 .
- Watanabe , Y. , Aburatani , K. , Mizumura , T. , Sakai , M. , Muraoka , S. , Mamegosi , S. and Honjoh , T. 2005 . Novel ELISA for detection of raw and processed egg using extraction buffer containing a surfactant and a reducing agent . Journal of Immunological Methods , 300 : 115 – 123 .