Abstract
The present study investigated the effects of selenium status on allergy in vivo. A total of 20 NC/Nga mice or 20 BALB/c mice were randomly assigned to one of four treatment groups and given a diet containing 0, 1, 2 or 3 µg/g selenomethionine. Their allergic responses were subsequently evaluated. Spontaneous dermatitis was inhibited in NC/Nga mice given a diet without selenomethionine, enhanced in mice given a diet containing 1 µg/g selenomethionine and suppressed in mice given a diet containing 3 µg/g selenomethionine. Further, active cutaneous anaphylaxis, a type-I allergic response of BALB/c mice, was inhibited in mice given a diet without selenomethionine, enhanced in mice given a diet containing 1 µg/g selenomethionine and suppressed in mice given a diet containing 3 µg/g selenomethionine. Allergies seem to be aggravated in the presence of a slight selenium deficiency, but abrogated when the diet is sufficient in, or supplemented with, selenomethionine.
Keywords:
Introduction
An increase in allergic response can be problematic, hence the epidemiological factors of allergy should be identified so that the cause of the allergy can be removed and the allergic response prevented. One of the main predisposing factors for allergy is the presence of an allergen; however, eliminating all allergens is an unlikely prospect. Individual differences in the sensitivity to allergens and allergy onset suggest that host/environmental factors including diet and nutritional status contribute significantly to allergic responses. Allergy or inflammation is enhanced by oxidative stress (Omata et al., Citation2001; Tsukahara et al., Citation2003), and selenium can reduce oxidative stress by inducing the production of glutathione peroxidase or other antioxidant enzymes (Moriarty, Reddy, & Maquat, Citation1998; Okuno, Motobayashi, Ueno, & Nakamuro, Citation2006; Perchellet, Abney, Thomas, Guislain, & Perchellet, Citation1987; Wingler, Böcher, Flohé, Kollmus, & Brigelius-Flohé, Citation1999). To date, only a few studies have investigated the relationship between allergies and the modification of redox status by selenium compounds. We previously found that selenite promotes the proliferation of lymphocytes in vitro (Ueno, Hasegawa, Ido, Okuno, & Nakamuro, Citation2008; Ueno, Kajihara, Nakamura, Yodoi, & Nakamuro, Citation2007) and reported that selenomethionine, a selenium compound usually found in vegetables, reduced oxidative stress in the pancreas and improved glucose tolerance in a mouse model of diabetes (Shimizu, Okuno, Sakazaki, Nakamuro, & Ueno, Citation2010; Shimizu, Ueno, Okuno, Sakazaki, & Nakamuro, Citation2009). Patients infected by the human immunodeficiency virus (HIV) (Hurwitz et al., Citation2007; Kamwesiga et al., Citation2011) and patients with cancer (Cheng et al., Citation2012) tend to have a low level of selenium. These observations suggest that selenium deficiency suppresses immune functions including antiviral and anticancer activities. Furthermore, epidemiological reports have shown controversial results concerning the relationship between selenium intake and allergies in children (Kamer et al., Citation2012; Thomson et al., Citation2012). However, the influence of selenium compounds on mouse models of allergy has not yet been evaluated in vivo.
The NC/Nga mouse model develops dermatitis under conventional conditions, and the symptoms resemble atopic dermatitis of humans; the NC/Nga mouse is therefore used for the investigation of allergic dermatitis (Jin, He, Oyoshi, & Geha, Citation2009). For type-I allergy models, passive cutaneous anaphylaxis (PCA) and active cutaneous anaphylaxis (ACA) are used (Inagaki, Miura, Nagai, & Koda, Citation1992; McCamish & Benedict, Citation1963). In PCA experiments, immunoglobulin E is artificially administered and histamine release from mast cells is examined, while in ACA experiments immunoglobulin E secretion from B lymphocytes and histamine release from mast cells are both investigated.
The present study investigated the effects of selenium status on spontaneous dermatitis in NC/Nga mice, and on ACA in BALB/c mice, a strain that is frequently used in immunological research.
