Abstract
Osteoporosis is recognised as one of the major hormonal deficiency diseases, especially in menopausal women and the elderly. The present study investigated whether treatment with sunflower (Helianthus annuus L.) seed extract (SSE) may affect the function of MC3T3-E1 osteogenic cells. In order to determine the growth and differentiation of osteoblast, MTT (3-(4,5-dimethyl-thiazol-2yl)-2,5-diphenyl tetrazolium bromide) assay, alkaline phosphatase (ALP) activity, collagen synthesis and osteocalcin secretion were performed. Also, the production of tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6) and nitric oxide (NO) in osteoblastic MC3T3-E1 cells was measured. SSE significantly (p<0.05) increased cell growth, ALP activity, collagen content and osteocalcin secretion compared with control. The effect of SSE (50 µg/ml) in increasing cell growth, ALP activity and collagen content was prevented by the presence of 10−6 M cycloheximide and 10−6 M ICI182780, suggesting that SSE's effect results from a newly synthesised protein component and might be partly involved in oestrogen action. Treatment with SSE (10 and 50 µg/ml) decreased the 5 µg/ml lipopolysaccharide-induced production of TNF-α, IL-6 and NO in osteoblasts. Our data indicate that the enhancement of osteoblast function by sunflower seed may result in the prevention for osteoporosis and inflammatory bone diseases.
Introduction
Osteoporosis associated with oestrogen deficiency after menopause is the most common cause of age-related bone loss. The current clinical treatment regimens for osteoporosis are anti-resorptive drugs, which maintain bone mass by inhibiting osteoclast function, such as oestrogen, oestrogen receptor analogues, calcitonin and bisphosphates (Pfister et al., Citation2006). The effect of these drugs in increasing bone mass or recovering bone loss is relatively minor, probably no more than 2% per year (Rodan & Martin, Citation2000). The potential complications, including breast cancer, uterine bleeding and cardiovascular events, also restrict their usage for osteoporosis. It is desirable, therefore, to identify better and safe anabolic agents with low cytotoxicity. Oestrogen replacement therapy seems to be the most effective method to reduce post-menopausal bone loss, but may be accompanied by side-effects. Thus, it would be most helpful to discover a natural dietary substance that minimises bone loss in post-menopausal women. The phytoestrogen, therefore, are potentially important in the prevention of post-menopausal osteoporosis caused by oestrogen deficiency (Newton & Grady, Citation2011).
There is a continuously increasing interest in assessing the role of nutritional constituents, present in small quantities in food, to prevent the risk of chronic disease (Atkinson & Ward, Citation2001). Many bioactive compounds have been discovered in food, among them phenolic compounds, that is, flavonoids, phenolic acids and phytoestrogens, which show antioxidant properties and may act as oestrogen agonist/antagonists with beneficial health effects, for example, reducing the risk of cancer, osteoporosis and cardiovascular disease (Kris-Etherton et al., Citation2002). There are numerous reports regarding the effects of individual nutritional constituents present in plant-derived food (Choi & Kim, Citation2008; Lee & Choi, Citation2008). Sunflower (Helianthus annuus L., Asteraceae) seed has a great potential for meeting the ever-increasing demand for edible oil and protein by virtue of the high oil content and relatively high protein content of its meal. Sunflower meal is primarily used as ruminant feed but its nutritional and functional properties make it potentially useful in human food. Phenolics content in sunflower defatted meal ranges from 3 to 4% (w/w), 79% of which is soluble and 21% bound to protein (Bau, Mohtadi-Nia, & Mejean, Citation1983). Lignans are widely distributed in plants and present a wide range of biological activities (Ward, Citation1999). Antitumour, antimitotic, antiviral, enzyme inhibitory, piscicidal, fungistatic, etc. properties have been reported (Rios, Giner, & Prieto, Citation2002). Ten lignans and a phenylpropanoid were isolated from H. annuus (Macias, López, Varela, Torres, & Molinillo, Citation2004). Chlorogenic and caffeic acids account for 70% of polyphenols present (Leung, Fenton, & Clandinin, Citation1981). In the present study, we investigated whether sunflower seed may affect the differentiation and production of bone-resorbing agents in MC3T3-E1 osteogenic cells.
Materials and methods
Extraction
Sunflower seed was purchased commercially (Kyungdong Pharmaceuticals, Korea); the identification of the plant was done by Dr. S.J. Koo at the Kyunghee University, Korea, and the herbarium specimen (KU04-22) has been stored in Department of Food & Nutrition, Kyung Hee University, Korea. The SSE was obtained by extraction with ethanol–water (70% ethanol) extraction method. The extract was filtered and concentrated with a rotary evaporator at temperature below 50°C and then freeze dried (yield: 7.9% w/w). All reagents were purchased from Sigma (USA), unless otherwise stated.
