Abstract
With the purpose of finding an immunoassay of similar sensitivity for type II pyrethroids such as fenpropathrin, cypermethrin, deltamethrin, esfenvalerate, cyhalothrin, fluvalinate and flucythrinate, we prepared three kinds of polyclonal antibodies (Pabs), and screened against nine coating antigens (CAgs). The final selected antibody–antigen combination based on heterologous assay was further optimised and tested for tolerance to methanol concentration, ionic strength and pH changes. Sensitivity of optimal immunoassay was found to be quite similar among cypermethrin, deltamethrin and esfenvalerate, as IC50 values were 23.2, 24.5 and 25.1 µg/L for the three pyrethroids, respectively. No cross-reactivity was measured to type I pyrethroids or other relative metabolites. This immunoassay was used to detect different combinations of cypermethrin, deltamethrin and esfenvalerate spiked in West Lake water, and recoveries were between 95.6% and 103.6%, which indicated that the chosen ELISA was feasible for multi-residue analysis.
Introduction
Pesticide residue is generally considered as a risk for people's health and environmental safety (Casida & Quistad, Citation1998). Pyrethroids are largely used because of its low dosage and high efficacy. However, excessive application of those insecticides would pollute water and soil (Zhang, Khan, Akhtar, & Ivarson, Citation1984), and influence survival of aquatic animals (Perry, Venners, Barr, & Xu, Citation2007; Schimmel, Garnas, Patrick Jr., & Moore, Citation1983) and terricolous mammals (Barr, Leng, & Angerer, 2007; Go, Garey, Wolff, & Pogo, Citation1999).
Chromatographic analysis is a major strategy to analyse the residues of pyrethroids (Barbini, Vanni, Girolimetti, & Dommarco, Citation2007; Mekebri, Crane, Blondina, Oros, & Rocca, Citation2008; You & Lydy, Citation2007; Zawiyah et al., Citation2007), however, this method is relatively time-consuming and need expensive apparatus. Immunoassay is considered as an alternative technique because of its high sensitivity, low cost and convenient operation. Enzyme-linked immunosorbent assay (ELISA) is a particularly powerful tool for on-site screening for pesticide monitoring (Watanabe et al., Citation2006).
Pyrethroids are usually grouped into two types. Type II pyrethroids can be distinguished from type I pyrethroids as the former one contains the cyano group (Mak et al., Citation2005). Immunoassays for single pyrethroid residue had been maturely established in previous studies (Ahn, Watanabe, Gee, & Hammock, Citation2004; Lee, Shan, Watanabe, Stoutamire, Gee, & Hammock, Citation2002; Shan, Leeman, Stoutamire, Gee, Chang, & Hammock, Citation2000; Shan, Stoutamire, Wengatz, Gee, & Hammock, Citation1999; Wengatz, Stoutamire, Gee, & Hammock, Citation1998). Moreover, many attempts were made to develop immunoassays in which several pesticides could be recognised by using generic haptens. Mak et al. (Citation2005) synthesised generic haptens to prepare broad selective antibodies, heterologous assay (the different molecular structures between immunising hapten and coating hapten) showed the best sensitivity for type II pyrethroids. Homologous assay (the same molecular structures with immunising hapten and coating hapten) also got perfect results in Hao's et al. (2009) and Wang's et al. (Citation2011) studies. Zhang, Zhang, Wang and Li (Citation2010) first discussed the situation of detecting mixed pyrethroids, but the recovery results were a bit low as the cross-reactivities (CRs) of certain insecticides varied much. Not like low CRs are required in immunoassays for single analyte, CRs expect to be closer in multiple pesticides analysis, because equal sensitivity would be helpful for stable recovery. However, in previous studies, although the antibodies were of broad selectivity or even with high sensitivity, sample analysis was frequently focused on single analyte because CRs varied considerably, total quantity of mixed pesticides were seldom discussed.
The purpose of this study is to develop a broad-selective immunoassay for type II pyrethroids. We hope the immunoassay can get similar or better sensitivity in comparison with former studies, and get as similar CRs as possible. Most importantly, we try to develop a method in which several pesticides could be detected simultaneously. In the present study, we synthesised three immunogens (with new hapten synthetic strategy) and nine coating antigens (CAgs) to make up 27 combinations, and the heterologous assays were emphasised during the screening process. The final chosen ELISA here could detect seven compounds (IC50s are shown in the bracket behind the pyrethroids) such as fenpropathrin (14.5 µg/L), cypermethrin (23.2 µg/L), deltamethrin (24.5 µg/L), esfenvalerate (25.1 µg/L), cyhalothrin (46.8 µg/L), fluvalinate (66.1 µg/L) and flucythrinate (72.4 µg/L). Cypermethrin, deltamethrin and esfenvalerate were found to be quite similar in sensitivity. These three pyrethroids were tried for recovery tests in water samples. All the pre-treatment processes were quite simple, and the acceptable results indicated that this immunoassay could be applied on quantitative multi-residue analysis.
