Abstract
Chitosan is obtained by N-deacetylation of chitin, which is the second most abundant natural biopolymer. In this study, an improved preparation and characterisation of a chitosan-based immunoaffinity chromatography (IAC) column were performed. The immunoaffinity adsorbent was prepared by covalently coupling monoclonal antibody (mAb) against methandrostenolone (MA) to glutaraldehyde cross-linked chitosan (chitosan CL). Scanning electron micrograph of chitosan CL indicated that the shape of the particles was spherical with a diameter range from about 300 to 500 µm. Infrared spectral analysis suggested that the immunoaffinity adsorbent was successfully prepared by coupling antibody with chitosan CL. For chromatographic extraction, 90% methanol was selected as eluant. Under optimum conditions, the maximum binding capacity of the IAC column was 3900 ng MA/mL adsorbent. The average recovery of 50, 250 and 500 ng of MA standards from IAC columns was 96.9% with a relative standard deviation among columns of 1.48%. After eight uses, the extraction recovery of the IAC column remained 82.1%. To further verify the effect of matrices on the extraction efficiency, the IAC columns were challenged with MA-fortified animal tissue and feed samples. Recoveries were 84.9–87.1%, demonstrating the effectiveness of these IAC columns for sample clean-up in MA residue determination.
Introduction
Chitosan is produced by the deacetylation of chitin, a polysaccharide consisting predominantly of unbranched chains of β-(1→4)-2-acetoamido-2-deoxy-d-glucose (N-acetylglucosamine). Chitin is known to be the second most abundant natural biopolymer derived from exoskeletons of crustaceans and also from cell walls of fungi and insect. These polysaccharides are renewable resources and are currently being explored intensively for their applications in pharmaceutical, cosmetics, agricultural, food and non-food industries as well (Kumar, Citation2000; Rinaudo, Citation2006; Singla & Chawla, Citation2001).
Compared with agarose, which is commonly used as support matrix for chromatography, chitosan is rather cheap but has many of the excellent chemical properties possessed by agarose, such as non-toxicity, chemical stability and bio-compatibility (Kumar, Citation2000). For the presence of multiple functional groups, such as amino and hydroxyl groups on its polysaccharide chain, which provide the flexibility for structural modifications and for coupling of various ligands, chitosan has been considered as one of the ideal natural supports for chromatographic adsorbents. Various chitosan-based chromatographic adsorbents have been successfully prepared for affinity, ion-exchange and hydrophobic interaction chromatographic separation during the past decades (Dhananjay & Mulimani, Citation2008; Liu, Zou, & Haginaka, Citation2006; Monier, Ayad, Wei, & Sarhan, Citation2010; Trimukhe, Mahadik, Gokhale, & Varma, Citation2008; Wang, Guo, & Jiang, Citation2002; Xi & Wu, Citation2006).
Immunoaffinity chromatography (IAC), a special sub-type of affinity chromatography, relies on the highly specific and reversible interaction between the antibody and target compound and has proven to be one of the most powerful techniques for single-step isolation and purification of individual compounds or classes of compounds from complex matrices (Moser & Hage, Citation2010). Because of its high selectivity, the approach of using IAC is very attractive as a clean-up method for determining a single or a limited number of analytes particularly in complex food or feed extracts containing potential interferences (Senyuva & Gilbert, Citation2010). IAC has been successfully used for the clean-up of various analytes, including mycotoxins, veterinary drugs, pesticides, vitamins and heavy metals, in a large range of different matrices (Abe et al., Citation2011; Chuang, Van Emon, Jones, Durnford, & Lordo, Citation2007; Heudi, Kilinç, Fontannaz, & Marley, Citation2006; Holtzapple, Buckley, & Stanker, Citation2001; Meletis, Meniades-Meimaroglou, & Markaki, Citation2007; Wang & Shen, Citation2007). A preliminary preparation of a chitosan-based immunoaffinity adsorbent using epichlorohydrin as a cross-linking reagent has been described in an earlier work of ours (Wang, Xu, Zhang, Wang, & Dong, Citation2011). However, the prepared immunoaffinity adsorbent was inadequate in size uniformity of the chitosan bead and stability to repeated use. In the present study, chitosan-based immunoaffinity adsorbent was prepared through optimised parameters for cross-linking and coupling with monoclonal antibody (mAb) against methandrostenolone (MA), aiming at obtaining a stable and specific immunoaffinity adsorbent and improving its potential application in real samples clean-up.
