Abstract
In this study, the effects of fermentation by Lactobacillus rhamnosus GG on the antigenicity and Immunoglobulin E (IgE)-binding inhibitions of α-lactalbumin (LA), β-lactoglobulin (LG), and α- caseins (CN) and β-CN were investigated, using polyclonal antibodies and milk-allergic patient sera in competitive enzyme-linked immunosorbent assay (ELISA). Meanwhile, the proteolysis of milk proteins was detected by trinitrobenzene sulfonic acid (TNBS) assay and sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). As a result, we found that fermentation by L. rhamnosus GG could significantly reduce the antigenicity and IgE binding of α-LA, β-LG, α-CN and β-CN in reconstituted milk. In addition, the antigenicity of four proteins decreased to a lower value at 12 h of fermentation and at 0.5 d of cold storage after fermentation. And the IgE-binding inhibitions of four milk proteins in fermented milk were reduced and ranged from 6.5 to over 70% compared to raw milk.
1. Introduction
Food allergy is now a major research subject because it can lead to serious health problems. It is estimated that in well-developed countries over 6% of children and 3–4% of adults suffer from this ailment (Sicherer & Sampson, Citation2009). Cow's milk allergy (CMA), an immunologically mediated reaction to cow's milk proteins (Bahna, Citation2002), has drawn much attention from the public since cow's milk is almost seen as a necessity of consuming food. All cow's milk proteins are potential allergens, among which α-lactalbumin (α-LA), β-lactoglobulin (β-LG) and caseins are the main (Lakshman, Tachibana, et al., Citation2011). Modifying these main proteins through fermentation is currently a feasible way to reduce the allergenicity.
Fermentation is known as one of the most traditional processing technologies in the food industry. Fermented milk products such as yoghourt are well received by the public for their health-promoting properties. Many studies have reported that fermented milk can reduce the allergenicity. Kefir is a traditional yoghourt which is enriched by the addition of transglutaminase (m-TG) to milk used for fermentation. Wróblewska, Kolakowski, Pawlikowska, Troszynska, and Kaliszewska (Citation2009) have improved that this type of yoghourt could be offered especially to those consumers who are searching for foodstuffs with low immunoreactivity. Yogurt fermented by lactic acid bacteria (LAB) is considered to have improved textures, flavours and tastes. El-Ghaish et al. (Citation2011) investigated the potential use of LAB for reduction of allergenicity and for longer conservation of fermented foods. Results indicated that the immunoreactivity of fermented dairy was reduced due to changing the allergen presentation or cleaving the allergenic protein epitopes. It has already been reported that fermentation by certain LAB can reduce the antigenic response of milk proteins (Ehn, Allmere, Telemo, Bengtsson, & Ekstrand, Citation2005; Kleber, Weyrich, & Hinrichs, Citation2006). Jedrychowaki and Wroblewaka (Citation1999) studied the reduction of the antigenicity of whey proteins by lactic acid fermentation. As a result, over 99% antigenicity of α-LA and β-LG disappeared compared to raw milk. Bu, Luo, Zhang, and Chen (2010) got a similar conclusion, and furthermore, Bu also found that the combination of Lactobacillus helveticus and Streptococcus thermophiles were the most effective starters. Since different bacterial strains have different specificities, the effect of other strains should be considered.
Lactobacillus rhamnosus GG is an important member of LAB, which is an experimentally and clinically well-documented probiotic used in different dairy products (Korpela, Moilanen, Saxelin, & Vapaatalo, Citation1997). Cross and Gill (Citation2001) found that the L. rhamnosus GG has the ability to reduce the development of allergy in at-risk infants during the first two years of life. In a mouse model, Thang et al. (Citation2011) has proved that LGG supplementation appeared to have reduced CMA. But, to the best of our knowledge, there are only few reports that have focused on the information of the effects of fermentation by L. rhamnosus GG on the antigenicity and allergenicity of caseins.
Therefore, this study will focus on the effects of the fermentation by L. rhamnosus GG on the antigenicity and allergenicity of caseins (α-CN and β-CN) and examine the effects on the antigenicity and allergenicity of whey proteins (α-LA and β-LG) as well.