Materials and methods
Chemicals
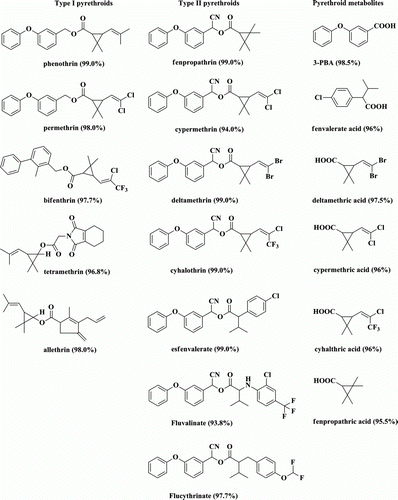
Pyrethroid standards, together with the pyrethroid metabolites ( displays the structural formula and purity) were obtained from the National Standards Company (Beijing, China). Starting products, such as Triethylamine (TEA), dimethylaminopurine (DMAP), ruthenium (III) trichloride hydrate, N,N′-dicyclohexylcar, N,N-dimethylformamide (DMF), isobutyl chloroformate, ovalbumin (OVA; MW, 45,000), bovine serum albumin (BSA; MW, 67,000), Tri-n-butylamine, goat anti-rabbit immunoglobulin conjugated to horseradish-peroxidase (HRP), complete and incomplete Freund's adjuvants for hapten synthesis were provided by Jiangsu Yangnong Chemical Group Co., Ltd. O-phenylenediamine (OPD), Tween-20 and other chemical reagents were purchased from Shanghai Chemical Reagents Company (China). Phosphate buffered saline (PBS, 10 mM, pH 7.4), carbonate-buffered saline (CBS, 50 mM, pH 9.5) and phosphate-citrate buffer (pH 5.6) were self-prepared. All other chemicals and organic solvents were of analytical grade or better.
Equipments
1H nuclear magnetic resonance (NMR) spectra were obtained with an AVANCE DMX 500 spectrometer (Bruke, Berlin, Germany); operating at 400 MHz for solutions in CDCl3. Chemical shifts were given relative to tertramethylsilane (TMS). Electron spray ion (ESI) mass spectra were measured using an Esquire-LC00075 mass spectrometer (Bruke, Berlin, Germany). Electron ion mass spectra were obtained with an HP 5890/5973 mass spectrometer (Agilent, Wilmington, DE, USA). Ultraviolet-visible (UV-vis) spectra were recorded on a spectrophotometer (Xinmao, Shanghai, China). The ELISA was carried out in 96-well polystyrene microplates (COSTAR, High Binding Plates, USA). Plates were washed with a DEM plate washer (Beijing Tuopu Analytical Instruments, Beijing, China). Absorbencies were measured with a 550 plate reader (Bio-Rad, Hercules, CA, USA). A gas chromatograph (GC) (Agilent 6890; Agilent Technologies Inc.) was used to validate the spiked samples be free of tested pyrethroids.
Synthesis of haptens
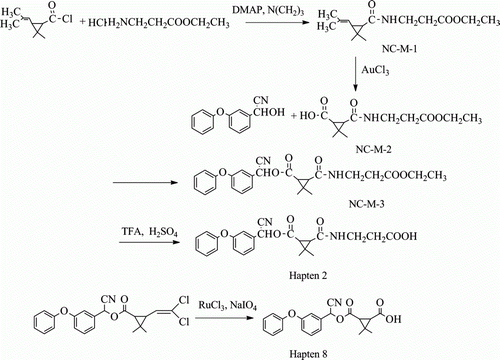
lists the molecular structure of nine haptens. The novel synthesis routes of immunising hapten 2 and hapten 8 are illustrated in , and the concrete procedure is described as follows. The rest of other seven haptens were synthesised referring to previous studies (ESI-MS data did not show here) (Hao et al., Citation2009; Lee, Beasley, & Skerritt, Citation1998).
Table 1. Structure of nine haptens.
Synthesis of 3-(2-carboxy-ethylcarbamoyl)-2,2-dimethyl-cyclopropanecarboxylic acid cyano- (3-phenoxy-phenyl)-methyl ester (Hapten 2)
NC-M-1
A solution of 1.28 g (6.9 mmol) of chrysanthemoyl chloride in 30 mL chloroform was added dropwise to a stirred mixture of 1.582 g (11 mmol) γ-amino butyric acid ethyl ester hydrochloride, 0.178 g (1.45 mmol) of DMAP, 1.547 g (15.3 mmol) of TEA in 120 mL chloroform. After reacting for 6 h at 20°C, the mixed solution was washed with dilute hydrochloric acid three times first, and then washed with water (5×100 mL) to neutral. After dried by Na2SO4 and purified by a column of silica gel (250 g), the product NC-M-1 (1.71 g, 6.7 mmol) was eluted with 1:6 (v/v) ethyl acetate–petroleum ether.
NC-M-2
A solution of 2.645 g (10.3 mmol) of NC-M-1, 0.166 g (0.61 mmol) of ruthenium (III) trichloride hydrate and 8.39 g (39 mmol) of sodium periodate were dissolved in 70 mL mixed solution (CCl4: CH3CN: H2O=10:10:15, V/V), and heated to reflux for 24 h while isolating air. After the solution was refrigerated, it was filtered and the filter cake was washed with saturated sodium chloride solution. The filtrate was combined and extracted with dichloromethane for three times. After dried by anhydrous Na2SO4, the concentrated solution was extracted by saturated sodium bicarbonate and adjusted pH to 2 with hydrochloric acid. And then the solution was extracted with ethyl acetate and dried by anhydrous Na2SO4 once again. After the extracting solution was concentrated and purified by a column of silica gel (250 g), the product NC-M-2 (1.78 g, 7.2 mmol) was eluted with 1:6 (v/v) ethyl acetate–petroleum ether.
