Abstract
This paper presents the generation of monoclonal antibodies (mAbs) against testosterone (TES) through cell fusion technology, and the development of a mAb-based indirect competitive enzyme-linked immunosorbent assay (icELISA) method to detect TES residue in bovine edible tissues. Under optimal conditions, this assay exhibited a working range of 0.04–19 ng/mL, with half-maximum inhibition (IC50) and limit of detection values of 0.27 and 0.02 ng/mL, respectively. After three sample pretreatment procedures were checked, a simple dilution method was adopted for further use. The stabilisation studies demonstrated that the icELISA kits can be stored for at least 180 to 240 days, at 4°C and −20°C, respectively. When applied to bovine samples, the data from icELISA and gas chromatography coupled to mass spectrometry showed excellent correlation (r2=0.9923 in muscle, 0.9884 in liver and 0.9957 in kidney). Therefore, this assay has the potential to be incorporated into a quantitative monitoring programme for the rapid screening of TES residue in foods.
Introduction
Testosterone (TES) is a steroid hormone from the androgen group and is found in mammals, reptiles, birds and other vertebrates (Cox & John-Alder, Citation2005). In mammals, TES is primarily secreted by the testes of males and the ovaries of females, although small amounts are also secreted by the adrenal glands. In men, TES plays a key role in the development of male reproductive tissues such as the testis and prostate and promotes development of secondary sexual characteristics such as increased muscle and bone mass and hair growth (Tehranipour & Moghimi, Citation2010). In addition, TES is essential for human health, especially for the prevention of osteoporosis (Bassil, Alkaade, & Morley, Citation2009). In people who have undergone TES deprivation therapy, TES increases beyond the castrate level have been shown to increase the rate of spread of an existing prostate cancer (Tuck & Francis, Citation2009). Nevertheless, maintaining normal TES levels in elderly men has been shown to improve many parameters that are thought to reduce cardiovascular disease risk, such as increased lean body mass, decreased visceral fat mass, decreased total cholesterol and glycolic control (Stanworth & Jones, Citation2008).
TES is known to have been administered to many athletes in order to improve their performance, and this ‘doping’ is banned in most organised sports. TES enhances muscle development, strength or endurance, by increasing protein synthesis in muscle tissue (Hartmann & Steinhart, Citation1997). As a result, muscle fibers become larger and repair faster than usual. Because exogenous TES may be used for illegal growth promotion in livestock farming, thereby causing potential health risks to consumers, regulations that prohibit the use of this anabolic steroid have been renewed or strengthened by many countries. TES and other anabolic steroids were designated ‘controlled substances’ by the United States Congress in 1990 (Lu et al., Citation2006). The use of steroids for growth-promoting purposes in animals is banned in the European Union (EU) (Directive 88/146/EEC), and the determination of residues is required in animals and in fresh meat by the EU Directive (86/849/EEC) (Lu, Kreuzer, Takkinen, & Guilbault, Citation2007).
These regulations have resulted in the development of a broad range of methods to detect this drug in biological matrices, including high-performance liquid chromatography (Choi, Kim, & Chung, Citation2003; Gonzalo-Lumbreras, Pimentel-Trapero, & Izquierdo-Hornillos, Citation2003), liquid chromatography coupled to mass spectrometric (Draisci, Palleschi, Ferretti, Lucentini, & Cammarata, Citation2000; Leinonen, Kuuranne, Kotiaho, & Kostiainen, Citation2004), gas chromatography coupled to mass spectrometric (GC-MS) (Hartmann, & Steinhart, Citation1997; Saudan, Baume, Mangin, & Saugy, Citation2004; Van Uytfanghe et al., Citation2004) and other quantitative methods. Whilst these techniques have been used as quantitative and confirmatory methods, they usually require highly skilled personnel and expensive equipment. Moreover, their pretreatment procedures involve numerous extraction steps that are time consuming and unsuitable for routine analysis of a large number of samples. Compared with these traditional chromatographic methods, immunoassays are typically portable and cost-effective, with adequate sensitivity, high selectivity and simple sample extraction procedures. Several immunoassays for the detection of TES residues in biological matrices have been developed recently (Al-Dujaili, Citation2006; Lu et al., Citation2006, Citation2007). However, there are few published immunoassay data for TES in edible bovine tissue. It was, therefore, the aim of this study to produce a monoclonal antibody (mAb) and develop and validate a sensitive competitive enzyme-linked immunosorbent assay (ELISA) for the detection of TES residue in animal tissues. Following the minimisation of sample pretreatment, we also investigated the stability of the new ELISA kits.