Materials and methods
Chemicals and instruments
Chitosan with an average molecular weight of 100 kD and a deacetylation degree of 93% was obtained from Golden-Shell Biochemical Co., Ltd. (Yuhuan, China). MA, trenbolone (TB), testosterone propionate (TP), glutaraldehyde, epichlorohydrin and sodium borohydride (NaBH4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). High-performance liquid chromatography (HPCL)-grade methanol was obtained from Fisher Scientific, Inc. (Pittsburgh, PA, USA). All other chemicals were of analytical grade and supplied by Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
The enzyme-linked immunosorbent assay (ELISA) plate reader (synergy HT) was obtained from Bio Tek Instrument, Inc. (Winooski, VT, USA). Scanning electron microscope (JSM-7001F) was supplied by JEOL (Beijing) Co., Ltd. (Beijing, China). IR spectroscopy (Nicolet Nexus 670) was obtained from Thermo Fisher Scientific (Shanghai) Inc. (Shanghai, China). The ultra-pure water system was supplied by Chengdu Ultrapure Technology Co., Ltd. (Chengdu, China).
Preparation of cross-linked chitosan
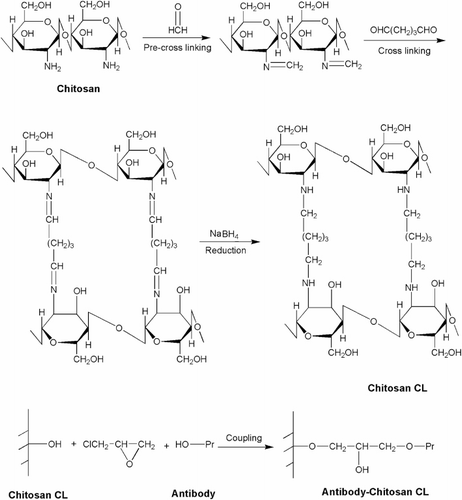
As shown in , chitosan was cross-linked using glutaraldehyde according to the previously described method, with modification (Wang et al., Citation2002; Zhang et al., Citation2008). Briefly, 2 g of chitosan powder was dissolved in 100 mL of 1% acetic acid. The resulting chitosan solution was mixed with 100 mL of paraffin oil/carbon tetrachloride (1:1, v/v) containing 0.3% span-80, and the mixture was stirred for 40 min. Then, a total of 0.6 mL of formaldehyde was added into the mixture as pre-cross-linking reagent, and stirring was maintained at room temperature for 40 min. Subsequently, glutaraldehyde solution was added dropwise into the mixture to the final concentration ranged from 0.5 to 2.0%. The cross-linking reaction was then agitated at 60°C for 3 h, with the pH maintained between 7 and 9 throughout. In order to reduce the products of the Schiff-base reaction, cross-linked chitosan (chitosan CL) was treated with 10 mL of 5 mol/L NaBH4 overnight. Finally, the chitosan beads were thoroughly washed with distilled water and dried in a vacuum drier.
Preparation of chitosan-based immunoaffinity adsorbent
For the antibody coupling process, the obtained chitosan CL beads were immersed in 10 mL of 0.1 mol/L NaHCO3 and mixed with 0.3 mg mAb against MA. After gentle agitation at room temperature for 3 h, epichlorohydrin was added to the above mixture to the final concentrations ranged from 10 to 25% and mechanically stirred at 40°C for another 5 h. Finally, the immunoaffinity adsorbent (mAb–chitosan CL) was obtained after filtering and washing with distilled water.
Characterisation of physical and chemical properties of the chitosan-based immunoaffinity adsorbent
The diameter and surface morphology of the chitosan beads were analysed using a scanning electron microscope. The functional groups of chitosan, chitosan CL and mAb-chitosan CL were determined by IR spectroscopy.
Characterisation of the chromatographic performances of the chitosan-based immunoaffinity adsorbent
The immunoaffinity adsorbent was packed into a column (30×10 mm i.d.). According to Wang et al. (Citation2011), the chromatographic performance of the column was characterised in terms of its eluting condition, binding capacity, specificity, reproducibility and reusability.
To examine the eluting condition, 200 ng of MA was dissolved in 2% methanol/water (v/v) and applied to IAC column. After removing the unspecific binding MA using PBS buffer, 4 mL of eluting solution (60, 70, 80, 90 or 100% methanol) was used to release the bound MA from the column. MA content in effluents was measured by ELISA method.