NC-M-3
3-phenoxy-benzaldehyde cyanohydrin dissolved in methylene chloride was combined with 5.01 g (20.2 mmol) of NC-M-2, 5.95 g (25.2 mmol) of N,N′-dicyclohexylcar and 1.1 g of (9 mmol) DMAP and reacted in ice bath. After stirring for 20 h, the solution was added in 20 mL of 3% HCl and extracted with ethyl acetate for twice. The organic phase was combined and washed with water to neutral. The concentrated solution was dried by anhydrous Na2SO4 and purified by a column of silica gel (250 g), the product NC-M-3 (4.60 g, 9.44 mmol) was eluted with 1:6 (v/v) ethyl acetate–petroleum ether.
Hapten 2
A solution of 0.958 g (2.0 mmol) of purified NC-M-3 was combined with 4 g trifluoroacetic acid and 1 g of concentrated H2SO4 and then heated to react for 18 h. The solution was adjusted to pH 3–5 with NaHCO3, extracted with ethyl acetate and purified by silica gel column to get the final product hapten 2 of 0.592 g (1.3 mmol). ESI-MS: 435 (M + H)+(100%); 1H-NMR(CDCl3):d8.02(s, 1H, -NH), 6.96–7.42 (m, 9H, -ArH), 6.31 (s, 1H, -CHCN), 3.59 (m, 2H, -CH2N), 2.57 (m, 2H, -CH2CO) and 1.26 (m, 6H, 2×-CH3).
Synthesis of 3,3-dimethyl-cyclopropane-1,2-dicarboxylic acid mono-[cyano-(3-phenoxy-phenyl)-methyl] ester (Hapten 8)
A solution of 2.2 g (5.2 mmol) of cypermethrin, 0.12 g of ruthenium (III) trichloride hydrate and 4.3 g (20 mmol) of sodium periodate were dissolved in 70 mL mixture of CCl4:CH3CN:H2O (10:10:15, V/V). After heating to reflux for 2 h, the solution was adjusted to pH 2–6 by 10% HCl and extracted by dichloromethane for three times. After that, the organic phase was combined and washed by water for three times. Then the solution was dried by anhydrous Na2SO4 and concentrated. The residue was purified by silica gel column (eluent as follows: ethyl acetate:petroleum ether=1:10 in volume ratio) to get the final product of hapten 8 (1.12 g). ESI-MS: 366 (M-H) +(100%); 1H-NMR(CDCl3): 7.402–6.999 (9H, m, Ph), 6.363–6.341 (1H, m, -CH-CN), 2.328–2.280 (2H, m,-CH-CH-COOH), 1.348 (3H, s, Me), 1.238 (3H, s, Me).
Preparation of immunogens and coating antigen
As small molecular weight compounds are not immunogenic alone, hapten 2, 8 and 9 with cyano group were conjugated with BSA by the mixed anhydride method (Gendloff, Casale, & Ram, Citation1986) to synthesise immunogens. Additionally, six other haptens, together with those other three, were conjugated with OVA by the same method to obtain CAgs. Each hapten (0.25 mmol) was dissolved in 1 mL of dry DMF, and then 60 µL of Tri-n-butylamine and 30 µL of isobutyl chloroformate was added dropwise. After the mixture was stirred at room temperature overnight, the precipitate was removed by centrifugation, and about 500 µL of the reaction solution was added dropwise to a solution of BSA/OVA [120 mg BSA/OVA was dissolved in 6 mL PBS (0.01 M, pH 7.4)] with vigorous stirring at 4°C. The reaction mixture was stirred gently at 4°C for 2 h to complete the conjugation. Finally, immunogens and coating antigen were purified by dialysis for three days using PBS (10 mM, pH 7.4). The conjugates were stored at−20°C until use. Conjugate formation was confirmed spectrophotometrically. UV-vis spectra showed qualitative differences between the carrier protein and the conjugates in the region of maximum absorbance of haptens. Molar ratios of those conjugates ranged from 3 to 21. The molar ratios were calculated according to this equation:
C means the concentration (mg/kg) of hapten or protein, and M means molecular weight of hapten or protein.
Immunisation protocol
New Zealand white rabbits were housed according to the EEC 609/86 Directives regulating the welfare of experimental animals. Female rabbits weighing 2–2.5 kg were used for raising polyclonal antibodies (Pabs) with each of the three immunogens (two rabbits per immunogen). The immunogens (500 µg) dissolved in PBS (0.5 mL) was emulsified with Freund's complete adjuvant (1:1 volume ratio) and injected intradermally at multiple points on the back. Two weeks later, each rabbit was boosted with an additional 500 µg of the immunogen emulsified with Freund's incomplete adjuvant and bled 7–10 days later. After that, boosting and bleeding were continued by turns at one week interval. Serum was isolated by centrifugation and added sodium azide as preservative with a final concentration of 0.02%. Antiserum was aliquoted and stored at−20°C.
Screening antibody–antigen combination that show high affinity
To select antibody–antigen combinations which show sufficient affinity among all the 27 possible combinations (three kinds of antibodies towards nine kinds of coating antigens) is an important precondition for later pesticide screening. The working concentration of antibody–antigen combination is all the same throughout the whole experiment (antibody dilution is 1:16,000 and concentration of CAg is 16 µg/mL). Besides, OVA was coated as a control to examine the background values of the reaction. The absorbent values applied to evaluate the affinity were shown on .
Table 2. Summary of optical density of titration tests.
Screening sensitive antibody–antigen combination for the type II pyrethroids
The three types of antibodies were screened against nine different kinds of CAg using a two-dimensional titration method with the coated antigen format. The combinations with high affinity were applied in a competitive inhibition experiment to select the most sensitive antibody for the type II pyrethroids. In this study two type I (tetramethrin and permethrin) and two type II pyrethroids (cypermethrin and esfenvalerate), with representative structure for each type respectively, were studied simultaneously.