Cell culture
MC3T3-E1 cells (RCB1126, an osteoblast-like cell line from C57BL/6 mouse calvaria) were obtained from the RIKEN Cell Bank (Tsukuba, Japan). MC3T3-E1 cells were cultured at 37°C in 5% CO2 atmosphere in α-modified minimal essential medium (α-MEM; GIBCO). Unless otherwise specified, the medium contained 10% heat-inactivated foetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin.
Cell viability
The cells were suspended in medium and plated at a density of 7.0×103 cells/well into a 96-well culture dish (Costar, Cambridge, MA). After 24 h, the medium was replaced with phenol red-free media containing 5% charcoal-dextran-treated FBS (CD-FBS) supplemented with glabridin. After 2 days of culture, cell proliferation was measured by MTT (3-(4,5-dimethyl-thiazol-2yl)-2,5-diphenyl tetrazolium bromide) assay. This assay is based on the ability of viable cells to convert soluble MTT into an insoluble dark blue formazan reaction product. In the bulk cell photometric MTT assay, the bulk conversion of MTT in the well plate was measured photometrically. MTT was dissolved in Dulbecco's Phosphate Buffered Saline (DPBS) at a concentration of 5 g/L and sterilised by passage through a 0.22 mm filter. This stock solution was added (1 part to 10 parts medium) to each well of culture plate, and the plate was incubated at 37°C for 2 h. Dimethylsulfoxide (DMSO) was added to all wells and mixed thoroughly to dissolve the dark blue crystals. After a few minutes at room temperature, to ensure that all the crystals were dissolved, the plates were read on a microplate reader at a wavelength of 570 nm.
ALP activity
After the osteoblasts reached confluence, the medium was replaced with differentiation medium containing 5 mM β-glycerophosphate and 50 µg/ml ascorbic acid, to initiate differentiation. After 6 days, the cells were cultured with sample for 2 days. The medium was removed, and the cell monolayer was gently washed twice with PBS. The cells were lysed with 0.2% Triton X-100 and the lysate was centrifuged at 14,000×g for 5 min. The clear supernatant was used for the measurement of ALP activity and protein concentration. ALP activity and protein concentration were determined using an ALP activity assay kit (Wako) and a bicinchoninic acid (BCA)-protein assay kit (Pierce, Rockford, IL), respectively.
Collagen contents
After the osteoblasts reached confluence, the medium was replaced with differentiation medium. After 6 days, the cells were cultured with sample for 2 days, and cellular collagen content was measured using Sircol Collagen Assay kit (Biocolor Ltd. Northern Ireland). This Sircol Collagen Assay is a quantitative dye-binding method designed for the analysis of collagens extracted from mammalian tissues and cells during in vitro culture. The dye reagent binds specifically to the [Gly-X-Y]n helical structure found in mammalian collagens (Types I–V).
Measurement of osteocalcin
After the osteoblasts reached confluence, the medium was replaced with differentiation medium. After 14 days, the cells were cultured with sample for 2 days, and osteocalcin content was measured using sandwich ELISA assay kit (Biomedical Technologies Inc., USA). Two mouse osteocalcin antibodies are employed, each directed towards an end of the (C or N terminal) osteocalcin molecule. The N-terminal antibody is bound to the well, which binds the mouse osteocalcin standard or sample. The biotin labelled C-terminal mouse osteocalcin antibody completes the sandwich. This sandwich ELISA kit is specific for intact mouse osteocalcin only. Both carboxylated and decarboxylated mouse osteocalcin are recognised.
Cytokines tumour necrosis factor-α and interleukin-6 (TNF-α and IL-6) immunoassay
After cells were treated with sample and 5 µg/ml lipopolysaccharide (LPS) for 48 h, TNF-α and IL-6 contents in the medium were measured with an enzyme immunoassay system (R&D System Inc., Minneapolis, MN, USA) according to the manufacturer's recommendation. In brief, cytokine present is bound by immobilised antibody pre-coated onto a microplate. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for cytokine is added to the well. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution is added to the wells. The enzyme reaction yields a blue product that turns yellow when stop solution is added. The intensity of the colour measured is in proportion to the amount of cytokine bound.
