Abstract
The gastrointestinal distribution of glycinin in pigs at different growth stages was investigated in this study. Fifteen healthy General No. 1 barrows weaned on the 28th day were randomly assigned to three groups with five replicates: five weanling, five growing and five finishing. All pigs received diets with non-soya bean ingredients in non-experimental periods, while the pigs received diets containing 4% purified glycinin in experimental periods. Binding of anti-glycinin antibody was detected using a labelled streptavidin–peroxidase complex method to determine the gastrointestinal distribution of glycinin. The results indicated that there was a significant difference on the gastrointestinal mucosal distribution of glycinin between pigs of different ages. Glycinin in growers was higher than piglets and lower than finishers (P<0.001). The highest content of glycinin was in the distal jejunum and ileum for piglets, and in distal jejunum for growers and finishers (P<0.05).
1. Introduction
Glycinin, the main storage globulin in soya bean, has been identified as a dietary allergen for humans (Burks, Brooks, & Sampson, Citation1988; Duke, Citation1934; Foucard & Yman, Citation1999) and young animals (Sun, Li, Li, Dong, & Wang, Citation2008; Zhao et al., Citation2008). It may cause a series of allergic reactions resulting in damage to the intestinal morphology, immune function disorders, growth depression and diarrhoea. Glycinin, with a molecular weight about 360 kDa, is composed of five subunits, and each contains an acidic and a basic polypeptide chain linked by a single disulphide bond with molecular weight of 34–44 and 20 kDa, respectively (Kitamura & Shibasaki, Citation1975).
Recently, several studies on the gastrointestinal digestion of glycinin in pigs have been reported (Wang, Qin, Sun, Zhao, & Zhang, Citation2010; Zhao et al., Citation2008; Zhao, Qin, Sun, Zhang, & Wang, Citation2010). As glycinin passes down the gastrointestinal tract in pigs, the normalized percent of glycinin remaining in digesta tends to decline and depends on the age (Wang et al., Citation2010). It is important to compare the differences of sensitivity to glycinin during the growth stages. Glycinin may cross the gastrointestinal tissue and induce allergy, but the gastrointestinal distribution of glycinin has not yet been reported to the best of our knowledge.
In this study, pigs were used as a large-animal model due to their physiological and immunological similarity to human beings. The objective of the present study was to investigate the gastrointestinal distribution of glycinin detected by the immunohistochemical staining in pigs of different growth stages. These studies are essential for exploring the mechanism of soya bean-induced hypersensitivity, and may have further implications for preventing and treating the related allergic disease in humans and animals.
2. Materials and methods
2.1. Purification of glycinin
Purified glycinin samples of over 85% purity in diets were kindly donated by Professor Shuntang Guo at the Food Institute of China Agricultural University (Patent number, 200410029589.4, Beijing, China).
2.2. Production of polyclonal antibody
Glycinin samples containing more than 95% glycinin were isolated and purified from defatted soya flour according to the method by Setsuko and Fumio (Citation1987). Polyclonal antibody against glycinin were prepared as previously described by He, Hu, and Gao (Citation2002). Polyclonal antisera were further purified as described in our previous study (Zhao et al., Citation2008).
2.3. Experimental design
The experiment using pigs from the same herd was conducted in the Jilin University Experimental Pig Farm (Changchun, China). In order to provide complete nutrition in a solid food, all the piglets were fed from 7th day of age with dry whole milk. Fifteen healthy General No.1 barrows weaned on the 28th day after birth were randomly allotted to three groups. Each group had five replicates: five weanling (7.06±0.18 kg), five growing (44.54±9.04 kg) and five finishing (78.93±7.37 kg). All pigs received diets without ingredients originating from soya bean products in non-experimental periods; while the pigs received diets containing 4% purified glycinin in experimental periods (Sun et al., Citation2008). After 3 days of adaptation, the experimental periods of each group started and lasted for 7 days. The composition and nutrient content of the diets are shown in . The diets were formulated to meet NRC (1998) requirements.
Table 1. Ingredient composition and nutrient levels of the diets.
2.4. Sample collection and chemical analysis
After the experimental periods, pigs were anaesthetised with excess procaine and killed 1 hour after the morning meal. Samples of small intestinal tissue were taken from the stomach, the duodenum, the proximal-jejunum, the mid-jejunum, the distal-jejunum and the ileum. After being washed in saline, the samples were fixed in formalin and embedded in paraffin. Five-micrometre-thick sections were stained for the immunohistochemistry.