Competitive indirect ELISA
The procedure of the competitive indirect ELISA is performed as previously described by Liu, Jin, Gui, Cheng, Guo, and Zhu (Citation2007). All incubations were carried out at 37°C and after each incubation the microtiter plates were washed three times with PBST (10 mM PBS containing 0.05% Tween 20, pH 7.4). The plates were coated with hapten-OVA conjugate (100 µL per well) in CBS (50 mM, pH 9.6) to incubate for 2 h. Then the plates were blocked by incubating with 2% skimmed milk in PBS (300 µL per well) for 30 min. Serial dilutions (50 µL per well) of the pyrethroid standards with concentration ranging from 3.125 to 200 µg/L in methanol-PBS were added, and followed by adding 50 µL per well of a previously determined antibody dilution. After incubation for 1 h, 100 µL per well of diluted (1/1000) goat anti-rabbit IgG-HRP was added. The mixture was kept on incubating for 1 h and then 100 µL per well of OPD solution (10 mg of OPD and 10 µL of 30% of H2O2 added in 25 mL of phosphate-citrate buffer, pH 5.4) was added. The reaction was paused 15 min later by adding 50 µL of 2 M H2SO4 and absorbance was measured at 450 nm. The standard curve was listed as: y= aln(x) + b (obtained from Microsoft Office Excel 2007, using linear regression model, inhibition rate is set as ‘y’, and logarithm of analyte concentration is set as ‘x’).
Assay optimisation
The assay optimisation was first performed on cypermethrin, and its final optimal conditions were applied to other pyrethroids.
Methanol concentration
As pyrethroids are hydrophobic, it is necessary to use organic solvent to improve the analyte solubility in assay medium. Methanol was considered as the most suitable solvent in formal studies (Hao et al., Citation2009; Watanabe, Shan, Stoutamire, Gee, & Hammock, Citation2001) and adopted here. The effect of methanol concentrations were tested by dissolving cypermethrin standards in distilled water containing different amount of methanol (10%, 20%, 30%, 40%, 50%, V/V), and incubating with antibody in 10 mM PBS buffer (10 mM sodium phosphate, 137 mM NaCl, 2.7 mM KCl, pH 7.4) on the coated plate.
Ionic strength
The effect of buffer capacity of assay solution on ELISA performance was studied using different concentrations (10, 20, 50, 100 and 200 mmol/L, pH kept between 7.3 and 7.5) of PBS to dissolve antibody, and 30% (v/v) methanol-water was used to dissolve the cypermethrin standards.
pH
The effect of pH on immunoassay performance was studied by using buffers of various pH values (5.0, 5.8, 6.6, 7.4 and 8.5) to dissolve antibody and 30% methonal-buffer to dissolve cypermethrin standards. The buffer was 50 mM PBS, which was prepared by changing the amounts of Na2HPO4 and KH2PO4 to adjust pH values, whereas the concentration of NaCl and KCl remaining at 685 and 13.5 mM.
Cross-reactivity
The optimised assays were applied to CR study by using the standard solution of cypermethrin and other structurally related pyrethroids and metabolites. The CR was calculated using the following equation:
Here, IC50 values (analyte concentration that reduces 50% maximum absorbance of the competitive ELISA) were determined by indirect competitive immunoassay.
Recovery test in West Lake water
There are plenty of tea trees grown on the hills around West Lake (Hangzhou, China). Pyrethroids are the main pesticides applied on tea trees and transport of these pesticides in the lake will influence the aquatic ecosystem. Therefore, monitoring residue levels of pyrethroid in West Lake water is required. The lake water sample tested was verified by GC to be free of tested pyrethroids residues. Standard and sample solutions were run in triplicate wells. Prior to the ELISA determination, 7 mL of water sample (filtered with filter paper) was spiked with 3 mL of pyrethroid standard (prepared in methanol), and then dissolved twice with 30% methanol-PBS, and a 50 µL portion was used for the selected ELISA. The same water sample, with absence of pyrethroids, was set as blank control with identical pre-treatment. This step was to examine whether the sample background show great matrix effect. Recovery tests here were first performed on individual analyte of cypermethrin, deltamethin and esfenvalerate (spiked concentrations were 5, 20, 50 µg/L, respectively), each standard curve of the three was employed for the right analyte itself as well as the other two ones in calculation, and single factor test (analysed by Duncans new multiple range method) was done as to certify the consistency in sensitivity as well as in calculation among the three pyrethroids (seen in Tables ). Finally, this certified method was applied to multi-residue in the same water samples ().