Methods
Common procedures for all animal treatments
The experimental protocol of our study was in accordance with the animal experiment guidelines of Setsunan University, Japan. These guidelines were adapted from the guidelines of the Japanese Society for Pharmacology. The Committee for the Ethical Use of Experimental Animals of Setsunan University approved the study. Efforts were made to minimise animal suffering as much as possible by reducing the number of animals used in experimentation and using alternatives for in vivo techniques.
A total of 20 mice of each strain were housed five per cage and allowed one week for acclimation while fed a normal diet (MF Certified Diet, Oriental Yeast Co., Ltd.) ad libitum with full access to water. After acclimation, the mice were randomly assigned to one of four treatment groups as follows: (1) selenomethionine-deficient diet without selenomethionine, (2) selenomethionine-deficient diet supplemented with 1 µg/g selenomethionine, (3) selenomethionine-deficient diet supplemented with 2 µg/g selenomethionine and (4) selenomethionine-deficient diet supplemented with 3 µg/g selenomethionine. The mice were kept in a pathogen-free room maintained on a 12-h light–dark cycle (lights on at 7:00 am), at an ambient temperature of 23° ± 1°C and a humidity level of 47–67%. All mice were allowed ad libitum access to water and to their respective diets.
Selenium-deficient feed, which was purchased from Oriental Yeast Co., Ltd. (Tokyo, Japan) contained 30% torula yeast (KR yeast, Kohjin Life Sciences Co., Ltd., Tokyo, Japan), 17% α-corn starch, 23.7% β-corn starch, 10% sucrose, 13% lard, 5% mineral mixture, 0.85% vitamin mixture, 0.30% dl-methionine and 0.15% choline chloride, as described previously (Shimizu et al., Citation2009, Citation2010). To prepare the selenomethionine-supplemented feed, approximately 25 g of the powdered feed was supplemented with 0–3 µg/g selenomethionine (Sigma-Aldrich, St. Louis, MO, USA). To achieve this, 1 mL of a 0–75 mg/mL selenomethionine aqueous solution was added to 25 g of selenium-deficient diet in covered feed boxes (Roden CAFÉ, Oriental Yeast Co., Ltd.). The dose of selenomethionine was calculated in accordance with the selenium content of normal feed and the mean intake of that feed (Shimizu et al., Citation2009, Citation2010). Briefly, in these studies, the normal MF diet contained 0.4 µg of selenium (equivalent to 0.99 µg selenomethionine) per gram of diet, and the mean intake of an Institute of Cancer Research (ICR) mouse (a commonly used outbred mouse strain) fed that diet was 4.7 g per day.
Spontaneous dermatitis in NC/Nga mice
Conventional three-week-old female NC/Nga mice weighing 18–20 g (Japan SLC Co., Shizuoka, Japan) received one of the four diets described above for a period of five weeks. The degree of dermatitis observed in the mice was scored depending on the severity according to a previous report (Hirasawa et al., Citation2004) with some modifications, as follows: (1) presence of slight blushing or minor hair loss; (2) presence of blushing or hair loss; (3) presence of a wide area of blushing, or of blushing plus hair loss; (4) presence of a skin lesion; and (5) presence of bleeding. After the five-week intervention period, we anaesthetised the mice by intraperitoneal administration of 0.1 mL of physiological saline with 10 mg/mL sodium pentobarbital (approximately 50 mg/kg body weight, 1 mg/mouse) and collected the plasma, spleen, thymus, mesenteric lymph nodes and back skin for evaluation of the selenium content.
ACA
Three-week-old female BALB/c mice weighing 18–20 g (Japan SLC Co.) were fed one of the four diets described above for a period of four weeks before we attempted to elicit an ACA response with subcutaneous administration of 1 µg ovalbumin (OVA; Sigma-Aldrich) and 1 mg alum (Sigma-Aldrich). This treatment was repeated one week later for additional sensitisation. After a further week (two weeks after the initial administration of OVA and alum), the mice were anaesthetised by intraperitoneal administration of 0.1 mL of physiological saline with 10 mg/mL sodium pentobarbital (approximately 50 mg/kg body weight, 1 mg/mouse), 250 µL of 0.5% Evan's blue solution was injected into the tail vein and 10 µL of 0.1 mg/mL OVA was injected subcutaneously into the right ear lobe. To measure the exudate of Evan's blue, the ear lobes of the mice were excised 30 min post-injection, incubated overnight in 0.3 mL of 1 mol/L KOH (potassium hydroxide) solution at 37°C, neutralised with 10 mol/L phosphoric acid–acetone (3:67) solution (0.7 mL) and centrifuged at 15,000g for 10 min at room temperature. The absorbance of the supernatant was measured at 620 nm (Inagaki et al., Citation1992).
