Abstract
In this study, tylosin (TYL) was derivatized with 4-aminophenylacetic acid to synthesise a new hapten and the hapten was used to produce the monoclonal antibody. The obtained antibody simultaneously recognised TYL, tilmicosin, acetylisovaleryltylosin and the metabolite of TYL (desmycosin) with cross-reactivities of 100%, 62%, 97% and 93%, respectively. After evaluation of two coating antigens, a heterologous competitive indirect enzyme linked immunosorbent assay was developed to determine the four analytes in milk simultaneously. The limits of detection for the four analytes were in the range of 1.5–3.1 ng mL−1. The recoveries from fortified milk were in the range of 76.3–97.4% with coefficients of variation of 5.3–15.7%.
1. Introduction
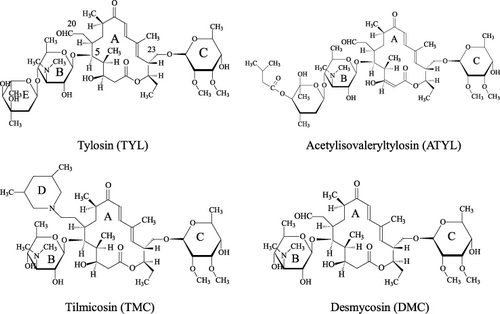
Macrolide antibiotics (MACs) are a class of drugs highly active against Gram-positive bacterial and Mycroplasma species. Therefore, MACs are widely used to treat various diseases in swine, cattle, sheep and poultry (Christodoulopoulos, Warnick, Papaioannou, & Fthenakis, Citation2002; Fajt et al., Citation2003; Laven and Andrews, Citation1991; Shryock, Staples, & DeRosa, Citation2002; Zhang et al., Citation2004). The commonly used MACs include tylosin (TYL), tilmicosin (TMC) and acetylisovaleryltylosin (ATYL; ). TYL is a traditional MAC that is generated by Streptomyces fradiae. ATYL and TMC are both semi-synthetic drugs derived from TYL. TYL is unstable and it can be degraded in sugar syrup (Kochansky, Knox, & Shimanuki, Citation1999), honey (Kochansky, Citation2004) and acidic media (Paesen, Cypers, Pauwels, Roets, & Hoogmartens, Citation1995) to yield its predominant metabolite, desmycosin (DMC), also referred to as tylosin B ().
The wide use of MACs in farm animals may produce residues in food of animal origin and induce the resistance of bacterial strains to antimicrobials in human use. For protection of consumer health, it is very important to monitor the residues of these MACs in foods of animal origin. In the last decade, high-performance liquid chromatography (Prats, Francesch, Arboix, & Perez, Citation2001) and liquid chromatography–mass spectrometry (Benetti, Dainese, Biancotto, Piro, & Mutinelli, Citation2004; Bogialli, Ciampanella, Curini, Di Corcia, & Laganà, Citation2009; Cherlet, De Baere, Croubels, & De Backer, Citation2002; Draisci, Palleschi, Ferretti, Achene, & Cecilia, Citation2001; Heller and Nochetto, Citation2004; Lucchetti et al., Citation2005; Martos, Lehotay, & Shurmer, Citation2008; Msagati and Nindi, Citation2004; Wang & Leung, Citation2007; Wang, Leung, & Butterworth, Citation2005) were used to determine the residues of MACs in various animal original foods. For detection of TYL residues, most reported methods were focused on the parent compound, TYL. In consideration of the structural instability of TYL, animal derived foods for human consumption should be analysed for both TYL and DMC rather than parent TYL alone. However, there has been only one article reporting the simultaneous determination of TYL and DMC, and that study was in honey (Thompson, Pernal, Noot, Melathopoulos, & van den Heever, Citation2007).