2.5. Immunostaining with anti-glycinin polyclonal antibody
Sections were placed on positively charged glass slides and deparaffinised. They were then exposed to microwave pre-treatment (in 10-mmol/L citrate buffer, pH 6 at 850 W for 20 min). To detect the gastrointestinal distribution of glycinin, binding of anti-glycinin polyclonal antibody was achieved using a labelled streptavidin–peroxidase complex method (Ultrasensitive Kit, KIT9706, Maixin, Fuzhou, China). The optimal concentration of anti-glycinin polyclonal antibodies in order to reduce the non-specific reaction was 5 µg mL−1. Sections were then counterstained with haematoxylin, dehydrated, cleared and permanently mounted. Negative control sections were treated without the primary antibody.
The specific brown immunohistochemical staining was scanned. Integrated optical density (IOD) at the site of mucosa, villus and crypt of different digestive tract parts were estimated using Image-Pro Plus 5.0 software. The control experiment resulted in negligible background staining (data not shown). The glycinin in the gastrointestinal tissue was quantified by measuring the IOD of the immunoreactive staining.
2.6. Statistical analysis
All data were analysed using the general linear model procedure of Statistical Package for Social Sciences version 11.5 (SPSS Inc., Chicago, USA). Duncan's multiple range test was employed to test the differences among the means.
3. Results
3.1. Location of glycinin
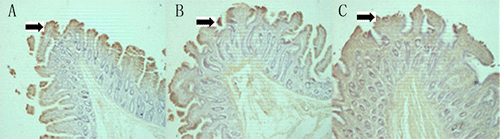
Glycinin was located in the mucosa of stomach, duodenum, jejunum and ileum as evinced by the results of immunochemistry (). There was much glycinin in mucous epithelium and lamina propria. Much glycinin was also found in the intestinal villi, but little in the crypt, central lacteal and connective tissues. No positive staining was found in the negative control (photo not shown).
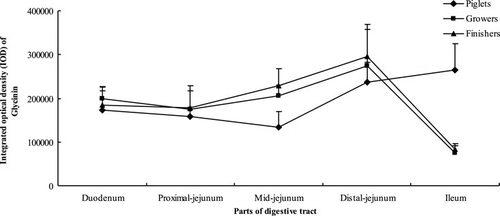
3.2. Distribution of glycinin in the gastrointestinal mucosa
shows that the growth phase had significant effects on the distribution of glycinin in the gastrointestinal mucosa (P<0.001). The glycinin in growers was higher than piglets and lower than finishers (P<0.001).
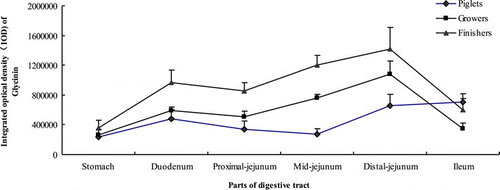
With the descending down of the digestive tract, the mucosal content of glycinin increased in piglets, but hardly changed between duodenum and mid-jejunum (P<0.05). Although it tended to go down, but did not result in a significant drop from duodenum to proximal jejunum, the mucosal content of glycinin in growers and finishers different from piglets increased slowly in the stomach and distal jejunum but fell sharply in the ileum (P<0.05). The highest content of glycinin was in the distal jejunum and ileum for piglets, and in distal jejunum for growers and finishers (P<0.05).
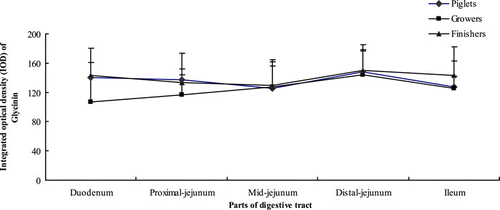
3.3. Distribution of glycinin in the small intestinal villi and crypt
As shown in , growth phase had no significant effects on distribution of glycinin in intestinal villi (P=0.077). The distribution of glycinin in intestinal villi of pigs at different growth phases was similar to that in intestinal mucosa. For pigs at different growth phases, the content of glycinin in the villi between the upper small intestine (duodenum, proximal jejunum and mid-jejunum) showed no significant differences (P>0.05). Moreover, the glycinin in distal jejunum was more than in the upper intestine (P<0.05). In ileum, it remained stable for piglets while dropped drastically for growers and finishers (P<0.05).