Determination of nitrite production
After cells were treated with sample and 5 µg/ml LPS for 48 h, nitrite production, an indicator of nitric oxide (NO) synthesis, was measured in the culture supernatant of osteoblasts. Briefly, after mixing 100 µl of Griess reagent (1% sulphanilamide and 0.1% naphthylethylenediamide in 5% phosphoric acid) with 100 µl culture supernatant, optical density at 540 nm was measured by using a microplate reader. Nitrite concentrations were calculated from the standard curve of sodium nitrite prepared in culture medium.
Statistics
The results are expressed as mean±SEM (n=6). Statistical analysis was performed using a one-way ANOVA followed by Duncan's multiple range test. A P<0.05 value was considered statistically significant.
Results
Effect of SSE on the growth of MC3T3-E1 cells
MC3T3-E1 cells were incubated with SSE, and cell growth was measured. MC3T3-E1 cell growth was promoted by stimulation with SSE at 20–200 µg/ml up to approximately 2-fold (). The effect of SSE (50 µg/ml) in increasing cell growth was eliminated in the presence of cycloheximide (10−6 M) or ICI182780 (10−6 M) (). On the basis of this observation, we evaluated the differentiation-inducing activity of SSE on MC3T3-E1 cells by assessing for intracellular ALP activity, collagen synthesis and osteocalcin secretion.
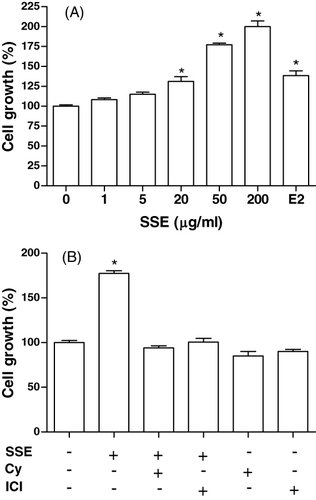
Effect of SSE on ALP activity in MC3T3-E1 cells
ALP activity was measured to study the effect of SSE on the osteoblastic differentiation in MC3T3-E1 cells (). Culture in the presence of SSE (20 and 50 µg/ml) caused a significant increase in the ALP activity of osteoblastic cells (). The effect of SSE (50 µg/ml) in increasing ALP activity was eliminated in the presence of cycloheximide (10−6 M) or ICI182780 (10−6 M) ().
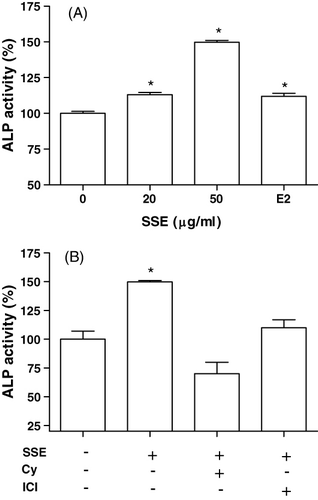
Effect of SSE on collagen synthesis in MC3T3-E1 cells
Cells were cultured in a medium containing SSE. The presence of 20 and 50 µg/ml SSE caused a significant increase in collagen content (). The SSE (50 µg/ml)-induced increase in collagen synthesis was eliminated by the presence of cycloheximide (10−6 M) or ICI182780 (10−6 M) ().
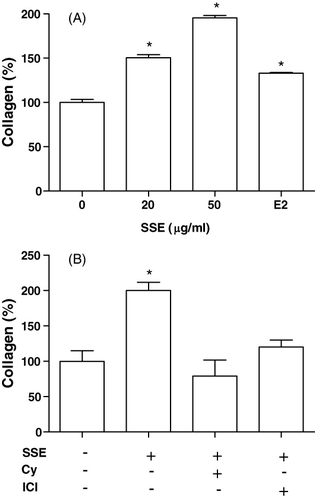
Effect of SSE on osteocalcin secretion in MC3T3-E1 cells
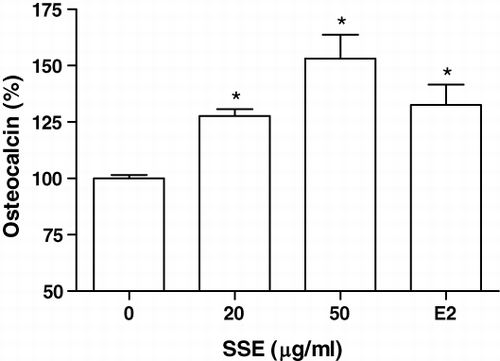
We examined the effects of SSE on the osteocalcin synthesis by MC3T3-E1 cells. The MC3T3-E1 cells were cultured with SSE (20 and 50 µg/ml), and the content of osteocalcin in medium was measured (). The increase in the osteocalcin content was significant at an SSE concentration of 10 and 50 µg/ml in MC3T3-E1 cell culture.