These analytical methods are time-consuming, and sophisticated and expensive instruments are required. In comparison, enzyme linked immunosorbent assay (ELISA) is a low cost and sensitive method capable of screening large numbers of samples in a single test. The core reagent of an ELISA method is antibody. By now, there have been several articles reporting the production of different antibodies to these MACs. Yao and Mahoney (Citation1989) produced a polyclonal antibody against 23-deoxy-23-amino-O-mycaminosyl-tylonolide. The antibody showed cross-reactivity (CR) to the 12-, 14- and 16-membered macrolides that contain amino sugar moieties. Wicker et al. (Citation1994) prepared a polyclonal antibody against TYL that cross-reacted with TMC. Jackman, Spencer, Silverlight, Marsh, and Bellerby (Citation1997) produced a polyclonal antibody against DMC and Silverlight, Brown, and Jackman (Citation1999) showed the anti-DMC antibody exhibited high CR (96%) with TYL. Creemer, Beier, and Kiehl (Citation2003) synthesised a hapten of TYL and a hapten of TMC. Beier, Creemer, Ziprin, and Nisbet (Citation2005) used the hapten of TMC to produce a monoclonal antibody that was specific to TMC and showed no CR with TYL and other MACs that do not contain the structure of 3,5-dimethylpiperidine (the D ring in TMC molecule, ). The authors did not determine the antibody reactivity to ATYL and DMC. Peng et al. (Citation2012) produced a monoclonal antibody against TYL that showed CR to TMC.
Recently, we have produced a monoclonal antibody against TYL that showed the following cross-reactivities: TYL (100%), ATYL (91%), DMC (76%) and TMC (49%) (Zhang, Liu, Wang, Chai, & Wang, Citation2012). Therefore, TYL can be regarded as a generic hapten of the four analytes to generate a broad-specific antibody. In this study, TYL was used to synthesise a new hapten with the aims to enhance the antibody CR and to develop a multi-determination immunoassay for the four analytes.
2. Materials and methods
2.1. Reagents and chemicals
Tylosin tartrate, ATYL, TMC and DMC, bovine serum albumin (BSA), Ovalbumin (OA), and Freund's adjuvant were all from Sigma-Aldrich (St. Louis, MO, USA). 3, 3′, 5, 5′-tetramethylbenzidine (TMB) was purchased from Serva (Heidelberg, Germany). Other chemical reagents were all analytical grade from Beijing Chemical Company (Beijing, China). The stock solutions of the four analytes were prepared by dissolving each compound in methanol to obtain a concentration of 100 µg mL−1. These solutions were stored at −20 °C in amber glass bottles. Working solutions of the four drugs with series concentrations were prepared by dilution of the stock solutions with PBS (1, 2, 5, 10, 20, 50, 100 and 200 ng mL−1). PBS (pH 7.2) was prepared by dissolving 0.2 g KH2PO4, 0.2 g KCl, 1.15 g Na2HPO4, and 8.0 g NaCl in 1000 mL water. Coating buffer was prepared with sodium carbonate and sodium hydrocarbonate (0.1 M, pH 9.6). Washing buffer was PBS containing 0.05% Tween (PBST). Substrate buffer was prepared with sodium hydrogen phosphate and citrate (0.1 M, pH 5.5). The substrate system was prepared by adding 200 µL 1% (w/v) TMB in DMSO (dimethyl sulfoxide) and 64 µL 0.75% (w/v) H2O2 to 20 mL substrate buffer.
2.2. Synthesis of the hapten
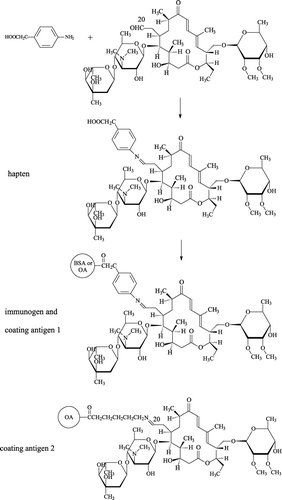
The hapten synthesis route is shown in . About 470 mg tylosin tartrate (0.5 mmol) and 75 mg 4-aminophenylacetic acid (0.5 mmol) were added to a mixture of 20 mL of water and 0.5 mL of acetic acid. The mixture was refluxed under heating until the solution turned yellow. Then the solvent was dried in drying box at 40 °C and the dry residue was dissolved in 10 mL of ethanol. After 10 mL of ethyl acetate was added into the ethanol solution, some lemon yellow sediment appeared. The mixture was filtered under vacuum and the residue was washed with 50 mL of water, and subsequently dried to yield the hapten PTYL (melt point of 220 °C; IR (KBr) Vmax 3426, 3100, 2971, 2933, 1704, 1594, 1375, 1155, 1266, 713, 680 cm−1).