2. Materials and methods
2.1. Materials
The antigen proteins used for sensitisation tests and enzyme-linked immunosorbent assay (ELISA) were all purchased from Sigma Chemical Company (St. Louis, MO, USA), including α-LA (L5385; purity ≥85%), β-LG (L3908; purity, approximately 90%), α-CN (C6780; purity ≥70%) and β-CN (C6905; purity ≥98%). Four kinds of sera from rabbits, which contained polyclonal antibodies corresponding to α-LA, β-LG, α-CN and β-CN, respectively, were self-made in our laboratory (Liu, Luo, & Li, Citation2012; Zheng, Shen, Bu, & Luo, Citation2008). The second antibody, conjugated to horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and anti-human IgE, was purchased from Sigma.
Sera from 77 CMA patients presenting various symptoms were selected from a children's hospital affiliated with Zhejiang University (Zhejiang, China), and their availability was approved by the internal ethical committee of the hospital. All patients had specific IgE antibodies towards milk from 0.4 to 11.8 IU ml−1. A pool containing the 77 sera was constituted for indirect competitive ELISA. Sera pool was dispensed in small aliquots and stored at −80 oC before use. Sera pool from six non-allergic people was used as a negative control. All chemicals were analytical grade.
2.2. The culture of micro-organism
L. rhamnosus GG used in this study was obtained from Junlebao Dairy Co. LTD in Shijiazhuang (Shijiazhuang, Hebei, China). The strain was freeze-dried and stored at −80 °C before use. The strain was cultured overnight at 37 °C in skim milk culture medium (12.5%) until the curd was formed. Then it was sub-cultured in the same medium as above for two or three generations in order to be activated. The coagulum could be used as a starter culture.
2.3. Fermentation test
Reconstituted skimmed milk (powder: water = 1: 7) was preheated in a water bath at 45 oC for 30 min and then sterilised at 90–95 °C for 5 min. After heat treatment, the milk was rapidly cooled to 45 °C, and then inoculated with the starter culture in a ratio of 3% (v/v) and cultured at 37 °C. After fermentation (when titratable acidity reached 70 °T), it was stored at 4 °C for three weeks. Samples were taken at specific times during fermentation and cold storage, then sub-packaged and frozen at −60 °C before being analysed.
2.4. Antigenicity analysis by the indirect competitive ELISA
The antigenicity of four main proteins was analysed by indirect competitive ELISA according to Bu et al. (Citation2010). Due to the difference of experiment personnel, everyone must determine the coating concentration of antigens, the dilution of corresponding serum and the standard curves of their own. In this study, the coating concentrations of antigens were 1µg ml−1, 2µg ml−1, 4 µg ml−1 and 2 µg ml−1 for α-LA, β-LG, α-CN and β-CN, respectively, and the dilutions of corresponding serum were 1: 30,000 for anti-α-LA serum, 1:60,000 for anti-β-LG serum, 1: 100,000 for anti-α-CN serum and 1: 40,000 for anti-β-CN serum. And the standard curves were 0.5– 64 µg ml−1 for α-LA, 4–512 µg ml−1 for β-LG, 1–128 µg ml−1 for α-CN and 2–256 µg ml−1 for β-CN. The antigenicity of milk proteins was expressed with concentration equivalent in milligrams per millilitre.