Materials and methods
Chemicals and materials
TES was purchased from Dr. Ehrenstorfer GmbH Company (Augsburg, Germany). Immunogen of TES-BSA and coating antigen of TES-OVA were preserved in our laboratory. Freund's complete adjuvant (FCA) and Freund's incomplete adjuvant (FIA) were obtained from Pierce (USA). The peroxidase-conjugated goat anti-mouse IgG (GaMIgG-HRP) was purchased from Sino-American Biotechnology Company (Shanghai, China). Hypoxanthine/thymidine/aminopterin (HAT) and hypoxanthine/thymidine were obtained from Sigma-Aldrich (USA). RPMI-1640 with L-glutamine was obtained from Gibco. Polyethylene glycol 1500 (PEG 1500, 50%) was from Roche Diagnostics Corporation (Indianapolis, USA). Fetal bovine serum (FBS) was from Hangzhou Sijiqing Biological Engineering Materials Co. Ltd. (Hangzhou, China). Transparent 96-well polystyrene microtiter plates (Boyang Experimental Equipment Factory, Jiangsu, China) were used for the colorimetric measurement. The buffers used were the same in our previous work (Jiang, Zhang, et al., Citation2011). A spectrophotometric microtiter reader (Multiskan MK3, Thermo company, USA), provided with a 450-nm filter, was used for absorbance measurements.
Production of monoclonal antibodies
Immunisation schedule
Five BALB/c female mice (8–10 weeks old) were immunised with TES-BSA conjugates by subcutaneous injection at multiple points. The first dose consisted of 60 µg of immunogen as an emulsion of phosphate buffered saline (PBS) and FCA. Four subsequent injections were given at three-week intervals with the same dosage of immunogen emulsified in FIA. After a resting period of at least three weeks from the last injection, mice were tail bled and selection was based on the ELISA determinations. Mouse showing the highest anti-TES activity was given an intravenous boost of immunogen without adding any adjuvant three days before spleens were removed.
Hybridoma production
Portions of the procedure were described previously (Ren, Zhang, Chen, & Yang, Citation2009) with some modifications. Briefly, splenocytes obtained from the final immunised mouse were fused with NS0 myeloma cells using PEG 1500 as a fusing agent, and then the fused cells were distributed into 96-well culture plates supplemented with HAT medium containing 15% FBS with peritoneal macrophages as feeder cells from young BALB/c mice. After 10–14 days, supernatants of hybridoma colonies were screened using a combination of noncompetitive and competitive indirect ELISA for the presence of significant TES recognition. Selected hybridomas were subcloned by limiting dilution, and colonies of interest were frozen in culture medium containing 10% dimethyl sulphoxide and cryopreserved in liquid nitrogen.
Generation and characterisation of mAb
A mature female BALB/c mouse was injected intraperitoneally (i.p.) with 0.5 mL of paraffin 10 days before receiving an i.p. injection of the positive hybridoma cells (1 to 5×106 cells). Ascites fluid was collected two weeks later and then stored at −20°C until use. Purification of mAb was performed by saturated ammonium sulfate precipitation method. mAb affinity (Ka) was measured by the method of a noncompetitive ELISA (Beatty, Beatty, & Vlahos, Citation1987).
ELISA procedures
Indirect ELISA was performed to determine the antibody titers (Le et al., Citation2009). TES-OVA were coated into a 96-well microplate (100 µL/well) for one hour at 37°C. After three washes with PBS + Tween 20 (PBST) (0.5% Tween 20), the wells were blocked with 1% BSA in PBS overnight at 4°C and washed again with PBST. Then 50 µL/well of antibody was added, and the plates were incubated for 15 minutes at 37°C. After another washing procedure, GaMIgG-HRP (50 µL/well) was added, followed by incubation for 25 minutes at 37°C. The final washing procedure was followed by adding 60 µL/well of freshly prepared substrate solution of TMB/H2O2 for 10 minutes, then the reaction was stopped by the addition of 2 M sulfuric acid (100 µL/well). The absorbance was measured at 450 nm, and the antibody titer was defined as the reciprocal of the dilution that resulted in an absorbance value that was twice higher than that of the background.