2.3. Preparation of the conjugates
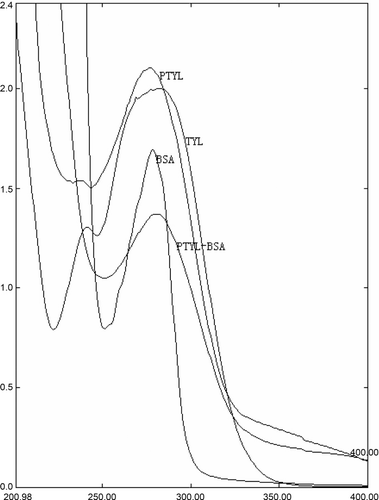
The conjugates were prepared as shown in . About 4 mL of N,N-dimethylformamide dissolving 53 mg hapten and 25 µL triethylamine were added into a glass jar. Then 20 µL of isobutyl chloroformate was added and the mixture was stirred for 60 min at 4 °C. Then, the mixture was added dropwise into 2 mL of sodium bicarbonate solution containing 74 mg BSA or 30 mg OA and stirred for 12 h at 4 °C. The resulting immunogen (PTYL-BSA) and coating antigen (PTYL-OA) were dialysed against three changes of PBS for 3 days and stored at −20 °C until used. TYL, PTYL, BSA and the conjugates were all scanned on a UV-Vis spectrophotometer to verify the conjugation. The hapten/protein coupling ratios were determined according to the previous 2,4,6-trinitrobenzene sulphonic acid method (Sashidhar, Capoor, & Ramana, Citation1994).
2.4. Production of the monoclonal antibody
Six 8-week-old female BALB/c mice were immunised subcutaneously with an emulsion of the immunogen (50 µg protein per mouse) in Freund's complete adjuvant. Beginning two weeks later, mice were boosted at 2-week intervals. The serum of each mouse was collected and the antibody titre was monitored. The spleen from the mouse with the highest titre after six boosters was removed and the splenocytes were fused with SP2/O myeloma cells and cultured in 96-well plates. Following a first screening by indirect ELISA, the positive hybridomas were rescreened using the competitive indirect ELISA described later with TYL as the competitor. Hybridomas producing the specific monoclonal antibody to TYL were sub-cloned twice by the limiting dilution method and the sub-cloned hybridoma cells were collected, centrifuged and frozen in liquid nitrogen. The ascites from hybridoma-induced mice were purified using saturated ammonium sulphate precipitation and used for development of the competitive indirect ELISA.
2.5. Competitive indirect ELISA
In this study, coating antigen PTYL-OA (coating antigen 1, ) and a coating antigen previously prepared in our laboratory (coating antigen 2, ) (Zhang et al., Citation2012) were used to develop the homologous and heterologous ELISA. The optimal dilutions of coating antigen and antibody were determined by using the checkerboard procedure, in which the well with an absorbance of 1.0 was defined as the optimal dilutions of the coating antigen and the antibody. After that, each well of a microtitre plate was coated with 100 µL of coating antigen, incubated overnight at 4 °C, and then blocked with 1% foetal calf serum. The plate was washed three times with PBST, and then, 50 µL of the optimal antibody dilution and 50 µL of TYL standard with series concentrations were added to the wells (in triplicate) for incubation for 1 h at 37 °C. The plate was washed as aforementioned. About 100 µL of horseradish peroxidase labelled goat anti-mouse IgG was added before incubation for 30 min at 37 °C. After washes, 100 µL of TMB substrate system was added prior to 15 min incubation at 37 °C. Finally, the reaction was stopped by the addition of 50 µL of 2 M H2SO4, and the plate was read on an ELISA plate reader at 450 nm to obtain the absorbance (B) of each well.
The other three analytes shown in and several other drugs (erythromycin, spiramycin and avermectin) were all determined by the ELISA. The limits of detection (LOD) for these analytes were defined as the concentrations showing 10% of inhibition, respectively. The competitive inhibition curves were developed by plotting the B/B0 values (mean absorbance of the standards divided by the mean absorbance of zero standards) verse the concentrations (Log C). The CR among these competitors was calculated from the half-maximal inhibition concentration (IC50) as follows: CR (%)=100×IC50 TYL/IC50 competitor.
2.6. Sample preparation
The extraction of MACs from milk sample was modified from the previous reports (Bogialli et al., Citation2009; Wang & Leung, Citation2007). A milk sample (5 mL) and 30 mL of acetonitrile were added into a 50 mL polypropylene centrifuge tube and the tube was shaken vigorously on a variable speed reciprocal shaker for 10 min. The mixture was centrifuged at 10,000 rpm for 10 min and the acetonitrile phase was collected and evaporated to dryness. The dry residue was dissolved in 5 mL of PBS and filtered through a 0.45 µm Millipore filter for ELISA analysis.