2.5. ELISA IgE-binding assay
Ninety-six well plates (Costar, Corning, NY, USA) were coated with 100 µl of antigen proteins in 50 mmol l−1 carbonate buffer (pH 9.8) and incubated at 4 °C overnight. The coating concentrations of antigens (500 µg ml−1, 100 µg ml−1, 10 µg ml−1 and 10 µg ml−1 for α-LA, β-LG, α-CN and β-CN, respectively) were determined earlier by ELISA. All fermented milk samples were diluted in 10 mmol l−1 phosphate-buffered saline solution (PBS, pH 7.4) (1/200 for α-LA and α-CN, 1/400 for β-LG and β-CN). Patient sera were diluted 1/5 in PBS to give an appropriate maximal absorbance. Fermented milk solution was mixed 1:1 with sera samples and incubated at 4 °C overnight to be used as the samples for ELISA. The following day, wells were blocked with blocking buffer [10 mmol l−1 PBS, pH 7.4, containing 1% (w/v) bovine serum albumin and 0.1% Tween 20] at 37 °C for 1 h. Subsequently, 100 µl of the samples were added in each well, incubated at 37 °C for 1 h. About 100 µl of second antibody (anti-human IgE, epsilon chain specific, peroxidase conjugate developed in goat, Sigma A 9667) diluted in 1/500 in blocking buffer was added to the wells and incubated for 1 h at 37 °C. The wells were added in with 100 µl 3, 3’, 5, 5’-tetramethylenbenzidine (TMB, Amresco, OH, USA) substrate solutions. After the plates were incubated at 37 °C for 10 min, 50 µl 2 mol l−1 H2SO4 were added into wells to stop the reaction. After each step, plates were washed four times with 10 mmol l−1 PBS (pH 7.4) containing 0.5% Tween 20. Uninhibited sera sample (no milk) was used as a control for the maximal absorbance of each antigen.
The absorbance was read at dual wavelengths of 450 and 630 nm by Multiskan MK3 ELISA plate reader (Thermo Labsystems, Franklin, MA, USA). The IgE binding was represented by the inhibition percentages (%) which were calculated using the following equation:
2.6. Free amino group determination
The content of free amino groups in fermented milk was determined according to the trinitrobenzene sulfonic acid (TNBS) method (Adler-Nissen, Citation1979). The absorbance was measured at 420 nm and a calibration curve within the range of 0.25–3.5 mmol l−1 was used. The dilution of milk sample was 1:20 (v/v) in 1% SDS.
2.7. Sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
A modified method was used (Li, Luo, & Feng, Citation2011). The acrylamide separating gel was 15% and sample volume loaded onto the gel was 10 µl.
2.8. Statistical analysis
All data were subjected to analysis of variance (ANOVA). The least significant difference (LSD) procedure was used to test for difference between means (significance was defined at P < 0.05) using SAS software (SAS Institute Inc, NC, USA).
Trend analysis was performed using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA).
3. Results and discussion
3.1. Effects of fermentation time and cold storage time on four milk proteins antigenicity
The antigenicity of four milk proteins (α-LA, β-LG, α-CN and β-CN) during fermentation and cold storage were determined, and the results are shown in and . In this study, the reconstituted milk was heated at 95 °C for 5 min to destroy most of the micro-organisms. It was reported that heating treatment brings about major changes in antigenicity (Bu, Luo, Zheng, & Zheng, Citation2009; Sharma, Kumar, Betzel, & Singh, Citation2001). Compared with the untreated milk, the heating treatment had a significant (P <0.05) reduction on the antigenicity of caseins (α-CN and β-CN) and α-LA. And above all the changes, the antigenicity of α-LA was the most significant, with the value of antigenicity reduced from 12.56 to 2.92 mg ml−1. But the antigenicity of β-LG increased probably because of the new potential allergen produced in heat processing (Davis, Smales, & James, Citation2001). Studies have shown that proteins heated to 90–100 °C will form aggregates and heat treatment of β-LG exposed some new epitopes (Coggins, Rowe, Wilson, & Kumari, Citation2010). The new epitopes may be potential antigenic determinant and give rise to higher antigenicity.
During the fermentation process, the antigenicity of α-CN and β-CN was reduced gradually along with the fermentation time (). The caseins (α-CN and β-CN) antigenicity decreased significantly (P <0.05) in the first 12 h and slowly in the later fermentation time. When fermented at 12 h, the antigenicity of α-LA, β-LG, α-CN and β-CN were all significantly (P < 0.05) lower than that of controls (unfermented reconstituted milk). The reduction percentage of α-LA, β-LG, α-CN and β-CN antigenicity were 67.12%, 16.79%, 46.91% and 28.30%, respectively. This indicated that fermentation by L. rhamnosus GG effectively reduced antigenicity of the four milk proteins. It is accepted that proteolytic enzymes can be produced during microbial fermentation (Wróblewska et al., Citation2009) and they can hydrolyse milk proteins with higher possibility for cleavage of epitopes to degrade milk protein allergens.