The indirect competitive ELISA (icELISA) was employed to determine the sensitivity, and the procedure was the same as the indirect ELISA except that after blocking, a competition step was introduced by adding 50 µL/well of analyte, followed by 50 µL/well of appropriate antibody. Titration checkerboard assays were performed to determine the coating antigen and antibody concentrations. With the icELISA format, analytes that do not react with the antibody would produce absorbance near 100%; conversely, analytes that do react with the antibody would decrease in percentage of absorbance. All samples were made in triplicate, and competition curves were obtained by plotting absorbance against the analyte concentrations, which were fitted to a four-parameter logistic equation.
Sample preparation for icELISA
Fresh bovine meat samples (muscle tissue, liver and kidney) from animals that had not been exposed to steroids according to the grower's log, were purchased in retail outlets in Xinxiang, China. After fat and connective tissues were removed by dissection, the samples were homogenised with a high-speed triturator, and collected in a 50 mL round-bottomed plastic flask. Two gram sample homogenate was accurately weighed into a glass centrifuge tube, and TES standard solution was added at this step. Subsequently, three different sample preparation methods were compared.
Simple dilution method
After 10 mL of methanol was added, the sample was mixed briefly and centrifuged at 5000 rpm for 10 minutes. The supernatant was separated and 2 mL of this suspension was diluted with 2 mL of distilled water, of which a 50 µL aliquot per well was pipetted into the microtiter plate for analysis.
Physicochemical method
The homogenate was mixed with 5 mL of methanol and heated in a water bath at 60°C for 15 minutes, then placed elsewhere in a freezer for temperatures below 0°C for two hours. The mixture was centrifuged at 3500 rpm for 5 minutes and this process was repeated one more time. The supernatant was extracted twice with 5 mL n-hexane to remove fat, and the aqueous methanol layer was transferred into the solid phase extraction C18 cartridges (Dalian Sipore Co., Ltd, China) pretreated with 3 mL of methanol and 3 mL of deionised water. The cartridge was then washed with 10 mL of the elution solution (n-hexane-ether [70:30, v/v]) at a flow rate of less than 0.5 mL/min. Next, the eluate was neutralised with 500 µL of 1.0 M sodium hydroxide. After centrifugation (3500 rpm, 5 min), the organic layer was collected in a tapered glass tube, and the solvents were removed under a stream of nitrogen in a water bath at 45°C. The extracts were redissolved in 20 mL of PBS with 10% methanol, and the solution was ready for ELISA detection.
Immunoaffinity chromatography (IAC) process
The sample preparation procedure was performed as a modification of that described before (Uchigashima et al., Citation2009; Wang, & Shen, Citation2007). Approximately 2 g of CNBr-activated agarose gel (Sigma, St. Louis, USA) was swollen in 4 mL of HCl (0.01 M) and equilibrated with NaHCO3 solution (0.1 M, pH 8.4). The gel was then transferred into the TES mAb solution (20 mg IgG dissolving in 10 mL of NaHCO3, 0.1 M, pH 8.4), and the mixture was gently agitated at 4°C for 24 hours to prepare the immunosorbent. The coupled gel was separated from the liquid phase by filtration, and then added to 30 mL of a blocking buffer (1 M monoethanolamine modified with 0.5 M NaCl, pH 8.0) for two hours at 25°C. After washed alternately with acetate buffer (0.1 M, pH 4.0) and Tris-HCl buffer (0.1 M, pH 8.0) for three cycles, the gel of 2 mL bed volume was transferred to a glass column (8×100 mm, i.d.). The prepared IACs were filled with PBS and used for the experiments described below. The hydrolysates collected above was mixed with 5 mL of methanol, and centrifuged at 5000 rpm for 10 minutes. The supernatant was removed by aspiration, of which 1 mL suspension was diluted with 9 mL of PBS, and then applied to the prepared IAC at a flow rate of 1 drop/s. After all of the solution had flowed through, the column was washed twice with 5 mL of PBS and 5 mL of distilled water to remove the impurities. The adsorbed TES was eluted with 5 mL of acetonitrile, the first 1 mL was retained in the column for 3 minutes, and the remaining 4 mL was flowed through. The eluate was evaporated to dryness under a stream of nitrogen in a 45°C water bath. Finally, the dry residue was dissolved in 20 mL of a 1:9 (v/v) mixture of methanol and PBS for screening by icELISA.