Blank milk samples were obtained from several controlled cows. In order to evaluate the matrix interference, matrix-matched standards prepared with the extracts of blank milk sample were used to develop the matrix-matched competitive curves. The accuracy was evaluated by determination of the recoveries from the four analytes fortified blank milk at concentrations of 20, 50, 100 ng mL−1. A total of 35 unknown milk samples from China (20 commercial packaged milk samples from several supermarkets and 15 raw milk samples from several dairy farms) were analysed by the developed ELISA method.
3. Results and discussions
3.1. Hapten and immunogen
As shown in , the three MAC drugs and DMC all contain a 16-atom macrocyclic lactone ring (A ring), a 5-O-mycaminosyl ring (B ring), and a neutral sugar (C ring). The antibody specific to the three rings should recognise the four analytes simultaneously. In a previous report, B ring and D ring (3,5-dimethylpiperidin at C20 position in the molecule of TMC) in the hapten of TMC were presented to the immune system and the resulting antibody only recognised TMC (Beier et al., Citation2005). This result is because among the four analytes only TMC contains the D ring (). In other reports, A ring and C ring in the molecule of DMC were presented to the immune system, resulting in an antibody that simultaneously recognised DMC, TYL and TMC (Jackman et al., Citation1997; Silverlight et al., Citation1999), analytes that contain the two rings. In our recent study, TYL was derivatized with 6-aminohexanoic acid linker at the C20 position to synthesise the hapten. The immunogen had A ring and C ring spaced far from the carrier (similar to the illustration of coating antigen 2, ) and resulted in an antibody that simultaneously recognised TYL, ATYL, DMC, and TMC (Zhang et al., Citation2012).
In the present study, a new hapten of TYL was synthesised by derivatization of TYL with 4-aminophenylacetic acid utilising the C20 aldehyde group in TYL molecule (). This was equivalent to introduction of a phenylacetic acid at C20 position and the free carboxyl group was used to couple with the carrier. This hapten contained the common structures of the four analytes (A ring, B ring and C ring) to elicit antibodies that recognise all four analytes.
The successful synthesis of the hapten PTYL was proven by following data. First, the melting point of the hapten (220°C) was higher than that of TYL (200 °C), indicating a new compound was obtained. Second, the infrared (IR) data showed the main chemical groups of parent TYL were still present and a carboxyl group, a benzene ring and a C=N bond were obtained. Third, the UV absorbance spectra of PTYL and TYL were similar (), indicating the general structure of TYL was untouched. Fourth, the UV absorbance spectra of the immunogen contained the characteristic peaks of PTYL and BSA (), indicating PTYL was coupled to BSA. The hapten density was 14 mol/mol in the immunogen and 11 in the coating antigen.
3.2. Antibody performance
Six hybridomas producing specific monoclonal antibody to TYL were obtained and the antibodies were named TY01, TY02, TY07, TY13, TY25 and TY56. Their IC50 and CRs to the four analytes are shown in (coating antigen 1). Antibody TY01 and TY13 showed high specificity for TYL and showed low CRs to ATYL (6.4% and 8.1%, respectively) and negligible CRs to TMC and DMC (< 2%). This pattern was possibly because the two antibodies mainly bind the E ring in the molecule of TYL (). The four other antibodies simultaneously recognised the four analytes. Antibody TY56 showed the best performance with IC50 in the range of 14.2–22.9 ng mL−1 and CRs in the range of 62–100% (). Based on these results, we speculated that the four antibodies mainly bind A ring and C ring, because they showed negligible cross-reactivity (CRs<1%) to the MACs that do not contain the two rings (erythromycin, spiramycin and avermectin, data not shown). The CRs of these antibodies to the four analytes were better than those of the previously reported antibodies (Beier et al., Citation2005; Jackman et al., Citation1997; Peng et al., Citation2012; Silverlight et al., Citation1999; Wicker et al., Citation1994; Yao and Mahoney, Citation1989).
Table 1. Performances of the six antibodies for the four analytes with different coating antigens.