Results and discussion
Hapten design
All the nine haptens were successfully synthesised. New catalysts such as DMAP and ruthenium (III) trichloride hydrate were used in synthesising hapten 2, and a convenient one-step synthesis was applied to hapten 8 by using pyrethroid technical. As a result, the reaction time and reagent was cut down and the yield was kept at similar level compared to former studies (Lee et al., Citation1998; Mak et al., Citation2005). The yields for each haptens are listed as: hapten 1 (76.7%), hapten 2 [39.3% (Yields for intermediate products NC-M-1, NC-M-2 and NC-M-3 are 96.9%, 69.7% and 58.2%, respectively.)], hapten 3 (68.8%), hapten 4 (62.1%), hapten 5 (67.9%), hapten 6 (71.5%), hapten 7 (68.8%), hapten 8 (57.9%) and hapten 9 (59.8%). The purpose of this study is to develop a broad-selective immunoassay for type II pyrethroids, more important, we expect to get antibodies with equal or similar sensitivity for each analyte. Heterology format did well in modifying sensitivity and selectivity especially in immunoassay of organophosphorus pesticide multi-residue analysis in this laboratory (Wang et al., Citation2010), which attribute to the stronger affinity of analyte to antibody in comparison with the CAg. In this study, we also focus on this strategy. Hapten 2, 8 and 9, all containing the α-cyano phenoxybenzyl group, were synthesised as immunising and coating haptens. The other six haptens without the cyano group were designed as coating haptens only. The optimum assay, which is of as lower and closer sensitivity as possible for each analyte, was obtained by changing the antibody–antigen combinations, that is, different immunising haptens versus different coating haptens. Those nine coating haptens can be simply classified into two groups according to their structure: one group preserves most of the pyrethroid molecular structure (i.e., hapten 2, 3, 4 and 8) while the other group preserves partial structure only (i.e., hapten 1, 5, 6, 7 and 9). In our opinion, the selectivity and selectivity modulation could be done as for the two reasons below: on the one hand, the two groups of coating haptens provide a broad selection spectrum as their structure are quite different on the whole, various degrees of heterology could be investigated as to see which combination is best. On the other hand, for each group itself, structural transformation is minor from one to another, the degree of heterology can be fine-tuning, and avoid drastic change in sensitivity for certain compound.
Screening antibody–antigen combinations of high affinity
Three PAbs and nine CAgs made up 27 combinations. Here both the homologous and heterologous assays were examined. Our first objective was to screen which CAg could be well recognised by the three antibodies. As shown on , background values of the reaction can nearly be ignored (control values are quite low). Both the homologous combinations (i.e., CAg-2/Pab-2, CAg-8/Pab-8 and CAg-9/Pab-9) exhibit great affinity, indicating that the immunisation process is successful. High affinity also can be observed in heterologous assays, especially when the two opposite haptens both contain the α-cyano groups, suggesting it is a strong haptenic determinant. Noticeably, heterologous combinations containing Pab-2 usually show good affinity (i.e., Pab-2/CAg 5, 7, 8 and 9), which implies that immunising hapten preserves major molecular structure of pyrethroid might be the best candidate for obtaining broad selective antibody. In addition, from this phenomenon we can conclude that Pab-2 is more tolerant to CAg heterology in getting good affinity, because Pab-8 and Pab-9 do not show so perfect recognition to CAgs without the cyano group. CAg-3, CAg-4 and CAg-6 show poor affinity to all of the PAbs, which indicates that too much heterology is not helpful. Finally, combinations of high affinity (absorbance values > 0.80) were selected to develop immunoassays for type II pyrethroids.
Screening sensitive antibody–antigen combination for the type II pyrethroids
Competitive inhibition experiments were performed to select which antibody and CAg combinations showed high sensitivity to type II pyrethroids. On the whole, sensitivity for cypermethrin and esfenvalerate is better than tetramethrin and permethrin in most of the combinations (). In addition, the only difference in structure between cypermethrin and permethrin is whether the cyano radical -CN exists, but the former one shows much stronger inhibition. These results indicate -CN is particularly a determinant group. For type II pyrethroids, it is noteworthy that heterologous assays obtained more perfect results while homologous assays all failed in getting good recognition. We also notice that systems with hapten homology or similarity (i.e., CAg-2/Pab-2, CAg-8/Pab-8 and CAg-9/Pab-2) cannot recognise any of the analytes well. This phenomenon can be concluded as: first of all, for pyrethroids immunoassay, haptens homology or highly similarity allow antibody to exhibit strong affinity for CAg, but once the recognition sites were largely occupied by the competitive antigens, there is only poor or no recognition for target compounds. Secondly, introducing a certain degree of heterology in the molecular structure of the competitor may result in advance of assay sensitivity even selectivity (Galve, Sanchez-Baeza, Camps, & Marco, Citation2002). Pab-2 displays broad applicability as the three combinations respectively with CAg-2, CAg-7 and CAg-8 all recognise the two type II pyrethroids. Finally, the combination of CAg-7/PAb-2 exhibiting lowest and most similar IC50s for cypermethrin and esfenvalerate (56.4 and 60.2 µg/L, respectively, much lower than other systems) was selected for further assay optimisation and residual analysis.
Table 3. Selected competitive ELISA screening data against type I and type II pyrethroids.
Assay optimisation results
Factors like cosolvent, ionic strength and pH value can directly affect the assay sensitivity by modifying the solubility of the analyte or by changing the interaction between the antibody and the competitive conjugate (Gui, Liu, Wang, Liang, & Zhu, Citation2009). Maximal absorbance (Amax), IC50 and Amax/IC50 are usually used as criteria to evaluate immunoassay performances. Amax/IC50 is a convenient estimate of the influence of some factors on the ELISA sensitivity, the higher ratio indicating higher sensitivity (Abad & Montoya, Citation1997), so this parameter was used as primary criteria to evaluate the optimal effect.