TES stock solution prepared in methanol at 1 mg/mL was stored at 4°C in amber glass bottles and to be used for meat spiking. For recovery studies, various concentrations of TES diluted in PBS were added into homogenised tissues to produce spiked concentrations of 2, 10 and 20 ng/g. To validate the selected method, concentration measured and concentration fortified were compared by analysing TES spiked in blank matrices of muscle, liver and kidney at six levels (0.5, 1, 2, 4, 8 and 16 ng/g).
Stabilisation evaluation
The stability character of antigen-coated plates and TES mAb were identified by freeze-thaw test. During the experiments both of them were equilibrated to room temperature for 30 minutes from a stored condition at −20°C, and then frozen for one hour. The procedure was repeated eight cycles, and the maximum absorbance (Amax) was checked each time. To estimate the effective shelf life of the icELISA kit, antigen-coated plates and TES mAb were preserved in a refrigerator at 4°C for 180 days, or frozen at −20°C for 240 days. The Amax and half-maximum inhibition concentration (IC50) values of standard curve were calculated every 30 days.
GC-MS analysis
To validate the developed icELISA, the samples were analysed by GC-MS for comparison, and the procedures were performed according to Van Uytfanghe et al. (2004). The sample extraction procedure for GC-MS analysis was the same as physicochemical pretreatment ways. In our optimised methods, enzymatic hydrolysis with β–glucuronidase from E.coli was performed in acetate buffer (pH 5.2, 0.2 mol/L). Next, the homogenate was mixed with methanol and heated at 60°C for 15 minutes, then placed in an ice bath at −18°C for two hours. After liquid-liquid extraction with n-hexane, the analytes were subjected to a normal-phase SPE C18 cartridge for clean-up. The dried organic extracts were derivatised with Heptafluorobutyric anhydride (Alfa Aesar, USA), and then the products were injected into GC-MS.
Results and discussion
mAbs production and characterisation
Twelve days after the fusion, growing hybridoma clones could be observed in many wells of the culture plates. The fusion rate of the mouse spleen cells with myeloma cells was about 82%. Supernatants of all wells were screened by simultaneous noncompetitive and competitive assays, and the positive well rate was 36%. Selection of clones from these positive cultures by limiting dilution led to nine stable hybridoma cell lines, in which clone T2B9 was the best. The protein concentrations of all mAbs were measured to be 2.8–4.6 mg/mL. The affinity of an antibody for its corresponding antigen is crucial parameters affecting the performance of an immunoassay, and high-affinity antibodies can lower the dissociation tendency of the antigen/antibody complex and produce sensitive IC50 values. In our study, the affinity constants (Kas) of nine hybridomas were between 4.2×109–2.7×1010 L/mol (data not shown). According to James (Citation1983), the nine hybridomas all produced high-affinity mAbs (between 107 and 1012 L/mol).
Establishment of icELISA standard curve
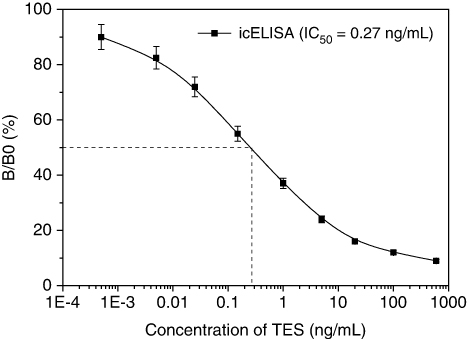
To ascertain the applicability of T2B9 mAb generated in this study, icELISA standard curve was developed. It is well known that working concentrations of antibody and coating antigen are crucial factors for the sensitivity of ELISA methods (Li et al., Citation2008). For this reason, checkerboard titrations were performed, taking into account the optimal dilutions. The optimal reagent concentrations were determined when the Amax was between 1.5 and 2.0, and the dose-response curve of inhibition ratio versus the TES concentration pursued the lowest IC50 values. From the checkerboard assays, the optimum concentration of coating antigen was 1.0 µg/mL and mAb was 0.4 µg/mL (1:10,000 dilutions). Based on the results of the checkerboard titration, a representative standard curve with icELISA format is shown in . The competitive curve allowed the detection of TES (20–80% inhibition rate) from 0.04 to 19 ng/mL, with an IC50 value of 0.27 ng/mL. The limit of detection value of the assay, which is represented by IC15 value (Jiang, Wang, et al., Citation2011), was calculated to be 0.02 ng/mL. The mAb-based icELISA had high sensitivity, with an IC50 about one order of magnitude lower than that of published ELISAs, which had IC50 values ranging from 2.8 to 74 ng/mL (Al-Dujaili, Citation2006; Lu et al., Citation2006, Citation2007).