The CRs of the four antibodies to ATYL and DMC (48–97%) were similar to our previous anti-TYL antibody (37–94%), but the CRs to TMC (29–62%) were higher than that antibody (16–49%) (Zhang et al., Citation2012). This was because of the different molecular structure of the two haptens. In the molecule of the previously described TYL hapten, there is a simple straight chain at C20 position and the structure at this position is different from that in TMC molecule ( and ), so the CRs were low. In the new hapten, PTYL, there is a benzene ring at C20 position and the general structure near this position is similar to that in the TMC molecule. The general structure of TMC could be regarded as a part of PTYL, so the CRs to TMC were high. Therefore, the new hapten of TYL improved the antibody selectivity, resulting in the antibody that could be used for multi-analyte immunoassay of the four analytes.
3.3. ELISA method
Nowadays, heterology in the coating antigen has been commonly used to improve the sensitivity and specificity of an immunoassay (Franek, Diblikova, Cernoch, Vass, & Hruska, Citation2006). Therefore, the four antibodies (TY02, TY07, TY25 and TY56), PTYL-OA (coating antigen 1) and the previously prepared coating antigen TYL-OA (coating antigen 2, ) were arranged into homologous and heterologous format to optimise the reagent combinations.
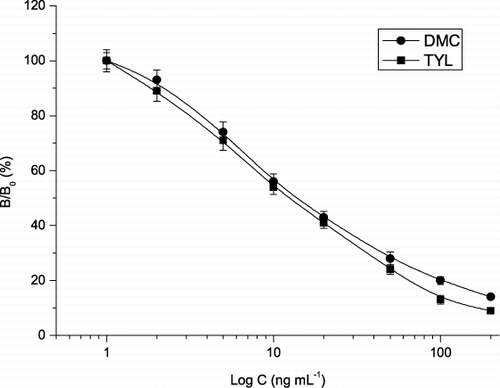
The performances of these antibodies in homologous format were described earlier. As shown in , the specificities and sensitivities of the four antibodies in heterologous format (using coating antigen 2) were generally better than that in homologous format (using coating antigen 1). For example, the CRs of antibody TY56 to the four analytes were in the range of 67–106% and IC50 were in the range of 11.9–18.8 ng mL−1. In addition, the CRs differences among these competitors in heterologous format were lower than that in homologous format. In heterologous format, the coating antigen 2 (containing a long straight chain) eliminated the influence of the antibody from the spacer arm in PTYL-BSA to show low competitive binding to the antibodies; thus increases the antibody binding for the competitors to achieve broad specificity and high sensitivity. Among these reagent combinations, antibody TY56 and coating antigen 2 produced the highest sensitivity, with LODs in the range of 1.5–3.1 ng mL−1 (). Therefore, this combination was used for the subsequent experiments. The competitive inhibition standard curves for the four analytes in the range of 1–200 ng mL−1 are shown in .
3.4. Sample extraction
An important step in the evaluation of an analytical procedure is to assess the matrix effect, which should be minimised by the appropriate sample preparation. In the present study, ATYL and TMC standards prepared with the extracts of blank milk were used to develop the matrix matched competitive curves. As shown in , the competitive inhibitory curves of the matrix matched ATYL and TMC were similar to that of their standards, indicating the extraction method was satisfactory. Then, recovery test was carried out by fortification of each analyte in blank milk (20–100 ng mL−1) for ELISA analysis. Intra-assay recoveries (six duplicates in a single day) ranged from 76.4% to 97.4% with coefficients of variation (CV) of 5.3–8.9%. Inter-assay recoveries (repeat once a day on six successive days) ranged from 76.3% to 94.6% with CVs of 7.1–15.7% (). These data meet the requirements for an analytical method for residue determination.
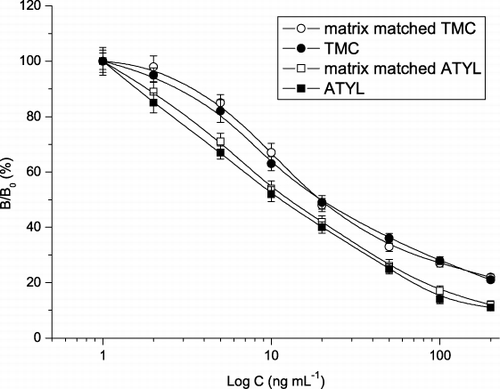
Table 2. Recoveries of the four drugs from blank fortified milk.