Selenium determination
Approximately 0.1 mL of plasma and 0.1 g of organ tissue were added to 3 mL mixed acid solution (HNO3/HClO4, 2:1), incinerated and the quantity of inorganic selenite was determined using a fluorometric method with diaminonaphthalene (Walkinson, Citation1966).
Statistical analysis
We calculated the mean ± standard deviation (SD) of values for four or five mice in each group and compared differences between the means using one-way analysis of variance (ANOVA) and t-tests with Bonferroni correction. We calculated the mean ± standard error (SE) of values for four or five mice in each group for the inflammation score. Differences in inflammation scores and dye exudation of ACA were analysed using Mann–Whitney tests. P values <0.05 were considered statistically significant.
Results
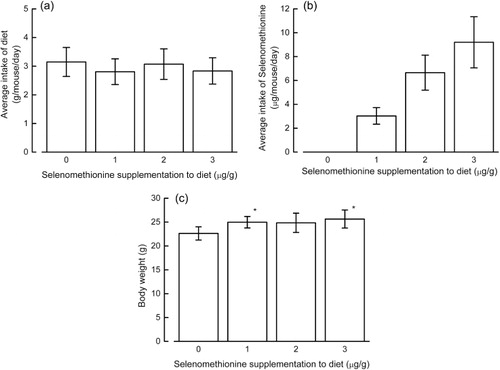
The average food intake and the estimated amount of selenium ingested by mice are shown in . The body mass increase in NC/Nga mice fed a diet without selenomethionine was lower than that in mice consuming any of the diets supplemented with selenomethionine, although this was not statistically significant (). We did not find an increase in urine excretion, a symptom of diabetes, in the present study, although it has previously been observed in streptozotocin-administered Nagoya-Shibata-Yasuda (NSY) mice fed different selenium-deficient diets (Shimizu et al., Citation2009; data not shown).
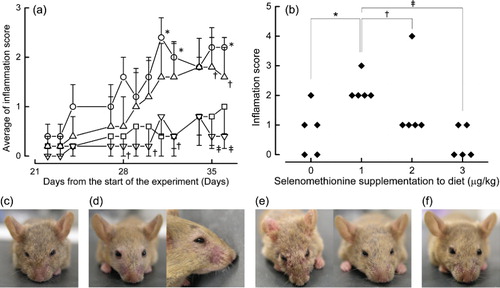
We have presented the degree of dermatitis as an inflammation score (, panels a and b). Mice given a diet without selenomethionine experienced slight hair loss, but did not have any signs of bleeding. Mice given a diet containing 1 µg/g selenomethionine experienced hair loss, bleeding and crust formation. Mice given a diet containing 2 µg/g selenomethionine experienced hair loss, but there were considerable differences between the individual mice, and mice given a diet containing 3 µg/g selenomethionine did not have hair loss or bleeding. The appearance of mice in the different treatment groups is shown in (panels c–f). The graph showing the relationship between selenomethionine dose and inflammation score had a reverse-U-shaped curve ().
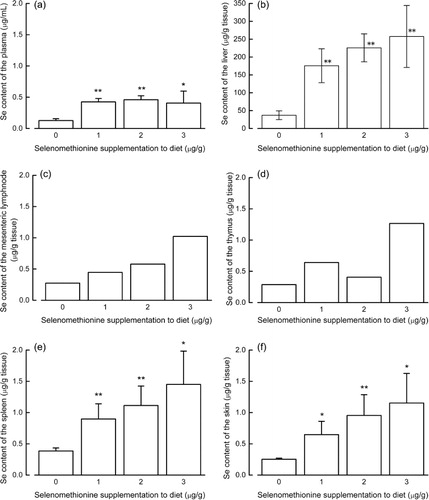
The plasma selenium level in mice given a diet without selenomethionine was significantly lower than that in mice fed a diet containing 1–3 µg/g selenomethionine, but it did not differ significantly between mice fed diets with 1, 2 or 3 µg/g selenomethionine (). Selenium levels in the liver, spleen and skin were proportional to the amount of selenium provided to the mice (). Because the selenium levels for both the mesenteric lymph nodes and the thymus were determined from a single pooled sample for each treatment group, it was not possible to perform a statistical analysis of these data. Nonetheless, we observed that the selenium level in the mesenteric lymph nodes tended to depend on selenium intake, whilst the selenium level in the thymus did not show such a tendency.