Sample pretreatment selection
In food samples, one of the most common challenges of immunosorbent assay is matrix interference (Sheng et al., Citation2009). Immunoassays often have a high potential for nonspecific binding to nontarget analytes, which would reduce the precision and accuracy of the analysis. Chemical substances present in sample or sample extracts, such as solvents, fat, salt and other compounds, might affect the binding between the antibody and analyte, reduce the sensitivity of the immunosorbent assay and lower the extent of colour development (Jin et al., Citation2008). So removing the matrix effects is important in the ELISA study. In this study, three pretreatment procedures were explored to reduce the matrix interferences, and the method effectiveness was assessed through spiking experiments. When the volume of the solution was adjusted accordingly, the spiked concentrations of 2, 10 and 20 ng/g corresponded to the concentrations in assay buffer of 0.2, 1.0 and 2.0 ng/mL, respectively. After diluting, these fortification levels entered into the dynamic range of the calibration curve, and favorable recoveries were obtained. At two higher spiked concentrations, the recoveries of TES were in the range of 88.7–126.5%, 88.2–110.4% and 99.5–117.4% for dilution method, physicochemical method and IAC process, respectively. The coefficients of variation (CV) values were all greater than 15%. When the lower spiked level was checked, the CVs obtained from IAC process were obviously higher than that of the other two methods. The explanation may be that the lower concentrations of TES are close to the margin of the elution range of the IAC column. These results proved the suitability of three preparation methods for assaying TES in bovine muscle. But in practice, it was not convenient to incorporate the ELISA kits with SPE cartridges or IAC columns. On the other hand, dilution method employed in this study is easy enough and can be performed in any laboratory with basic equipments. Therefore, it was preferred to apply in sample analysis.
Dilution method verification
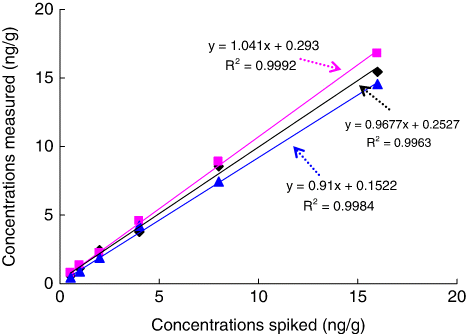
Dilution is an often used process to reduce the matrix interferences, but dilution of samples causes reduction of the sensitivity, due to shifting of the dynamic range. Therefore, dilution method for reducing the matrix effect is applicable only when the assay sensitivity is sufficient. Fortunately, our icELISA method was still sensitive enough after 10-fold dilution of extracts with PBS, and the concentration spiked (0.5, 1, 2, 4, 8 and 16 ng/g) was still within the range of the assay. A correlation between measured and fortified concentrations in fresh meat samples of bovine (muscle tissue, liver and kidney) were calculated, and correlation indices (recovered spike) are detailed in . It indicated that there was a little tendency to overestimate spiked concentrations in liver samples and underestimate in kidney samples. But in general, the statistical comparison showed no significant differences between spiked and determined concentrations in these matrices, and the correlation values were excellent. This means the joint use of dilution method and the optimised icELISA is a very promising tool for the food industry and regulatory agencies.
Freeze-thaw test
The stability of antigen-coated plates and TES mAb was checked. During eight freeze-thaw cycles the activity of antigen-coated plates was stable, while the binding ability of TES mAb decreased slightly. The fluctuation was acceptable, and we therefore recommend a limit of eight freeze/thaws for the plates coated with TES-OVA and for the mAbs.
Preservation period of icELISA kits
For icELISA kits, the storage conditions are a critical factor. It is possible that the reason for this is that the antibody easily loses its activity during storage. Two simple storage schedules (4°C and −20°C in refrigerator) were studied and the results are depicted in . It can be seen that the two methods showed no obvious effects on the Amax and IC50 values during the storage periods. On 180 day, the coated plates in 4°C had a little lower Amax than that in −20°C, suggesting that the antibody titers dropped slightly. The results indicate that the icELISA kits could be effective for at least 180 and 240 days, at 4°C and −20°C in storage, respectively.
Table 1. Influence of storage time on icELISA standard curve under 4°C and −20°C.