Under optimum eluting condition, a total of 3 µg of MA dissolved in 10 mL of 2% methanol was continuously loaded (1 mL for each) on the IAC column packed with 0.5 mL of immunoaffinity adsorbent. The MA content of the flow-through was determined by ELISA to calculate the column capacity.
To determine column-to-column variability (reproducibility), MA standards (200, 400 and 600 ng in 2 mL of 2% methanol) were loaded on six IAC columns. Using optimised eluting solution, MA content in effluents was determined by HPLC method and compared with loading amount.
The stability of the IAC column was estimated from the variation of its binding capacity and extraction recovery through 10 cycles of usage.
Evaluation of matrix effect on IAC extraction
The effect of matrix on IAC extraction was evaluated according to a published method with modification (Wang et al., Citation2013). In brief, animal tissue and animal feed samples were fortified with MA at the final concentrations of 1.5 and 6.0 ng/g. After homogenisation and ultrasonication, the supernatants were collected, and the pellets were pre-extracted with tert-butyl methyl ether. All the supernatant fractions were pooled and evaporated to dryness. The residues were dissolved in 2% methanol and loaded on the IAC column. Immunoaffinity extraction of MA was performed under the optimised conditions described above.
Results and discussion
Preparation of chitosan-based immunoaffinity adsorbent
The amount of glutaraldehyde and epichlorohydrin used in chitosan cross-linking and antibody coupling reaction affected the bead size, stability under acidic conditions and antibody coupling capacity to a large extent. As shown in , chitosan adsorbent partially dissolved in 1% acetic acid solution when the final concentration of glutaraldehyde was less than 0.5. With increasing glutaraldehyde, the stability in acidic conditions increased, and the colour of the chitosan matrix became brown. As more glutaraldehyde was used, more amino groups in chitosan reacted, and fewer free amino groups were left for antibody coupling. With this in mind, 1.0% glutaraldehyde was selected for subsequent cross-linking reaction.
Table 1. The colour and stability of the cross-linked chitosan when using different glutaraldehyde concentration.
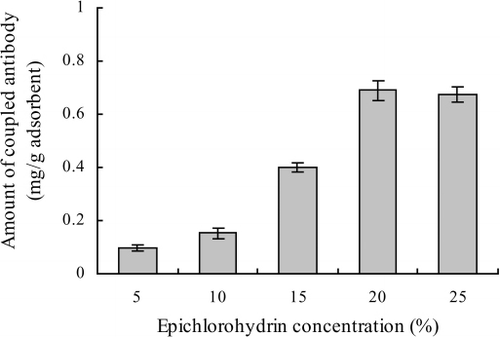
The binding capacity of the prepared immunoaffinity adsorbent mainly depends on the amount of antibody coupled to the cross-linked chitosan matrix. The effect of epichlorohydrin on the antibody coupling efficiency was examined. As shown in , the amount of antibody coupled to chitosan CL matrix increased with the increasing epichlorohydrin concentration used in coupling reaction until a plateau was reached. Indeed, the solution that contained 20% epichlorohydrin was found to yield sufficient antibody coupling (about 0.69 mg antibodies per gram cross-linked chitosan) and, therefore, was used in subsequent antibody coupling reaction.
Physicochemical properties of chitosan-based immunoaffinity adsorbent
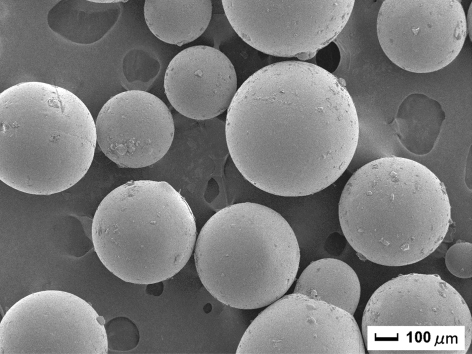
As shown in , the shape of the beads was spherical and rather homogenous in size, ranging between about 300 and 500 µm. The size homogeneity of the chitosan beads prepared in this study is much better than that of chitosan beads obtained in our earlier work using epichlorohydrin as cross-linking reagent that ranged from 20 to 80 µm (Wang et al., Citation2011).