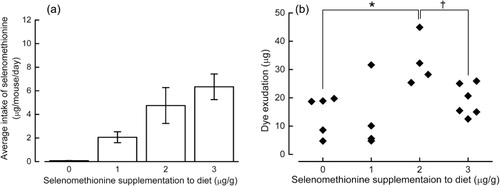
Because BALB/c mice ate less than NC/Nga mice (probably due to a strain difference), their intake of selenomethionine was less than that of NC/Nga mice (). Assessment of ACA revealed that the dye exudate, which indicates the permeability of blood vessels at a site of inflammation, was reduced in mice given a diet containing 0, 1 or 3 µg/g selenomethionine, whereas it was enhanced in mice given a diet containing 2 µg/g selenomethionine ().
Discussion
In humans, atopic dermatitis is a type-I allergy that is aggravated by oxidative stress (Omata et al., Citation2001; Tsukahara et al., Citation2003). Selenium compounds are known to reduce oxidative stress (Moriarty et al., Citation1998; Okuno, Kawai, Hasegawa, Ueno, & Nakamuro, Citation2001; Perchellet et al., Citation1987), but their role in allergic reactions is not well understood.
The present study investigated the effects of selenium deficiency and selenomethionine supplementation in two mouse models of allergy; NC/Nga mice spontaneously develop dermatitis and therefore serve as a model of atopic dermatitis, whereas ACA is an experimental mouse model of type-I allergy ACA which was induced in BALB/c mice, a strain that is commonly used in immunological research.
Severe selenium deficiency induced by a diet without selenomethionine caused inhibition of spontaneous dermatitis in NC/Nga mice, and of ACA in BALB/c mice. The body mass increase in mice given a diet without selenomethionine was less than that in mice given a diet with selenomethionine. These mice also had dull fur.
Our findings do not indicate that selenium deficiency can cure allergy, rather they indicate that severe selenium deficiency causes immunodeficiency. Our previous studies showed that lymphocyte proliferation in response to concanavalin A is enhanced when the culture medium is supplemented with selenite (Ueno et al., Citation2007, Citation2008). In vivo, a lack of selenium would inhibit the lymphocyte proliferation that is necessary for allergy, whilst selenomethionine supplementation is likely to enable this proliferation to recover. Delayed-type dermatitis, a type-IV allergy, is also inhibited in selenium-deficient humans (Hawkes, Hwang, & Alkan, Citation2009).
Although NC/Nga and BALB/c mice were fed different amounts of selenomethionine, BALB/c mice eat less than NC/Nga mice, and thus the two experiments represent similar dose dependencies.
In the NC/Nga mice fed with conventional food, the onset of spontaneous lesions occurred at eight weeks of age as previously described (Jin et al., Citation2009; Yamaguchi et al., Citation2001; data not shown). We found that the onset of spontaneous dermatitis was hastened in NC/Nga mice that became slightly selenium-deficient (diet with 1 µg/g selenomethionine), and this was retarded in mice provided a diet supplemented with selenomethionine to a level similar to that of mice provided with the conventional diet (diet with 3 µg/g selenomethionine). In addition, ACA was enhanced in BALB/c mice that were slightly selenium-deficient (diet with 2 µg/g selenomethionine), but reduced in selenomethionine-sufficient mice (diet with 3 µg/g selenomethionine). Selenite and selenomethionine moderate oxidative stress by inducing the expression of antioxidant enzymes, including glutathione peroxidase (Okuno et al., Citation2006; Shimizu et al., Citation2010). Thus, inflammation in spontaneous dermatitis and ACA may be moderated by selenomethionine because it regulates oxidative stress.