Correlation between icELISA and GC-MS analysis
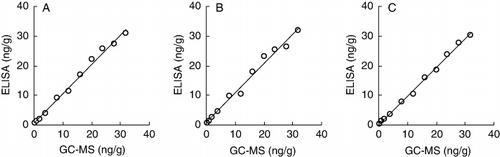
displays the performance of icELISA in comparison with the confirmatory GC-MS method. The linear regressions show excellent correlation of the data-sets for icELISA and GC-MS data, with R2=0.9923 in muscle, 0.9884 in liver and 0.9957 in kidney.
Conclusions
In summary, we successfully generated hybridomas that stably produced TES-specific mAbs and developed the icELISA standard curve. Following simple sample preparation, the assay accuracy was validated, and satisfied recoveries were observed in bovine edible tissues. Therefore, this icELISA can be used as a screening method for detecting TES residue in bovine tissues, and provides a practical advantage over methods requiring a tedious sample cleanup procedure.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. U1204310) and the Key Scientific & Technological Project of Education Department in Henan Province of China (Grant No. 2011A230003).
References
- Al-Dujaili, E. A. (2006). Development and validation of a simple and direct ELISA method for the determination of conjugated (glucuronide) and non-conjugated testosterone excretion in urine. Clinica Chimica Acta, 364, 172–179. doi:10.1016/j.cccn.2005.06.019
- Bassil, N., Alkaade, S., & Morley, J. E. (2009). The benefits and risks of testosterone replacement therapy: A review. Therapeutics & Clinical Risk Management, 5, 427–448. Retrieved from http://dx.doi.org/10.2147/TCRM.S3025.
- Beatty, J. D., Beatty, B. G., & Vlahos, W. G. (1987). Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. Journal of Immunological Methods, 100, 173–179. doi:10.1016/0022-1759(87)90187-6
- Choi, M. H., Kim, J. N., & Chung, B. C. (2003). HPLC-electrospray tandem mass spectrometric assay for urinary testosterone and dihydrotestosterone glucuronides from patients with benign prostate hyperplasia. Clinical Chemistry, 49, 322–325. doi:10.1373/49.2.322
- Cox, R. M., & John-Alder, H. B. (2005). Testosterone has opposite effects on male growth in lizards (Sceloporus spp.) with opposite patterns of sexual size dimorphism. Journal of Experimental Biology, 208, 4679–4687. doi:10.1242/jeb.01948
- Draisci, R., Palleschi, L., Ferretti, E., Lucentini, L., & Cammarata, P. (2000). Quantitation of anabolic hormones and their metabolites in bovine serum and urine by liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 870, 511–522. doi:10.1016/S0021-9673(99)01293-5
- Gonzalo-Lumbreras, R., Pimentel-Trapero, D., & Izquierdo-Hornillos, R. (2003). Development and method validation for testosterone and epitestosterone in human urine samples by liquid chromatography applications. Journal of Chromatographic Science, 41, 261–265. doi:10.1093/chromsci/41.5.261
- Hartmann, S., & Steinhart, H. (1997). Simultaneous determination of anabolic and catabolic steroid hormones in meat by gas chromatography-mass spectrometry. Journal of Chromatography B: Biomedical Sciences and Applications, 704, 105–117. doi:10.1016/S0378-4347(97)00460-X
- James, W. G. (1983). Monoclonal antibodies: Principles and practice, New York, NY: Academic Press.