Effect of methanol concentration
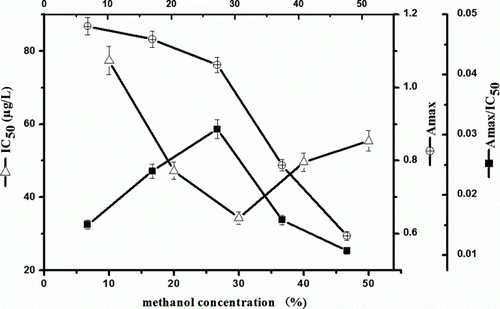
Some water-compatible organic solvents are often used to improve the solubility of hydrophobic analyte. In this study, the most appropriate solvent methanol, as mentioned in former reports, was selected for the cypermethrin ELISA. As shown on , the maximal absorbance (Amax) slowly decreases between 10 and 30% of methanol content, but it declines sharply when the concentrations range from 40 to 50%. Accordingly, IC50 values first decrease and go up later. The explanations maybe list in two points: on the one hand, with methanol concentration increases in a proper extent, solubility of analyte will be improved and easier to be recognised by antibody. On the other hand, sensitivity will retrogress under abundant cosolvent, because excessive organic solvent weaken the antigen–antibody interaction. Considering the maximum Amax/IC50 ratio appears at 30% methanol, and the lowest IC50 is found at 30% methanol (28.5 µg/L), which is approximately two times lower than that at 10% methanol (58.3 µg/L) and 50% methanol (52.3 µg/L), we select 30% methanol in water as final solution.
Effect of ionic strength
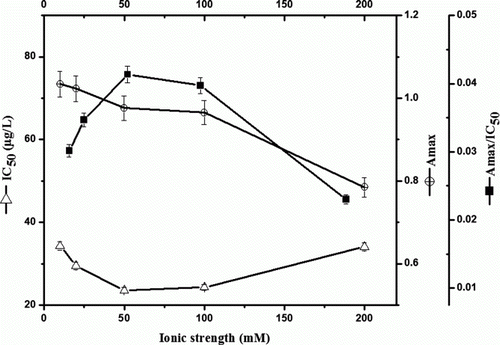
Regarding the effect of the ionic strength, immunoassay performance was investigated in medium at a concentration of PBS ranging from 10 to 200 mM. It is observed that the Amax of the assay is approximately 1.00 when the PBS concentration is 10 mM (). Then the value diminishes slightly when the concentrations beyond 100 mM. Sharp decline occurs at 200 mM, which indicates high ionic concentration also will affect the affinity of antibody to antigen. On the whole, the PBS concentration had an insignificant effect on the sensitivity of the assay, as the IC50 values transfer not so obviously (from 24.6 to 34.3 µg/L). We notice that Amax/IC50 ratios together with the other two parameter values (Amax and IC50) are very close at 50 and 100 mM here. Considering that the real samples might have high ionic strength values, PBS of 100 mM was adopted.
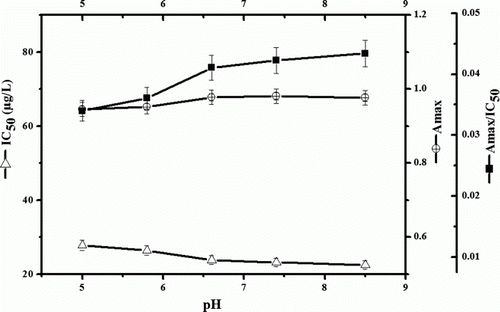
Effect of pH
It was observed that Amax values are increased until pH 7.4, then nearly stay constant (), and Amax/IC50 values exhibit similar tendency. On the basis of analyte stability [pyrethroids probably isomerise under alkaline environment (Lee et al., Citation1998)], pH 5.8 was selected as the best condition.
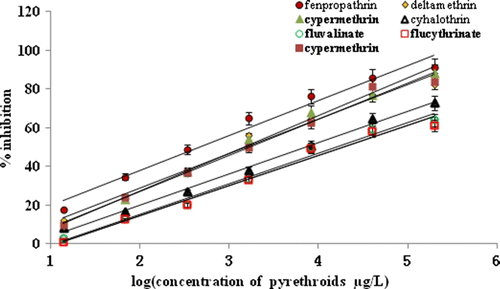
To sum up, the optimal conditions for cypermethrin were listed as: 30% of methanol, 100 mM PBS and pH 5.8. The parameters were applied to other pyrethroids like fenpropathrin, cypermethrin, deltamethrin, esfenvalerate, cyhalothrin, fluvalinate and flucythrinate, those compounds show nearly the same variation regularity (data does not exhibit here), indicating these optimal conditions could apply to all of the targets. exhibits the standard curves of the seven tested pyrethroids after being optimised. We notice that the curves of cypermethrin, deltamethrin, and esfenvalerate show similar slopes, exhibiting quite similar tendency of inhibition. Although the curves of fenpropathrin, cyhalothrin, fluvalinate and flucythrinate deviate from each other, they keep parallel with those three ‘coincident’ ones. These results suggest that screen by heterologous assay can modify the sensitivity to be as consistent as possible, especially for those of high similarity in molecular structure. This result is very helpful for multi-residue analysis.
Cross-reactivity determination
To evaluate the selectivity and specificity of the optimal ELISA system CAg-7/Pab-2, 10 pyrethroids were brought into the CR experiment (). This system shows high CRs with the type II pyrethroids such as cypermethrin (100.0%), deltamethrin (105.6%), esfenvalerate (108.2%), fenpropathrin (62.5%), fluvalinate (284.9%), flucythrinate (312.1%) and cyhalothrin (201.7%). Cypermethrin, deltamethrin and esfenvalerate show not only similar IC50 values and CRs, but also similar equation parameters (slope and intercept), exhibiting proximal dynamic characteristic. This result probably attributes to the similar molecular space dimension and binding site of the three. Quite poor IC50 and high CRs are measured for the non-cyano type I pyrethroids together with the metabolites, indicating that this combination is applicable to type II pyrethroids.
Table 4. Cross-reactivities (CRs) of pyrethroids and relative metabolites.