A selenomethionine-induced reduction in oxidative stress was previously illustrated in a mouse model of streptozotocin-induced diabetes; streptozotocin administration caused an increase in oxidised nucleotides in the pancreas (Shimizu et al., Citation2010). However, evaluation of oxidative stress by measuring glutathione levels was not successful, and assessing oxidative stress in vivo is difficult (Shimizu et al., Citation2009). The present study does not offer any direct evidence of changes in oxidative stress, and hence further investigations are warranted.
In conclusion, our results suggest the following: (1) severe selenium deficiency can cause immunodeficiencies, (2) a mild selenium deficiency can exacerbate allergy and (3) dietary supplementation with selenomethionine can abrogate allergy.
References
- Cheng, W.-H., Holmstrom, A., Li, X., Wu, R. T. Y., Zeng, H., & Xiao, Z. (2012). Effect of dietary selenium and cancer cell xenograft on peripheral T and B lymphocytes in adult nude mice. Biological Trace Element Research, 146, 230–235. doi:10.1007/s12011-011-9235-2
- Hawkes, W. C., Hwang, A., & Alkan, Z. (2009). The effect of selenium supplementation on DTH skin responses in healthy North American men. Journal of Trace Elements in Medicine and Biology, 23, 272–280. doi:10.1016/j.jtemb.2009.04.002
- Hirasawa, Y., Ori, K., Yamada, T., Ohtsu, S., Matsui, Y., Miwa, Y., … Higo, S. (2004). Anti-allergic action effect of Pseudolarix amabilis Rehd. extract and its efficacy on atopic dermatitis. Folia Phamacol. Jpn, 124, 271–283. doi:10.1254/fpj.124.271
- Hurwitz, B. E., Klaus, J. R., Llabre, M. M., Gonzalez, A., Lawrence, P. J., Maher, K. J., … Schneiderman, N. (2007). Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: A randomized controlled trial. Archives of Internal Medicine, 167(2), 148–154. doi:10.1001/archinte.167.2.148
- Inagaki, N., Miura, T., Nagai, H., & Koda, A. (1992). Active cutaneous anaphylaxis (ACA) in the mouse ear. The Japanese Journal of Pharmacology, 59, 201–208. doi:10.1254/jjp.59.201
- Jin, H., He, R., Oyoshi, M., & Geha, R. S. (2009). Animal models of atopic dermatitis. Journal of Investigative Dermatology, 129, 31–40. doi:10.1038/jid.2008.106
- Kamer, B., Wąsowicz, W., Pyziak, K., Kamer-Bartosińska, A., Gromadzińska, J., … Pasowska, R. (2012). Role of selenium and zinc in the pathogenesis of food allergy in infants and young children. Archives of Medical Science, 8, 1083–1088. doi:10.5114/aoms.2012.32420
- Kamwesiga, J., Mutabazi, V., Kayumba, J., Tayari, J.-C. K., Smyth, R., Fay, H., … Warren, D. (2011). Effect of selenium supplementation on CD4 T-cell recovery, viral suppression, morbidity and quality of life of HIV-infected patients in Rwanda: Study protocol for a randomized controlled trial. Trials, 12, 192. doi:10.1186/1745-6215-12-192
- McCamish, J., & Benedict, A. A. (1963). Studies on immediate cutaneous hypersensitivity in mice: I. Active cutaneous hypersensitivity. Journal of Immunology, 91, 651–657. Retrieved from http://www.jimmunol.org/content/91/5/651.abstract
- Moriarty, P. M., Reddy, C. C., & Maquat, L. E. (1998). Selenium deficiency reduces the abundance of mRNA for Se-dependent glutathione peroxidase 1 by a UGA-dependent mechanism likely to be nonsense codon-mediated decay of cytoplasmic mRNA. Molecular and Cellular Biology, 18, 2932–2939. Retrieved from http://mcb.asm.org/content/18/5/2932.full