- Jiang, J., Zhang, H., Li, G., Wang, Z., Wang, J., & Zhao, H. (2011). Preparation of anti-nortestosterone antibodies and development of an indirect heterologous competitive enzyme-Linked immunosorbent assay to detect nortestosterone residues in animal urine. Analytical Letters, 44, 2373–2393. doi:10.1080/00032719.2010.551694
- Jiang, J., Wang, Z., Zhang, H., Zhang, X., Liu, X., & Wang, S. (2011). Monoclonal antibody-based ELISA and colloidal gold immunoassay for detecting 19-nortestosterone residue in animal tissues. Journal of Agricultural and Food Chemistry, 59, 9763–9769. doi:10.1021/jf2012437
- Jin, R. Y., Gui, W. J., Guo, Y. R., Wang, C. M., Wu, J. X., & Zhu, G. N. (2008). Comparison of monoclonal antibody-based ELISA for triazophos between the indirect and direct formats. Food and Agricultural Immunology, 19, 49–60. doi:10.1080/09540100801933454
- Le, T., Yu, H., Guo, Y. C., Ngom, B., Shen, Y. A., & Bi, D. (2009). Development of an indirect competitive ELISA for the detection of doxycycline residue in animal edible tissues. Food and Agricultural Immunology, 20, 111–124. doi:10.1080/09540100902849740
- Leinonen, A., Kuuranne, T., Kotiaho, T., & Kostiainen, R. (2004). Screening of free 17-alkyl-substituted anabolic steroids in human urine by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids, 69, 101–109. doi:10.1016/j.steroids.2003.10.007
- Li, Y. L., Ji, B. Q., Chen, W., Liu, L. Q., Xu, C. L., Peng, C. F., & Wang, L. B. (2008). Production of new class-specific polyclonal antibody for determination of fluoroquinolone antibiotics by indirect competitive ELISA. Food and Agricultural Immunology, 19, 251–264. doi:10.1080/09540100802471538
- Lu, H. H., Conneely, G., Crowe, M. A., Aherne, M., Pravda, M., & Guilbault, G. G. (2006). Screening for testosterone, methyltestosterone, 19-nortestosterone residues and their metabolites in bovine urine with enzyme-linked immunosorbent assay (ELISA). Analytica Chimica Acta, 570, 116–123. doi:10.1016/j.aca.2006.03.108
- Lu, H., Kreuzer, M. P., Takkinen, K., & Guilbault, G. G. (2007). A recombinant fab fragment-based electrochemical immunosensor for the determination of testosterone in bovine urine. Biosensors and Bioelectronics, 22, 1756–1763. doi:10.1016/j.bios.2006.08.002
- Ren, X. F., Zhang, F. M., Chen, F. J., & Yang, T. B. (2009). Development of a sensitive monoclonal antibody-based ELISA for the detection of clenbuterol in animal tissues. Food and Agricultural Immunology, 20, 333–344. doi:10.1080/09540100903365852
- Saudan, C., Baume, N., Mangin, P., & Saugy, M. (2004). Urinary analysis of 16(5alpha)-androsten-3alpha-ol by gas chromatography/combustion/isotope ratio mass spectrometry: implications in anti-doping analysis. Journal of Chromatography B, 810, 157–164. Retrieved from http://dx.doi.org/10.1016/j.jchromb.2004.07.029.
- Sheng, W., Xu, T., Ma, H. X., Wang, X. T., Li, Q. X., & Li, J. (2009). Development of an indirect competitive enzyme-linked immunosorbent assay for detection of danofloxacin residues in beef, chicken and pork meats. Food and Agricultural Immunology, 20, 35–47. doi:10.1080/09540100802657581
- Stanworth, R. D., & Jones, T. H. (2008). Testosterone for the aging male; current evidence and recommended practice. Clinical Interventions in Aging, 3, 25–44. Retrieved from http://www.dovepress.com/articles.php?article_id=227.
- Tehranipour, M., & Moghimi, A. (2010). Neuroprotective effects of testosterone on regenerating spinal cord motoneurons in rats. Journal of Motor Behavior, 42, 151–155. doi:10.1080/00222891003697921
- Tuck, S. P., & Francis, R. M. (2009). Testosterone, bone and osteoporosis. Frontiers of Hormone Research, 37, 123–132. doi:10.1159/000176049
- Uchigashima, M., Saigusa, M., Yamashita, H., Miyake, S., Fujita, K., Nakajima, M., Nishijima, M. (2009). Development of a novel immunoaffinity column for aflatoxin analysis using an organic solvent-tolerant monoclonal antibody. Journal of Agricultural and Food Chemistry, 57, 8728–8734. doi:10.1021/jf901826a
- Van Uytfanghe, K., Stöckl, D., Kaufman, J. M., Fiers, T., Ross, H. A., De Leenheer, A. P., & Thienpont, L. M. (2004). Evaluation of a candidate reference measurement procedure for serum free testosterone based on ultrafiltration and isotope dilution-gas chromatography-mass spectrometry. Clinical Chemistry, 50, 2101–2110. doi:10.1373/clinchem.2004.037358
- Wang, J. P., & Shen, J. Z. (2007). Immunoaffinity chromatography for purification of Salbutamol and Clenbuterol followed screening and confirmation by ELISA and GC-MS. Food and Agricultural Immunology, 18, 107–115. doi:10.1080/09540100701596609