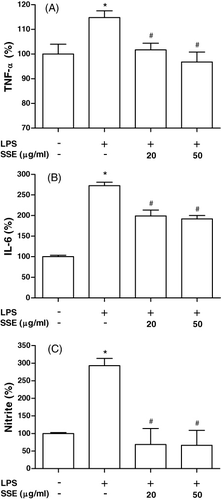
Effect of SSE on cytokines and NO production in MC3T3-EI cells
We investigated whether SSE modulates osteoblast production of TNF-α, IL-6 and NO (). When 5 µg/ml LPS was added to cells, production of TNF-α, IL-6 and NO increased significantly. However, LPS-induced TNF-α, IL-6 and NO productions were significantly inhibited by treatment of SSE (20 and 50 µg/ml).
Discussion
We investigated the ability of ethanol extract derived from SSE to induce osteoblast differentiation, as well as its inhibitory effect on the production of inflammatory mediators in osteoblasts. To assess the effects of sunflower seed on bone cells, we used the MC3T3-E1 cell line cloned from mouse calvaria, which undergoes in vitro osteoblast differentiation (Quarles, Yohay, Lever, Caton, & Wenstrup, Citation1992). Alkaline phophatase activity, collagen and osteocalcin are indicative of the differentiation process. Alkaline phosphatase is the most widely recognised biochemical marker for osteoblastic activity (Bellows, Aubin, & Heersche, Citation1991). Although its precise mechanism of action is poorly understood, this enzyme is believed to play a role in bone mineralisation. SSE significantly increased cell growth, ALP activity and collagen synthesis. Therefore, SSE seems to stimulate the proliferation and differentiation of osteoblasts. In addition, SSE augmented the osteocalcin secretion of osteoblastic MC3T3-E1 cells. This result also supports the stimulatory nature of SSE towards the function of osteoblastic cells. Osteocalcin is the specific product of osteoblasts and is believed to have a role in bone formation. However, its exact role in bone metabolism is not known (Ma et al., Citation2011). On the other hand, the stimulatory effects of SSE on ALP activity and collagen synthesis were blocked by the presence of cycloheximide (protein synthesis inhibitor) and ICI182780 (anti-oestrogen reagent). These results suggest that the effect of SSE in increasing bone formation results from a newly synthesised protein component and is mediated through an oestrogen-like action. Our study, therefore, based on reliable biological tests and biochemical markers reflective of osteoblastic activity, reveals that SSE stimulated osteoblast differentiation significantly.
To analyse the inhibitory effect of SSE on bone resorption, we tested the effect of the SSE on the production of bone-resorbing mediators in osteoblasts. SSE significantly inhibited the production of TNF-α, IL-6 and NO induced by LPS in osteoblasts, suggesting that SSE may block osteoclast formation at a point common to all mechanisms that induce it. Many investigators have shown that various agents modulate the synthesis and secretion by osteoblasts of mediators that stimulate or inhibit osteoclast formation. For example, parathyroid hormone stimulates the secretion of soluble or insoluble factors (BRSA) from osteoblast-like cells that stimulate osteoclastic activity (Fuller, Gallagher, & Chambers, Citation1991), and oestrogen stimulates the secretion of an inhibitory factor of BRSA (Ishii et al., Citation1993). TNF-α stimulates IL-6 production, and oestrogen inhibits IL-6 secretion in osteoblasts (Girasole et al., Citation1992). Thus, SSE may also modulate the synthesis and secretion of such mediators in osteoblasts to regulate bone resorption. Another possibility is that SSE may act indirectly on pre-osteoclasts to inhibit their maturation, or directly inhibit the activity of the mature osteoclast in bone. Our results show that SSE may have beneficial effect on bone through the stimulation of bone mineralisation and inhibition of bone resorption.
In our experiments, we cannot attribute the biological effects observed to particular constituents, as many other compounds are present in the plant extract. Our study investigates the effect of the ethanol extract of sunflower seed as a whole on bone metabolism. Some extracts derived by other plants, such as licorice root extract, soy extract and Chinese herbal extracts, demonstrate oestrogen-like effects on osteoblasts, findings that are in agreement with our results (Boue et al., Citation2003; Choi, Citation2005). Some extracts may show greater effect than the phytoestrogen compound alone, implicating that combinations of constituents present in plant extracts may be highly important in the final biological activity. We are currently undertaking extensive experiments necessary for the identification of compounds and their active effects on bone, as well as experiments to delineate their mechanism of action.
In conclusion, our results indicated that sunflower seed had an effect in accelerating the growth and differentiation of bone cells and inhibited the release of TNF-α, IL-6 and NO, which are all known to be stimulators of bone resorption.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012-0002531).