Table 1. Effect of fermentation by Lactobacillus rhamnosus GG on the antigenicity of four milk proteins.
Yogurt is a perishable cultured dairy product that has a shelf life up to 80 days under cold storage (Coggins et al., Citation2010). Yogurt during cold storage can obtain aroma, flavour, texture and many other characteristics. As is shown in , during refrigeration at 4 °C after the fermentation for 24 h, the antigenicity of the four milk proteins decreased at first and increased for a while and finally decreased slowly with cold storage time increasing. It was observed that at 0.5 d of cold storage, the antigenicity decreased to a lower value. This may be related to the result that the micro-organism activity was higher and produced more proteases to decline the proteins antigenicity. Fermentation milk with a proper cold storage time can reduce the milk proteins antigenicity more effectively.
Table 2. Effect of cold storage time on the antigenicity of four milk proteins by Lactobacillus rhamnosus GG.
3.2. Changes in the allergenicity (IgE-binding inhibition) of four cows' milk proteins during fermentation and cold storage
shows changes in IgE-binding inhibitions of four proteins during fermentation. After heat treatment, the IgE-binding inhibition of four proteins (α-LA, β-LG, α-CN and β-CN) decreased to 50.28%, 68.75%, 79.08% and 65.87%, respectively. These results are similar with the antigenicity. Taheri-Kafrani et al. (Citation2009) also reported that heat-induced denaturation of β-LG was associated with weaker binding of IgE from CMA patients and low binding of β-LG-specific IgE to heated β-LG was confirmed. In the process of fermentation (), the IgE binding of α-CN and β-CN was inclined to decrease significantly (P < 0.05) and increase slightly at the end of fermentation. The slight increase in proteins antigenicity after 24 h of fermentation was probably due to the further cleaved smaller peptides and amino acids, resulting in the exposure of some hidden epitopes or linear epitopes (Bu et al., Citation2010). According to , the reconstituted milk fermented by L. rhamnosus GG could reduce the allergenicity of α-CN and β-CN, while the IgE-binding inhibitions of α-LA and β-LG had no significant tendency of variation. When fermented at 12 h, the IgE-binding inhibitions of β-LG, α-CN and β-CN were reduced by 6.66, 19.30 and 9.55% and the α-LA allergenicity increased by 19.5%. It was likely that α-LA-specific IgE binding increased in the early stage of fermentation. But the IgE-binding inhibition of α-LA decreased with the increasing of fermentation time.
Table 3. Effect of fermentation by Lactobacillus rhamnosus GG on the IgE-binding inhibitions of four milk proteins.
L. rhamnosus GG was still very active within 24 h of cold storage after fermentation, while the micro-organism growth became slow with increasing cold storage time. In this study, changes in the allergenicity (IgE-binding inhibition) of the four cows' milk proteins during cold storage were investigated. And the results are shown in . The IgE-binding inhibitions of the four proteins decreased without regularity during the whole cold storage. This result may be attributed to the complexity and high nonlinearity of microbial fermentation process. However, it was observed that the IgE binding of α-CN and β-CN decreased obviously in the early stage of cold storage (0–1 d).
Table 4. Effect of cold storage time on the IgE-binding inhibitions of four milk proteins by Lactobacillus rhamnosus GG.
3.3. Changes of free amino groups
The content of free amino groups in fermented milk samples was determined so that we could investigate protease activity of L. rhamnosus GG and estimate the degree of hydrolysis of milk proteins during fermentation. shows the changes in the content of free amino groups during fermentation and cold storage process. It can be seen from that, the hydrolysis degree of milk proteins increased gradually with the fermentation process. This result is consistent with a previous study, which is committed to the idea that some proteases were released during LAB fermentation (Bu et al., Citation2010). We can simply view that the decrease of the four milk proteins antigenicity is because of the proteolysis. However, the antigenicity of the four proteins varied little in fermentation after 24 h, which was inconsistent with the changes of free amino groups. In addition, the tendency of the four proteins allergenicity and the hydrolysis degree of milk proteins were disaccord. This may result from the fact that although microbial proteases still hydrolysed milk proteins, the hydrolysis ability of specific enzymes on α-LA, β-LG, α-CN and β-CN was reduced. So, in conclusion, there was not a linear correlation between the hydrolysis degree of milk protein and the antigenicity of α-LA, β-LG, α-CN and β-CN during the fermentation.