- Okuno, T., Kawai, H., Hasegawa, T., Ueno, H., & Nakamuro, K. (2001). Enhancement of hydroxyl radical formation from superoxide anion radical in the presence of organic selenium compounds. Journal of Health Science, 47, 240–247. doi:10.1248/jhs.47.240
- Okuno, T., Motobayashi, S., Ueno, H., & Nakamuro, K. (2006). Cystathionine γ-lyase contributes to seleno methionine detoxification and cytosolic glutathione per-oxidase biosynthesis in mouse liver. Biological Trace Element Research, 109, 155–171. doi:10.1385/BTER:109:2:155
- Omata, N., Tsukahara, H., Ito, S., Ohshima, Y., Yasutomi, M., Yamada, A., … Mayumi, M. (2001). Increased oxidative stress in childhood atopic dermatitis. Life Sciences, 69, 223–228. doi:10.1016/S0024-3205(01)01124-9
- Perchellet, J.-P., Abney, N. L., Thomas, R. M., Guislain, Y. I., & Perchellet, E. M. (1987). Effects of combined treatments with selenium, glutathione, and vitamin E on glutathione peroxidase activity, ornithine decarboxylase induction, and complete and multistage carcinogenesis in mouse skin. Cancer Research, 47, 477–485. Retrieved from http://cancerres.aacrjournals.org/content/47/2/477
- Shimizu, R., Okuno, T., Sakazaki, F., Nakamuro, K., & Ueno, H. (2010, May 31–Jun. 04). Effect of sodium selenite supplementation on glucose intolerance in type 2 diabetic mice under different selenium status. Paper presented at the 9th International Symposium on Selenium in Biology and Medicine (Selenium 2010) Kyoto, Japan.
- Shimizu, R., Ueno, H., Okuno, T., Sakazaki, F., & Nakamuro, K. (2009). Effect of sodium selenite supplementation on glucose intolerance and pancreatic oxidative stress in type 2 diebetic mice under different selenium status. Journal of Health Science, 55, 271–280. doi:10.1248/jhs.55.271
- Thomson, C. D., Wickens, K., Miller, J., Ingham, T., Lampshire, P., Epton, M. J., … Year six New Zealand Asthma and Allergy Cohort Study Group (NZAACS6). (2012). Selenium status and allergic disease in a cohort of New Zealand children. Clinical & Experimental Allergy, 42, 560–567. doi:10.1111/j.1365-2222.2012.03924.x
- Tsukahara, H., Shibata, R., Ohshima, Y., Todoroki, Y., Sato, S., Ohta, N., … Mayumi, M. (2003). Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sciences, 72, 2509–2516. doi:10.1016/S0024-3205(03)00145-0
- Ueno, H., Hasegawa, G., Ido, R., Okuno, T., & Nakamuro, K. (2008). Effects of selenium status and supplementary seleno-chemical sources on mouse T-cell mitogenesis. Journal of Trace Elements in Medicine and Biology, 22(1), 9–16. doi:10.1016/j.jtemb.2007.10.002
- Ueno, H., Kajihara, H., Nakamura, H., Yodoi, J., & Nakamuro, K. (2007). Contribution of thioredoxin reductase to T-cell mitogenesis and NF-kB DNA-binding promoted by selenite. Antioxidants and Redox Signaling, 9(1), 115–121. doi:10.1089/ars.2007.9.115
- Walkinson, J. H. (1966). Fluorometric determination of selenium in biological material with 2,3-diaminonaphthalene. Analytical Chemistry, 38(1), 92–97. doi:10.1021/ac60233a025
- Wingler, K., Böcher, M., Flohé, L., Kollmus, H., & Brigelius-Flohé, R. (1999). mRNA stability and selenocysteine insertion sequence efficiency rank gastrointestinal glutathione peroxidase high in the hierarchy of selenoproteins. European Journal of Biochemistry 259(1–2), 149–157. doi:10.1046/j.1432-1327.1999.00012.x
- Yamaguchi, T., Maekawa, T., Nishikawa, Y., Nojima, H., Kaneko, M., Kawakita, T., … Kuraishi, Y. (2001). Characterization of itch-associated responses of NC mice with mite-induced chronic dermatitis. Journal of Dermatological Science, 25(1), 20–28. doi:10.1016/S0923-1811(00)00099-2