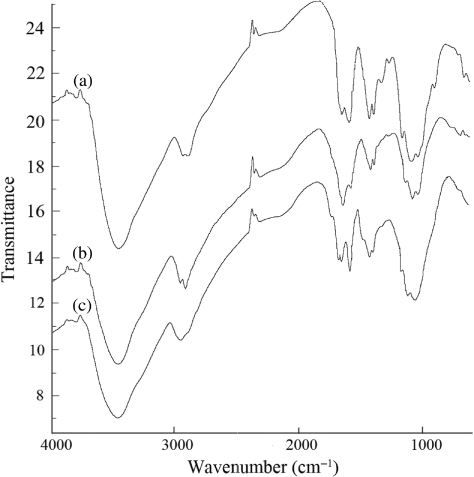
The infrared spectra of chitosan, chitosan CL and mAb-chitosan CL are shown in . As compared with chitosan, two strengthened bands at 2924.16 and 2880.40 cm−1 (υC–H) and a weakened band at 3372.76 cm−1 (υN–H) were observed in the IR spectrum of cross-linked chitosan, indicating that the cross-linking reaction had taken place between the amino group of chitosan and the aldehyde group of glutaraldehyde. In a spectrum of mAb-chitosan CL, the absorbent bands at 1642 and 1570 cm−1 corresponding to amides I and II became stronger, suggesting that the antibody was coupled to the cross-linked chitosan successfully.
Evaluation of the immunoaffinity chromatographic conditions
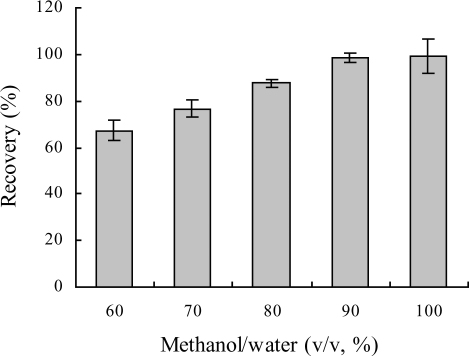
Loading and eluting conditions have a strong effect on immunoaffinity chromatographic separation. Due to the low water solubility of MA, 2% methanol was chosen as a loading buffer. After washing with PBS buffer, the trapped analyte could be released from the IAC column by dissociating the antibody–analyte complex with eluting buffer. In this study, different concentration of methanol was tested to release the MA from the column with a volume of 4 mL. As shown in , with the increasing of methanol concentration, the recovery of MA from the column increased. A higher concentration of methanol in the eluting solution might enhance the recovery efficiency, but it would probably be more harmful to the antibody. Therefore, 90% methanol was chosen as an eluting solution, and more than 97% of MA bound on the column was recovered by 4 mL of this solution.
Characterisation of the chitosan-based immunoaffinity column
Under optimum chromatographic conditions, the performance of the prepared IAC column was evaluated in terms of maximum binding capacity, column-to-column variability, specificity and reusability.
To determine the maximum binding capacity, 10 mL of MA standard solution (300 ng/mL) was continuously loaded (1 mL for each) on the IAC column. Nearly all the MA in the first 6 mL of loading solutions were completely retained on the column, and about 150 ng of MA from the seventh 1 mL of loading solution was detected in the flow-through. Therefore, the maximum binding capacity of the immunoaffinity adsorbents for MA was estimated as [6×300 (completely retained) + 150 (partially retained)]/0.5 = 3900 ng MA/mL adsorbents, more than twice the 1790 ng MA/mL adsorbents in binding capacity obtained in our earlier report (Wang et al., Citation2011).
In order to evaluate the column-to-column variability, 200, 400 and 600 ng of MA standard were loaded on six IAC columns. Under optimum chromatographic conditions, the MA content in the fraction of eluting was measured and compared with that of total loading. As summarised in , the recoveries of MA from IAC columns ranged from 94.61 to 98.96% with a relative standard deviation among columns of 1.48%, indicating a good reproducibility among columns.
Table 2. MA immunoaffinity column-to-column variability.
In order to determine the specificity of the immunoaffinity adsorbent, 1 mL of mixed solution containing MA and its analogues of TP and TB (100 ng for each compound) was loaded on the IAC column. After thorough washing, the amount of MA, TP and TB in elution fraction was measured by HPLC. It was found that about 96.6% of MA was retained on the IAC column, whereas only about 5.9% of TP and 9.5% of TB were adsorbed on the column. The results suggest that the effect of covalent coupling reaction on the specificity of the antibody can be tolerated.