References
- Atkinson, A.S., & Ward, E.W. (2001). Clinical nutrition: 2. The role of nutrition in the prevention and treatment of adult osteoporosis. Canadian Medical Association Journal, 165, 1511–1514.
- Bau, H.M., Mohtadi-Nia, D.J., & Mejean, L.G. (1983). Preparation of colorless sunflower protein products: Effect of processing on physicochemical and nutritional properties. Journal of the American Oil Chemists' Society, 60, 1141–1147.
- Bellows, C.G., Aubin, J.E., & Heersche, J.N.M. (1991). Initiation and progression of mineralization of bone nodules formed in vitro: The role of alkaline phosphatase and organic phosphate. Bone and Mineral, 14, 27–40.
- Boue, S.M., Wiese, T.E., Nehls, S., Burow, M.E., Elliott, S., Carter-Wientjes, C.H., et al. (2003). Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. Journal of Agricultural and Food Chemistry, 51, 2193–2199.
- Choi, E.M. (2005). The licorice root derived isoflavan glabridin increases the function of osteoblastic MC3T3-E1 cells. Biochemical Pharmacology, 70, 363–368.
- Choi, E.M., & Kim, Y.H. (2008). Hesperetin attenuates the highly reducing sugar-triggered inhibition of osteoblast differentiation. Cell Biology and Toxicology, 24, 225–231.
- Fuller, K., Gallagher, A.C., & Chambers, T.J. (1991). Osteoclast resorption stimulating activity is associated with the osteoblast cell surface and/or the extracellular matris. Biochemical and Biophysical Research Communications, 181, 67–73.
- Girasole, G., Jilka, R.L, Passeri, G., Boswell, S., Boder, G., Williams, D.C., et al. (1992). 17-β oestradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: A potential mechanism for the antiosteoporotic effect of estrogens. Journal of Clinical Investigation, 89, 883–891.
- Ishii, T., Saito, T., Morimoto, K., Takeuchi, Y., Asano, S., Kumegawa, M., et al. (1993). Estrogen stimulates the elaboration of cell/matrix surface-associated inhibitory factor of osteoclastic bone resorption from osteoblastic cells. Biochemical and Biophysical Research Communications, 191, 495–502.
- Kris-Etherton, M.P., Hecker, K.D., Bonanome, A., Coval, S.M., Binkoski, A.E., Hilpert, K.F., et al (2002). Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. American Journal of Medicine, 113, 71S–88S.
- Lee, K.H., & Choi, E.M. (2008). Myricetin, a naturally occurring flavonoid, prevents 2-deoxy-D-ribose induced dysfunction and oxidative damage in osteoblastic MC3T3-E1 cells. European Journal of Pharmacology, 591, 1–6.
- Leung, J., Fenton, T.W., & Clandinin, D.R. (1981). Phenolic compounds of sunflower flour. Journal of Food Science, 46, 1386–1393.
- Ma, H.P., Ming, L.G., Ge, B.F., Zhai, Y.K., Song, P., Xian, C.J., et al. (2011). Icariin is more potent than genistein in promoting osteoblast differentiation and mineralization in vitro. Journal of Cellular Biochemistry, 112, 916–923.
- Macias, F.A., López, A., Varela, R.M., Torres, A., & Molinillo, J.M. (2004). Bioactive lignans from a cultivar of Helianthus annuus. Journal of Agricultural and Food Chemistry, 52, 6443–6447.
- Newton, K.M., & Grady, D. (2011). Soy isoflavones for prevention of menopausal bone loss and vasomotor symptoms: Comment on “Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms”. Archives of Internal Medicine, 171, 1369–1370.
- Pfister, A.K., Welch, C.A., Lester, M.D., Emmett, M.K., Saville, P.D., & Duerring, S.A. (2006). Cost-effectiveness strategies to treat osteoporosis in elderly women. Southern Medical Journal, 99, 123–131.
- Quarles, L.D., Yohay, D.A., Lever, L.W., Caton, R., & Wenstrup, R.J. (1992). Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. Journal of Bone and Mineral Research, 7, 683–690.
- Rios, J.L., Giner, R.M., & Prieto, J.M. (2002). New findings on bioactivity of lignans. Studies in Natural Products Chemistry, 26, 183–292.
- Rodan, G.A., & Martin, T.J. (2000). Therapeutic approaches to bone diseases. Science, 289, 1508–1514.
- Ward, R.S. (1999). Lignans, neolignans and related compounds. Natural Product Reports, 16, 75–96.