Growth phase and part of digestive tract had no significant effects on the content of glycinin in the small intestinal crypt (P>0.05) ().
4. Discussion
The major soya bean storage globulin glycinin has been identified as an allergen (Burks et al., Citation1988; Shibaski, Suzuki, Tajima, Nemoto, & Kuroume, Citation1980). Its allergenicity contributes to hypersensitivity in humans, whose clinical symptoms range from gastrointestinal distress to life-threatening asthma and death (Foucard & Yman, Citation1999). There have been some studies using pigs as an animal model to investigate the gastrointestinal variation of immunoreactive glycinin (Wang et al., Citation2010; Zhao et al., Citation2008). These studies showed that the immunoreactivity of glycinin decreases as the glycinin descends down the gastrointestinal tract. The extent of the decrease becomes more pronounced with increasing age of the pigs. However, the reports about the gastrointestinal distribution of glycinin are scarce.
The data in this study indicate that the mucosal distribution of glycinin from stomach to mid-jejunum for pigs at different ages increased slowly, following the general rules of protein absorption. However, there was a significant difference on the gastrointestinal mucosal distribution of glycinin between pigs at different ages has been found in this study, which may attribute to the great discrepancy in the digestive physiology of these pigs. The immature digestive function (Efird, Armstrong, & Dennis, Citation1982; Han, Citation2002; Hartman, Hays, Baker, Neagle, & Catron, Citation1961), the weaning stress (Lallès, Bosi, Smidt, & Strokes, Citation2007; Lallès, Boudry, & Favier, Citation2004) and the rapid stomach emptying time (Han, Citation2002) might result in the lower gastric absorption of glycinin for piglets. With increasing age, the digestibility and absorbability of protein are improved.
The present work clearly demonstrates that the piglets presented a different pattern of glycinin distribution in the gastrointestinal tissue compared with growers and finishers. Much glycinin in the distal jejunum and the ileum tissue for piglets was found in this study. It could be explained in two ways. On one hand, glycinin-induced allergy might change the distal intestinal permeability. Glycinin could elevate the intestinal mast cell numbers (Ashida & Denda, Citation2003; Mathan & Mathan, Citation1986). These mast cells can be activated by soya bean glycinin-specific IgE, resulting in an excessive release of histamine. This may contribute to both the change of intestinal motility (Rangachari, Citation1992) and the occurrence of allergic symptoms. The mast cell numbers in the ileum were greater than those in the proximal intestine (in the duodenum and jejunum; Sun et al., Citation2008). Therefore, much release of histamine occurred in the distal intestine. The glycinin-induced allergy might enlarge the intestinal permeability, resulting in more glycinin in the ileum than in the proximal intestine. Moreover, many lymphoid patches are located in the distal jejunum and the ileum, and the M cells, specialised epithelial cells, are found exclusively in the lymphoid follicle-associated epithelium (FAE) of Peyer's patches (Bockman & Cooper, Citation1973; Owen & Jones, Citation1974). The M cells could transfer the allergens to the lymphoid cells of the lamina propia, and disturb the permeability of the adjacent epithelium to allergic proteins. When Salmonella typhimurium invades M cells, destruction of an M cell formed a gap in the FAE, allowing organisms to invade the adjacent enterocytes (Jones, Ghori, & Falkow, Citation1994). On the other hand, the lower tissue concentration of glycinin in the ileum of grower–finisher pigs might be attributed to the low level of immunoreactive glycinin in ileum. Immunoreactive glycinin was digested almost completely in grower–finisher pigs because of normal protease activity and gastric emptying time (Wang et al., Citation2010).
The villi could increase intestinal absorptive surface area, providing exceptionally efficient absorption of nutrients in the lumen. Therefore, the concentration of glycinin in villi is higher than that in crypt.
Taken together, the mucosal distribution of glycinin from stomach to mid-jejunum for pigs at different ages changed slowly. There was a significant difference in the gastrointestinal mucosal distribution of glycinin among pigs at different ages. The high glycinin in the distal jejunum and the ileum tissue for piglets is indicative of differences in the gastrointestinal tissue between piglets and grower–finisher pigs.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of P.R. China (No. 30871802 and No. 31101719) and ‘Eleventh Five-year Plan’ for Sci & Tech Research Program of Jilin Education Department of P.R. China No. 201044.