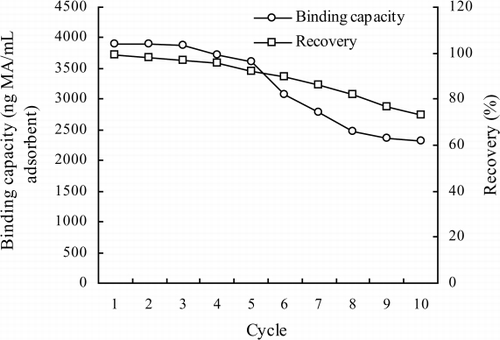
Because it is arduous and time consuming to obtain a highly sensitive and specific mAb, especially for the antibody against a small molecular compound (Basta et al., Citation2004; Clementi, Marini, Condò, & Giardina, Citation1991), IAC columns are expected to be used multiple times. Therefore, the IAC column, with its maximum binding capacity of 3900 ng MA/mL adsorbents, was tested for stability. The data for binding capacity and recovery after 10 uses are illustrated in . Although column capacity gradually decreases to about 75% with use, more than 80% of the recovery efficiency still remained after eight cycles. The loss of capacity may be attributed to the damage of binding site of the antibodies and the detachment of the antibodies from the immunoaffinity support. The stability of the IAC column prepared in this study is much higher than that obtained in our previous work (Wang et al., Citation2011).
Application of IAC columns for real samples
To further evaluate the effect of matrix on immunoaffinity extraction, the prepared IAC columns were challenged with fortified feed and animal tissue samples. Samples were spiked with MA at the levels of 1.5 and 6.0 ng/g according to the maximum residue level (2.0 ng/g) established for its structurally related compound (TB) by FAO/WHO (Joint FAO/WHO Expert Committee on Food Additives, Citation1988). As shown in , the recoveries of MA from the fortified samples were found to be in the range of 84.9–87.1% with RSD less than 7.5%.
Table 3. Extraction recoveries of MA from spiked animal tissue and feed samples by IAC column cleanup (n=3).
Conclusions
By covalently coupling the anti-MA antibody on glutaraldehyde cross-linked chitosan, an improved chitosan-based immunoaffinity adsorbent can be prepared. The resulting chitosan beads were spherical and rather homogenous in size. Under optimal conditions, the maximum binding capacity of the IAC column for MA was 3900 ng/mL adsorbent, and column-to-column variability was less than 1.48%. Even after eight uses, the recovery remained over 80%. When the IAC columns were challenged with samples of animal tissue and feed, satisfactory analyses were obtained. These results suggest that the use of glutaraldehyde as cross-linking reagent produces chitosan-based immunoaffinity adsorbents with improved performance that could be used for regulatory monitoring of MA in market samples.
Acknowledgements
Gratitude is expressed to Dr Afoakwah Akowuah Newlove for a critical reading of the manuscript. This work was co-supported by Natural Science Foundation of Jiangsu Province, China (BK2008242), China Postdoctoral Science Foundation (20070420974) and PAPD of Jiangsu Higher Education Institutions.
References
- Abe, K., Nakamura, K., Arao, T., Sakurai, Y., Nakano, A., Suginuma, C., … Sasaki, K. (2011). Immunochromatography for the rapid determination of cadmium concentrations in wheat grain and eggplant. Journal of the Science of Food and Agriculture, 91, 1392–1397. 10.1002/jsfa.4321
- Basta, P. V., Adcock, A. F., Tallent, C. R., Fleming, D. N., Seltzman, H. H., Whisnant, C. C., & Cook, C. E. (2004). Preparation of monoclonal antibodies reactive to the endogenous small molecule, anandamide. Journal of Immunological Methods, 285, 181–195. 10.1016/j.jim.2003.12.004
- Clementi, M. E., Marini, S., Condò, S. G., & Giardina, B. (1991). Antibodies against small molecules. Annali dell'Istituto Superiore di Sanità, 27, 139–143.
- Chuang, J. C., Van Emon, J. M., Jones, R., Durnford, J., & Lordo, R. A. (2007). Development and application of immunoaffinity column chromatography for atrazine in complex sample media. Analytica Chimica Acta, 583, 32–39. 10.1016/j.aca.2006.09.060
- Dhananjay, S. K., & Mulimani, V. H. (2008). Evaluation of modified chitosan as matrix for hydrophobic interaction chromatography. Process Biochemistry, 43, 647–653. 10.1016/j.procbio.2008.02.010
- Heudi, O., Kilinç, T., Fontannaz, P., & Marley, E. (2006). Determination of vitamin B12 in food products and in premixes by reversed-phase high performance liquid chromatography and immunoaffinity extraction. Journal of Chromatography A, 1101, 63–68. 10.1016/j.chroma.2005.09.059
- Holtzapple, C. K., Buckley, S. A., & Stanker, L. H. (2001). Determination of fluoroquinolones in serum using an on-line clean-up column coupled to high-performance immunoaffinity-reversed-phase liquid chromatography. Journal of Chromatography B, 754, 1–9. 10.1016/S0378-4347(00)00575-2
- Joint FAO/WHO Expert Committee on Food Additives. 1988. Residues of some veterinary drugs in animals and foods. Trenbolone acetate. FAO Food and Nutrition Paper, 41, 29–37.