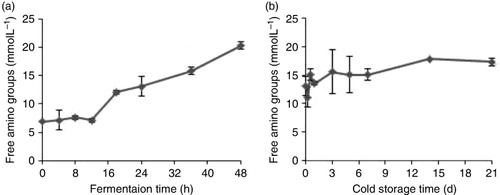
The changes of free amino groups’ content in fermented milk during cold storage at 4 °C were also determined (). During the whole cold storage process of fermented milk, the amount of free amino groups increased without regularity. The results may be attributed to the possible cases that micro-organisms still continued to grow vigorously and the strains needed to absorb the free amino acids during their own growth (Jiaping & Chengxing, Citation1999). During the cold storage process, the proteolytic activity of microbial proteases became weaker and the free amino acids released from the proteins were almost depleted or used up while hydrolysing. Then it presented a dynamic balance. Similarly, it could be seen from and that there was no defined correlation between the hydrolysis degree of milk proteins and the changes of protein antigenicity and allergenicity during cold storage.
3.4. SDS-PAGE analysis of milk proteins after fermentation and cold storage
The degradation of milk proteins was analysed by SDS-PAGE. shows the electrophoretic patterns of fermented milk at different fermentation and cold storage time. It could be seen from that untreated reconstituted milk is composed of four main proteins including α-CN, β-CN, α-LA and β-LG. After heat sterilisation, the concentration of α-LA and β-LG decreased obviously, while the α-CN and β-CN bands showed no obvious change. This may explain the result that the changes of antigenicity and IgE binding of α-LA and β-LG were more remarkable than α-CN and β-CN after heating. It is already known that α-LA and β-LG are thermolabile with heat-induced denaturation and aggregation, while α-CN and β-CN are more resistant to heating and fermentation (Anema & Klostermeyer, Citation1997; Jiaping & Chengxing, Citation1999; Lakshman, Toyokawa, et al., Citation2011). As is shown in , the concentration of the four milk proteins (α-CN, β-CN, α-LA and β-LG) decreased to different degrees compared with unfermented heated milk. The concentration of the four milk proteins had an obvious reduction during the fermentation process (), possibly resulting in the change tendency of the antigenicity and allergenicity of the four milk proteins during fermentation ( and ). It was seen from the electrophoresis pattern that there were many new bands appearing with the increasing fermentation time, this result was consistent with the free amino groups (). However, when the reconstituted milk fermented from 36 to 48 h, the newly presented bands decreased and disappeared indeed. These bands probably represented several types of peptides originating from hydrolysis of milk proteins during fermentation.
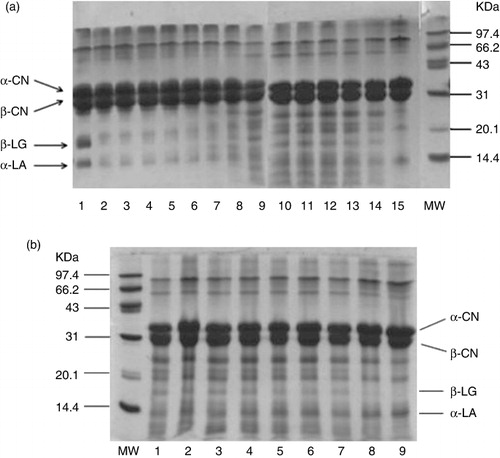
Changes of the four milk proteins concentration at different cold storage time were observed in (). It could be seen that the changes of bands were inconspicuous throughout cold storage, the concentration of the four milk proteins decreased only slightly after cold storage for 5 d. Although the band of β-LG almost disappeared at cold storage for 14 d, the antigenicity of β-LG still existed, possibly because of the fact that the high ability of L. rhamnosus GG produced new allergenic epitopes. As is shown in (), the changes in concentration of caseins were more significant than whey proteins with the increasing of cold storage time. These results showed that L. rhamnosus GG used in this study could hydrolyse whey proteins effectively during fermentation while the hydrolysis of caseins (α-CN and β-CN) was limited.