3.5. Unknown samples
The 35 unknown milk samples were analysed by the ELISA, and four samples were determined as positive. The residue levels calculated as TYL were 12, 31, 35 and 62 ng mL−1, but the specific analyte cannot be verified due to the antibody's high CR. A milk sample containing any of the four analytes could be detected as positive, but the result can only be expressed as TYL equivalents. Therefore, the ELISA positive results need to be confirmed with an instrumental method, e.g. LC-MS/MS.
4. Conclusion
Immunoassay is a commonly used method for rapid screening the residues of veterinary drugs in animal derived foods, but the use of immunoassay to determine MACs in foods is rare. In this study, a new hapten of TYL was synthesised and was used to produce a monoclonal antibody that simultaneously recognised three MACs (TYL, ATYL, TMC) and DMC, a metabolite of TYL. A heterologous competitive indirect ELISA was developed to detect the four analytes in milk simultaneously. This method could be used as a practical tool for routine screening of large numbers of milk samples, with positive results confirmed by instrumental methods.
Acknowledgements
This study was financed by Hebei Scientific and Technological Project (11221001D).
References
- Beier, R.C., Creemer, L.C., Ziprin, R.L., & Nisbet, D.J. (2005). Production and characterization of monoclonal antibodies against the antibiotic tilmicosin. Journal of Agricultural and Food Chemistry, 53, 9679–9688.
- Benetti, C., Dainese, N., Biancotto, G., Piro, R., & Mutinelli, F. (2004). Unauthorised antibiotic treatments in beekeeping: Development and validation of a method to quantify and confirm tylosin residues in honey using liquid chromatography-tandem mass spectrometric detection. Analytica Chimica Acta, 520, 87–92.
- Bogialli, S., Ciampanella, C., Curini, R., Di Corcia, A., & Laganà, A. (2009). Development and validation of a rapid assay based on liquid chromatogaphy-tandem mass spectrometry for determining macrolide antibiotic residues in eggs. Journal of Chromatography A, 1216, 6810–6815.
- Cherlet, M., De Baere, S., Croubels, S., & De Backer, P. (2002). Quantitation of tylosin in swine tissues by liquid chromatography combined with electrospray ionization mass spectrometry. Analytica Chimica Acta, 473, 167–175.
- Christodoulopoulos, G., Warnick, L.D., Papaioannou, N., & Fthenakis, G.C. (2002). Tilmicosin administration to young lambs with respiratory infection: Safety and efficacy considerations. Journal of Veterinary Pharmacology and Therapeutics, 25, 393–397.
- Creemer, L.C., Beier, R.C., & Kiehl, D.E. (2003). Facile synthesis of tilmicosin and tylosin related haptens for use as protein conjugates. Jounal of Antibiotics, 56, 481–487.
- Draisci, R., Palleschi, L., Ferretti, E., Achene, L., & Cecilia, A. (2001). Confirmatory method for macrolide residues in bovine tissues by micro-liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 926, 97–104.
- Fajt, V.R., Apley, M.D., Roth, J.A., Frank, D.E., Brogden, K.A., Skogerboe, T.L., et al. (2003). The effects of danofloxacin and tilmicosin on neutrophil function and lung consolidation in beef heifer calves with induced Pasteurella (Mannheimia) haemolytica pneumonia. Journal of Veterinary Pharmacology and Therapeutics, 26, 173–179.
- Franek, M., Diblikova, I., Cernoch, I., Vass, M., & Hruska, K. (2006). Broad-specificity immunoassays for sulfonamide detection: Immunochemical strategy of generic antibodies and competitors. Analytical Chemistry, 78, 1559–1567.
- Heller, D.N., & Nochetto, C.B. (2004). Development of multiclass methods for drug residues in eggs: Silica SPE cleanup and LC-MS/MS analysis of ionophore and macrolide residues. Journal of Agricultural and Food Chemistry, 52, 6848–6856.
- Jackman, R., Spencer, Y.I., Silverlight, J.J., Marsh, S.A., & Bellerby, P.J. (1997). Development of antibodies to tilmicosin and their use in the immunolocalization of the antibiotic in porcine lung tissue. Journal of Agricultural and Food Chemistry, 20(Suppl. 1), 131–132.
- Kochansky, J. (2004). Degradation of tylosin residues in honey. Jounal of Apicultural Research, 43, 65–68.
- Kochansky, J., Knox, D., & Shimanuki, H. (1999). Comparative stability of oxytetracycline and tylosin in sugar syrup. Apidologie, 30, 321–325.