- Kumar, M. N. V. R. (2000). A review of chitin and chitosan applications. Reactive and Functional Polymers, 46, 1–27. 10.1016/S1381-5148(00)00038-9
- Liu, Y., Zou, H., & Haginaka, J. (2006). Preparation and evaluation of a novel chiral stationary phase based on covalently bonded chitosan for ligand-exchange chromatography. Journal of Separation Science, 29, 1440–1446. 10.1002/jssc.200600015
- Meletis, K., Meniades-Meimaroglou, S., & Markaki, P. (2007). Determination of ochratoxin A in grapes of Greek origin by immunoaffinity and high-performance liquid chromatography. Food Additives & Contaminants, 24, 1275–1282. 10.1080/02652030701413852
- Monier, M., Ayad, D. M., Wei, Y., & Sarhan, A. A. (2010). Immobilization of horseradish peroxidase on modified chitosan beads. International Journal of Biological Macromolecules, 46, 324–330. 10.1016/j.ijbiomac.2009.12.018
- Moser, A. C., & Hage, D. S. (2010). Immunoaffinity chromatography: An introduction to applications and recent developments. Bioanalysis, 2, 769–790. 10.4155/bio.10.31
- Rinaudo, M. (2006). Chitin and chitosan: Properties and applications. Progress in Polymer Science, 31, 603–632. 10.1016/j.progpolymsci.2006.06.001
- Senyuva, H. Z., & Gilbert, J. (2010). Immunoaffinity column clean-up techniques in food analysis: A review. Journal of Chromatography B, 878, 115–132. 10.1016/j.jchromb.2009.05.042
- Singla, A. K., & Chawla, M. (2001). Chitosan: Some pharmaceutical and biological aspects – An update. Journal of Pharmacy and Pharmacology, 53, 1047–1067. 10.1211/0022357011776441
- Trimukhe, K. D., Mahadik, N. D., Gokhale, D. V., & Varma, A. J. (2008). Environment friendly crosslinked chitosan as a matrix for selective adsorption and purification of lipase of Aspergillus niger. International Journal of Biological Macromolecules, 43, 422–425. 10.1016/j.ijbiomac.2008.08.005
- Wang, J. P., & Shen, J. Z. (2007). Immunoaffinity chromatography for purification of salbutamol and clenbuterol followed screening and confirmation by ELISA and GC-MS. Food and Agricultural Immunology, 18, 107–115. 10.1080/09540100701596609
- Wang, Y., Xu, Y., Zhang, X., Wang, E., & Dong, Y. (2011). Development and characterization of a chitosan-supported immunoaffinity chromatography column for the selective extraction of methandrostenolone from food and feed samples. International Journal of Biological Macromolecules, 49, 428–432. doi:10.1016/j.ijbiomac.2011.05.027
- Wang, Y., Xu, Y., Zhang, X., Wang, E., Wu, J., & Dong, Y. (2013). Development and characterization of an immunoaffinity column for the selective extraction of methandrostenolone from food and feed samples. Food and Agricultural Immunology. doi:10.1080/09540105.2012.682565
- Wang, Y., Guo, M., & Jiang, Y. (2002). Evaluation of n-valeraldehyde modified chitosan as a matrix for hydrophobic interaction chromatography. Journal of Chromatography A, 952, 79–83. 10.1016/S0021-9673(02)00081-X
- Xi, F. N., & Wu, J. M. (2006). Preparation of macroporous chitosan layer coated on silica gel and its application to affinity chromatography for trypsin inhibitor purification. Reactive and Functional Polymers, 66, 682–688. 10.1016/j.reactfunctpolym.2005.10.028
- Zhang, L., Zhang, B., Lin, H., Liu, P., Yu, L., & Wang, D. (2008). Preparation of trypsin-immobilised chitosan beads and their application to the purification of soybean trypsin inhibitor. Journal of the Science of Food and Agriculture, 88, 2332–2339. 10.1002/jsfa.3354