References
- Ashida, Y., & Denda, M. (2003). Dry environment increases mast cell number and histamine content in dermis in hairless mice. British Journal of Dermatology, 149, 240–247.
- Bockman, D.E., & Cooper, M.D. (1973). Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer's patches. An electron microscopic study. American Journal of Anatomy, 136, 455.
- Burks, A.W., Brooks, J.R., & Sampson, H.A. (1988). Allergenicity of major component proteins of soybean determined by enzyme linked immunosorbent assay (ELISA) and immunoblotting in children with atopic dermatitis and positive soy challenges. Journal of Allergy and Clinical Immunology, 81, 1135–1142.
- Duke, W.W. (1934). Soybean as a possible important source of allergy. Journal of Allergy, 5, 300–302.
- Efird, R.C., Armstrong, W.D., & Dennis, L.H. (1982). The development of digestive capacity in young pigs: Effects of age and weaning system. Journal of Animal Science, 55, 1380–1387.
- Foucard, T., & Yman, I.M. (1999). A study on severe food reactions in Sweden-is soy protein an underestimated cause of food anaphylaxis? Allergy, 54, 261–265.
- Han, Z.K. (Ed.). 2002. Livestock physiology. (3rd ed.). Beijing: China Agricultural Publisher.
- Hartman, P.A., Hays, V.W., Baker, R.O., Neagle, L.H., & Catron, D.V. (1961). Digestive enzyme development in the young pig. Journal of Animal Science, 20, 114–123.
- He, Z., Hu, G., & Gao, Y. (2002). Polyclone antibodies preparation technique. In Z. He, G. Hu, & C. Wang (Eds.), Technique of animal immunology (pp. 71–76). Changchun: Jilin Science and Technology Publishing House.
- Jones, B.D., Ghori, N., & Falkow, S. (1994). Salmonella tryhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Payer's patches. The Journal of Experimental Medicine, 180, 15–23.
- Kitamura, K., & Shibasaki, K. (1975). Isolation and some physicochemical properties of acidic subunits of soybean 11S globulin. Agricultural and Biological Chemistry, 39, 945–951.
- Lallès, J.P., Bosi, P., Smidt, H., & Strokes, C.R. (2007). Weaning a challenge to gut physiologists. Livestock Science, 108, 82–93.
- Lallès, J.P., Boudry, G., & Favier, C. (2004). Gut functions and dysfunction in young pigs: Physiology. Animal Research, 53, 301–316.
- Mathan, M.M., & Mathan, V.I. (1986). Ultrastructural pathology of the rectal mucosa in Shigella dysentery. American Journal of Pathology, 123, 25–38.
- Owen, R.L., & Jones, A.L. (1974). Epithelial cell specialization within human Peyer's patches: An ultrastructural study of intestinal lymphoid follicles. Gastroenterology, 66, 189.
- Rangachari, P.K. (1992). Histamine: Mercurial messenger in the gut. American Journal of Pathology, 262, G1–G13.
- Setsuko, I., & Fumio, Y. (1987). Determination of glycinin and β-conglycinin in soybean protein by immunological methods. Journal of Agricultural and Food Chemistry, 35, 200–205.
- Shibaski, M., Suzuki, S., Tajima, S., Nemoto, H., & Kuroume, T. (1980). Allergenicity of major component proteins of soybeans. International Archives of Allergy & Applied Immunology, 61, 441–448.
- Sun, P., Li, D.F., Li, Z.J., Dong, B., & Wang, F.L. (2008). Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine. The Journal of Nutritional Biochemistry, 19, 627–633.
- Wang, T., Qin, G.X., Sun, Z.W., Zhao, Y., & Zhang, B. (2010). Comparative study on the residual rate of immunoreactive soybean glycinin (11S) in the digestive tract of pigs of different ages. Food and Agricultural Immunology, 21(3), 201–208.
- Zhao, Y., Qin, G.X., Sun, Z.W., Zhang, X.D., Bao, N., Wang, T., et al. (2008). Disappearance of immunoreactive glycinin and β-conglycinin in the digestive tract of piglets. Archives of Animal Nutrition, 62, 322–330.
- Zhao, Y., Qin, G.X., Sun, Z.W., Zhang, B., & Wang, T. (2010). Stability and Immunoreactivity of glycinin and β-conglycinin to hydrolysis in vitro. Food and Agricultural Immunology, 21(3), 253–263.