Sample analysis
To make the pre-treatment process more convenient and rapid, the spiked samples were applied to indirect competitive ELISA without any sample clean-up procedures. Control samples without pyrethroids were also systematically included in the analysis process. Some authors had suggested that dilution of samples was an effective means to reduce matrix interference, especially at the lower concentrations that are to the left of the IC50 value (Kim, Kim, Lee, & Lee, Citation2007). However, dilution of samples causes reduction of assay sensitivity due to the shift of the dynamic range. Therefore, concerning the sensitivity and the dynamic range of the immunoassay, water samples were diluted to 1:5 (v/v).
Single pyrethroid analysis results
For the ultimate purpose of multi-residue analysis, trial on single pyrethroid should be workable first. Cypermethrin, deltamethrin and esfenvalerate, those three pyrethroids with similar CRs, were spiked in West Lake water samples respectively. Standard curve of each pyrethroids was used to calculate the recoveries of those three analytes at three spiked concentrations (5, 20 and 50 µg/L), that is, each standard curve was employed for the analyte itself, as well as for the other two in calculation. The purpose is to check whether identical sensitivity will lead to identical results in a recovery test. As can be seen from Tables , recovery values are between 83.6 and 105.5%, and when the standard curve of cypermethrin, deltamethrin and esfenvalerate is selected for calculating the same compound at the same spiked concentration respectively, the results among them shows no significant difference. This result indicates that consistency of sensitivity and calculation for the three pyrethroids can avoid wide fluctuations (too low or two high) in recovery, which is quite feasible for multi-residue analysis as multiple analytes can be regarded as a single analyte.
Table 5. Recoveries of single residue of cypermethrin, deltamethrin and esfenvalerate in West Lake water samples calculated by the standard curve of cypermethrin.a
Table 6. Recoveries of single residue of cypermethrin, deltamethrin and esfenvalerate in West Lake water samples calculated by the standard curve of deltamethrin.a
Table 7. Recoveries of single residue of cypermethrin, deltamethrin and esfenvalerate in West Lake water samples calculated by the standard curve of esfenvalerate.a
Multiple pyrethroids analysis results
In previous reports, methods to illuminate multi-residue were usually based on one selected standard curve, and recovery tests for each pesticide were calculated using this fixed curve, but the situations of mixed pyrethroids were seldom discussed, as the IC50s was diverse and the recoveries (too high or too low) did not meet the criteria of analytical methods. These three type II pyrethroids, with proximal IC50 or CRs can avoid this problem. There are four possible multi-residue combinations as shown on . Here, standard curves of cypermethrin, deltamethrin and esfenvalerate are applied in multi-residue analysis for West Lake samples. The recoveries range from 95.6 to 103.6%. So, we can conclude that no matter which standard curve is used for calculation, and no matter what the compositions of the mixtures are, similar and suitable results are available. In a word, this immunoassay can be used for semi-quantitative multi-residue analysis of cypermethrin, deltamethrin and esfenvalerate.
Table 8. Recoveries of multiple analytes in West Lake samples.a
Acknowledgements
This study was financially supported by the National High Technology Research and Development Program (863) of China (2008) AA10Z422, the Programme of Science and Technology Innovation Team of Zhejiang province (2010) R50028 and the Fundamental Research Fund for the Central Universities.
References
- Abad , A. and Montoya , A. 1997 . Development of an enzyme-linked immunosorbent assay to carbaryl. 2. assay optimization and application to the analysis of water samples . Journal of Agricultural and Food Chemistry , 45 : 1495 – 1501 .
- Ahn , K.C. , Watanabe , T. , Gee , S.J. and Hammock , B.D. 2004 . Hapten and antibody production for a sensitive immunoassay determining a human urinary metabolite of the pyrethroid insecticide permethrin . Journal of Agricultural and Food Chemistry , 52 : 4583 – 4594 .
- Barbini , D.A. , Vanni , F. , Girolimetti , S. and Dommarco , R. 2007 . Development of an analytical method for the determination of the residues of four pyrethroids in meat by GC-ECD and confirmation by GC-MS . Analytical and Bioanalytical Chemistry , 389 : 1791 – 1798 .
- Casida , J.E. and Quistad , G.B. 1998 . Golden age of insecticide research: Past, present, or future? . Annual Review of Entomology , 43 : 1 – 16 .
- Galve , R. , Sanchez-Baeza , F. , Camps , F. and Marco , M.P. 2002 . Indirect competitive immunoassay for trichlorophenol determination: Rational evaluation of the competitor heterology effect . Analytica Chimica Acta , 452 : 191 – 206 .
- Gendloff , E.H. , Casale , W.L. and Ram , B.P. 1986 . Hapten-protein conjugates prepared by the mixed anhydride method. Cross-reactive antibodies in heterologous antisera . Journal of Immunological Methods , 92 : 15 – 20 .
- Go , V. , Garey , J. , Wolff , M.S. and Pogo , B.G.T. 1999 . Estrogenic potential of certain pyrethroid compounds in the MCF-7 human breast carcinoma cell line . Environmental Health Perspectives , 107 : 173 – 177 .
- Gui , W.J. , Liu , Y.H. , Wang , C.M. , Liang , X. and Zhu , G.N. 2009 . Development of a direct competitive enzyme-linked immunosorbent assay for parathion residue in food samples . Analytical Biochemistry , 393 : 88 – 94 .
- Hao , X.L. , Kuang , H. , Li , Y.L. , Yuan , Y. , Peng , C.F. Chen , W. 2009 . Development of an enzyme-linked immunosorbent assay for the a-cyano pyrethroids multiresidue in Tai lake water . Journal of Agricultural and Food Chemistry , 57 : 3033 – 3039 .