- Laven, R., & Andrews, A.H. (1991). Long-acting antibiotic formulations in the treatment of calf pneumonia: A comparative study of tilmicosin and oxytetracycline. Veterinary Record, 129, 109–111.
- Lucchetti, D., Fabrizi, L., Esposito, A., Guandalini, E., Di Pasquale, M., & Coni, E. (2005). Simple confirmatory method for the determination of erythromycin residues in trout: A fast liquid-liquid extraction followed by liquid chromatography-tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 53, 9689–9694.
- Martos, P.A., Lehotay, S.J., & Shurmer, B. (2008). Ultratrace analysis of nine macrolides, including tulathromycin A (Draxxin), in edible animal tissues with minicolumn liquid chromatography tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 56, 8844–8850.
- Msagati, T.A.M., & Nindi, M.M. (2004). The use of supported liquid membranes in the extraction of macrolides in biomatrices. Microchimica Acta, 148, 199–214.
- Paesen, J., Cypers, W., Pauwels, K., Roets, E., & Hoogmartens, J. (1995). Study of the stability of tylosin A in aqueous solutions. Journal of Pharmaceutical and Biomedical Analysis, 13, 1153–1159.
- Peng, D., Ye, S., Wang, Y., Chen, D., Tao, Y., Huang, L., et al. (2012). Development and validation of a competitive indirect enzyme-linked immunosorbent assay for the screening of tylosin and tilmicosin in muscle, liver, milk, honey and eggs. Journal of Agricultural and Food Chemistry, 60, 44–51.
- Prats, C., Francesch, R., Arboix, M., & Perez, B. (2001). Determination of tylosin residues in different animal tissues by high performance liquid chromatography. Journal of Chromatography B, 766, 57–65.
- Sashidhar, R.B., Capoor, A.K., & Ramana, D. (1994). Quantitation of amino groups using amino acids as reference standards by trinitrobenzene sulfonic acid: A simple spectrophotometric method for the estimation of hapten to carrier protein ratio. Journal of Immunological Methods, 167, 121–127.
- Shryock, T.R., Staples, J.M., & DeRosa, D.C. (2002). Minimum inhibitory concentration breakpoints and disk diffusion inhibitory zone interpretive criteria for tilmicosin susceptibility testing against Pasteurella multocida and Actinobacillus pleuropneu-moniae associated with porcine respiratory disease. Journal of Veterinary Diagnostic Investigation, 14, 389–395.
- Silverlight, J.J., Brown, A.J., & Jackman, R. (1999). Antisera to tilmicosin for use in ELISA and for immunohistochemistry. Food and Agricultural Immunology, 11, 321–328.
- Thompson, T.S., Pernal, S.F., Noot, D.K., Melathopoulos, A.P., & van den Heever, J.P. (2007). Degradation of incurred tylosin to desmycosin-Implications for residue analysis of honey. Analytica Chimica Acta, 586, 304–311.
- Wang, J., Leung, D., & Butterworth, F. (2005). Determination of five macrolide antibiotic residues in eggs using liquid chromatography/electrospray ionization tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 53, 1857–1865.
- Wang, J., & Leung, D. (2007). Analyses of macrolide antibiotic residues in eggs, raw milk, and honey using both ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry and high-performance liquid chromatography/tandem mass spectrometry. Rapid Communication in Mass Spectrometry, 21, 3213–3222.
- Wicker, A.L., Mowrey, D.H., Sweeney, D.J., Coleman, M.R., Morris, D.K., & Brockus, C.L. (1994). Particle concentration fluorescence immunoassay for determination of tylosin in premix, feeds, and liquid feed supplement: Comparison with turbidimetric assay. Journal of AOAC International, 77, 1083–1095.
- Yao, R.C., & Mahoney, D.F. (1989). Enzyme immunoassay for macrolide antibiotics: Characterization of an antibody to 23-amino-O-mycaminosyltylonolide. Applied Environmental Microbiology, 55, 1507–1511.
- Zhang, Y., Jiang, H., Jin, X., Shen, Z., Shen, J., Fu, C., et al. (2004). Residue depletion of tilmicosin in chicken tissues. Journal of Agricultural and Food Chemistry, 52, 2602–2605.
- Zhang, J.K., Liu, J.X., Wang, L.L. Chai, L., & Wang, J.P. (2012). Production of the monoclonal antibody against tylosin for immunoassay of macrolide antibiotics in milk. Journal of Environmental Science and Health, Part B, 47, 876–882.