4. Conclusion
Fermentation by L. rhamnosus GG could significantly reduce the antigenicity and human IgE residual allergenicity of four cows' milk proteins: α-LA, β-LG, α-CN and β-CN in the reconstituted milk. The reduction of proteins antigenicity was greater than that of IgE-binding ability in fermented milk. The antigenicity of the four proteins decreased to a lower value at 12 h of fermentation and at 0.5 d of cold storage. Furthermore, the heat treatment of milk before fermentation reduced the antigenicity of α-LA and IgE binding of α-LA and β-LG.
Funding
This study was financially supported by the National Natural Science Foundation of China [award number 31171715]; National Science and Technology Ministry of China [award number 2011BAD09B03]; Chinese University Scientific Fund [2013QJ030]; and Specialized Research Fund for the Doctoral Program of Higher Education [20120008110020].
Additional information
Funding
References
- Adler-Nissen, J. (1979). Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. Journal of Agricultural and Food Chemistry, 27, 1256–1262. doi:10.1021/jf60226a042
- Anema, S. G., & Klostermeyer, H. (1997). Heat-induced, pH-dependent dissociation of casein micelles on heating reconstituted skim milk at temperatures below 100 C. Journal of Agricultural and Food Chemistry, 45, 1108–1115. doi:10.1021/jf960507m
- Bahna, S. L. (2002). Cow's milk allergy versus cow milk intolerance. Annals of Allergy, Asthma & Immunology, 89(6), 56–60. doi:10.1016/S1081-1206(10)62124-2
- Bu, G. H., Luo, Y. K., Zhang, Y., & Chen, F. (2010). Effects of fermentation by lactic acid bacteria on the antigenicity of bovine whey proteins. Journal of the Science of Food and Agriculture, 90, 2015–2020.
- Bu, G. H., Luo, Y. K., Zheng, Z., & Zheng, H. (2009). Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food and Agricultural Immunology, 20, 195–206. doi:10.1080/09540100903026116
- Coggins, P. C., Rowe, D. E., Wilson, J. C., & Kumari, S. (2010). Storage and Temperature effects on appearance and textural characteristics of conventional milk yogurt. Journal of Sensory Studies, 25, 549–576.
- Cross, M., & Gill, H. (2001). Can immunoregulatory lactic acid bacteria be used as dietary supplements to limit allergies? International Archives of Allergy and Immunology, 125(2), 112–119. doi:10.1159/000053804
- Davis, P., Smales, C., & James, D. (2001). How can thermal processing modify the antigenicity of proteins? Allergy, 56(s67), 56–60. doi:10.1034/j.1398-9995.2001.00918.x
- Ehn, B.-M., Allmere, T., Telemo, E., Bengtsson, U., & Ekstrand, B. (2005). Modification of IgE binding to β-lactoglobulin by fermentation and proteolysis of cow's milk. Journal of Agricultural and Food Chemistry, 53, 3743–3748. doi:10.1021/jf048121w
- El-Ghaish, S., Ahmadova, A., Hadji-Sfaxi, I., El Mecherfi, K. E., Bazukyan, I., Choiset, Y., … Haertlé, T. (2011). Potential use of lactic acid bacteria for reduction of allergenicity and for longer conservation of fermented foods. Trends in Food Science & Technology, 22, 509–516. doi:10.1016/j.tifs.2011.05.003
- Jedrychowski, L., & Wroblewaka, B. (1999). Reduction of the antigenicity of whey proteins by lactic acid fermentation. Food and Agricultural Immunology, 11(1), 91–99. doi:10.1080/09540109999951
- Jiaping, L., & Chengxing, L. (1999). Studies on the proteolytic activity of various lactic acid bacteria fermented milk. Journal of Northeast Agricultural University, 1, 68–74.