- Kim , Y.J. , Kim , Y.A. , Lee , Y.T. and Lee , H.S. 2007 . Enzyme-linked immunosorbent assays for the insecticide fenitrothion. Influence of hapten conformation and sample matrix on assay performance . Analytica Chimica Acta , 591 : 183 – 190 .
- Lee , H.J. , Shan , G. , Watanabe , T. , Stoutamire , D.W. , Gee , S.J. and Hammock , B.D. 2002 . Enzyme-linked immunosorbent assay for the pyrethroid deltamethrin . Journal of Agricultural and Food Chemistry , 50 : 5526 – 5532 .
- Lee , N. , Beasley , H.L. and Skerritt , J.H. 1998 . Development of immunoassays for type II synthetic pyrethroids. 2. Assay specificity and application to water, soil, and grain . Journal of Agricultural and Food Chemistry , 46 : 535 – 546 .
- Liu , Y.H. , Jin , M.J. , Gui , W.J. , Cheng , J.L. , Guo , Y.R. and Zhu , G.N. 2007 . Hapten design and indirect competitive immunoassay for parathion determination: Correlation with molecular modeling and principal component analysis . Analytica Chimica Acta , 591 : 173 – 182 .
- Mak , S.K. , Shan , G. , Lee , H.J. , Watanabe , T. , Stoutamire , D.W. Gee , S.J. 2005 . Development of a class selective immunoassay for the type II pyrethroid insecticides . Analytica Chimica Acta , 534 : 109 – 120 .
- Mekebri , A. , Crane , D.B. , Blondina , G.J. , Oros , D.R. and Rocca , J.L. 2008 . Extraction and analysis methods for the determination of pyrethroid insecticides in surface water, sediments and biological tissues at environmentally relevant concentrations . Bulletin of Environmental Contamination and Toxicology , 80 : 455 – 460 .
- Perry , M.J. , Venners , S.A. , Barr , D.B. and Xu , X. 2007 . Environmental pyrethroid and organophosphorus insecticide exposures and sperm concentration . Reproductive Toxicology , 23 : 113 – 118 .
- Schimmel , S.C. , Garnas , R.L. , Patrick , J.M. Jr and Moore , J.C. 1983 . Acute toxicity, bioconcentration, and persistence of AC 222, 705, benthiocarb, chlorpyrifos, fenvalerate, methyl parathion, and permethrin in the estuarine environment . Journal of Agricultural and Food Chemistry , 31 : 104 – 113 .
- Shan , G. , Leeman , W.R. , Stoutamire , D.W. , Gee , S.J. , Chang , D.P.Y. and Hammock , B.D. 2000 . Enzyme-linked immunosorbent assay for the pyrethroid permethrin . Journal of Agricultural and Food Chemistry , 48 : 4032 – 4040 .
- Shan , G. , Stoutamire , D.W. , Wengatz , I. , Gee , S.J. and Hammock , B.D. 1999 . Development of an immunoassay for the pyrethroid insecticide esfenvalerate . Journal of Agricultural and Food Chemistry , 47 : 2145 – 2155 .
- Wang , C. , Li , X. , Liu , Y. , Guo , Y. , Xie , R. Gui , W. 2010 . Development of a mab-based heterologous immunoassay for the broad-selective determination of organophosphorus pesticides . Journal of Agricultural and Food Chemistry , 58 : 5658 – 5663 .
- Wang , J. , Yu , G. , Sheng , W. , Shi , M. , Guo , B. and Wang , S. 2011 . Development of an enzyme-linked immunosorbent assay based a monoclonal antibody for the detection of pyrethroids with phenoxybenzene multiresidue in river water . Journal of Agricultural and Food Chemistry , 59 : 2997 – 3003 .
- Watanabe , E. , Miyake , S. , Ito , S. , Baba , K. , Eun , H. Ishizaka , M. 2006 . Reliable enzyme immunoassay detection for chlorothalonil: Fundamental evaluation for residue analysis and validation with gas chromatography . Journal of Chromatography A , 1129 : 273 – 282 .
- Watanabe , T. , Shan , G. , Stoutamire , D.W. , Gee , S.J. and Hammock , B.D. 2001 . Development of a class-specific immunoassay for the type I pyrethroid insecticides . Analytica Chimica Acta , 444 : 119 – 129 .
- Wengatz , I. , Stoutamire , D.W. , Gee , S.J. and Hammock , B.D. 1998 . Development of an enzyme-linked immunosorbent assay for the detection of the pyrethroid insecticide fenpropathrin . Journal of Agricultural and Food Chemistry , 46 : 2211 – 2221 .
- You , J. and Lydy , M.J. 2007 . A solution for isomerization of pyrethroid insecticides in gas chromatography . Journal of Chromatography A , 1166 : 181 – 190 .
- Zawiyah , S. , Che Man , Y.B. , Nazimah , S.A.H. , Chin , C.K. , Tsukamoto , I. Hamanyza , A.H. 2007 . Determination of organochlorine and pyrethroid pesticides in fruit and vegetables using SAX/PSA clean-up column . Food Chemistry , 102 : 98 – 103 .
- Zhang , L.Z. , Khan , S.U. , Akhtar , M.H. and Ivarson , K.C. 1984 . Persistence, degradation, and distribution of deltamethrin in an organic soil under laboratory conditions . Journal of Agricultural and Food Chemistry , 32 : 1207 – 1211 .
- Zhang , Q. , Zhang , W. , Wang , X. and Li , P. 2010 . Immunoassay development for the class-specific assay for types I and II pyrethroid insecticides in water samples . Molecules , 15 : 164 – 177 .