- Kleber, N., Weyrich, U., & Hinrichs, J. (2006). Screening for lactic acid bacteria with potential to reduce antigenic response of β-lactoglobulin in bovine skim milk and sweet whey. Innovative Food Science & Emerging Technologies, 7(3), 233–238. doi:10.1016/j.ifset.2005.12.005
- Korpela, R., Moilanen, E., Saxelin, M., & Vapaatalo, H. (1997). Lactobacillus rhamnosus GG (ATCC 53103) and platelet aggregation in vitro. International Journal of Food Microbiology, 37(1), 83–86. doi:10.1016/S0168-1605(97)00049-4
- Lakshman, P. L., Tachibana, S., Toyama, H., Taira, T., Suganuma, T., Suntornsuk, W., & Yasuda, M. (2011). Application of an acid proteinase from Monascus purpureus to reduce antigenicity of bovine milk whey protein. Journal of Industrial Microbiology & Biotechnology, 38, 1485–1492. doi:10.1007/s10295-010-0933-0
- Lakshman, P. L. N., Toyokawa, Y., Tachibana, S., Toyama, H., Taira, T., & Yasuda, M. (2011). Reducing the antigenicity of milk whey protein using acid proteinases from Monascus pilosus. Process Biochemistry, 46, 806–810. doi:10.1016/j.procbio.2010.11.014
- Li, Z., Luo, Y. K., & Feng, L. G. (2011). Effects of Maillard reaction conditions on the antigenicity of α-lactalbumin and β-lactoglobulin in whey protein conjugated with maltose. European Food Research and Technology, 233, 387–394. doi:10.1007/s00217-011-1532-7
- Liu, X. Y., Luo, Y. K., & Li, Z. (2012). Effects of pH, temperature, enzyme-to-substrate ratio and reaction time on the antigenicity of casein hydrolysates prepared by papain. Food and Agricultural Immunology, 23(1), 69–82. doi:10.1080/09540105.2011.604770
- Sharma, S., Kumar, P., Betzel, C., & Singh, T. P. (2001). Structure and function of proteins involved in milk allergies. Journal of Chromatography B: Biomedical Sciences and Applications, 756, 183–187. doi:10.1016/S0378-4347(01)00107-4
- Sicherer, S. H., & Sampson, H. A. (2009). Food allergy: recent advances in pathophysiology and treatment. Annual Review of Medicine, 60, 261–277. doi:10.1146/annurev.med.60.042407.205711
- Taheri-Kafrani, A., Gaudin, J.-C., Rabesona, H., Nioi, C., Agarwal, D., Drouet, M., … Haertle, T. (2009). Effects of heating and glycation of beta-lactoglobulin on its recognition by IgE of sera from cow milk allergy patients. Journal of Agricultural and Food Chemistry, 57, 4974–4982. doi:10.1021/jf804038t
- Thang, C. L., Baurhoo, B., Boye, J. I., Simpson, B. K., & Zhao, X. (2011). Effects of Lactobacillus rhamnosus GG supplementation on cow's milk allergy in a mouse model. Allergy, Asthma, and Clinical Immunology: Official Journal of the Canadian Society of Allergy and Clinical Immunology, 7(1), 20. doi:10.1186/1710-1492-7-20
- Wróblewska, B., Kolakowski, P., Pawlikowska, K., Troszynska, A., & Kaliszewska, A. (2009). Influence of the addition of transglutaminase on the immunoreactivity of milk proteins and sensory quality of kefir. Food Hydrocolloids, 23, 2434–2445. doi:10.1016/j.foodhyd.2009.06.023
- Zheng, H., Shen, X., Bu, G. H., & Luo, Y. K. (2008). Effects of pH, temperature and enzyme-to-substrate ratio on the antigenicity of whey protein hydrolysates prepared by Alcalase. International Dairy Journal, 18, 1028–1033. doi:10.1016/j.idairyj.2008.05.002
