Abstract
The immune activation and toxicity of fresh Cordyceps militaris extracts (HFCM) treated by high-pressure processing (HPP) were evaluated by spleen and thymus index, macrophages phagocytosis activity, lymphocyte proliferation, interleukin-2, interferon-γ level, and human kidney 293 cells, hepatic and kidney function, with the ultrasonic treatment as control (UFCM). The results showed the spleen and thymus index, macrophage function, lymphocyte proliferation, IL-2, and IFN-γ levels in HFCM groups were higher than that in UFCM groups by 11.45–12.43%, 11.52–13.95%, 8.92–11.05%, 8.98–9.23%, 9.3–12.66%, and 12.69–17.83%. HFCM was 21.97%, 21.5%, and 20.74% higher than UFCM in the contents of polysaccharide, cordycepin, and total flavonoids. HFCM showed no toxicity to the cell viability, kidney, and hepatic, while the cell viability in UFCM groups was decreased to 81.54%. So the HFCM was stronger than UFCM in immune activation with no toxicity, and can be used as fresh health food.
Introduction
Cordyceps militaris (L.) Link (CM), a traditional Chinese medicinal and edible mushroom, has a similar physiological effect to Cordyceps sinensis (Yu, Wang, Huang, & Duh, Citation2006). In recent years, CM is in great demand as drug materials and health food products in China and South East Asia due to the health-stimulating properties and medicinal effects (Won & Park, Citation2005). The biological actions of CM were reported, including immune response, anti-phlogosis, anti-bacterium, anti-inflammatory, and anti-arrhythmia, which are closely related to the bioactive components, such as C. militaris polysaccharides (CMP), adenosine, cordycepin (3′-deoxyadenosine), cordycepic acid, protein, total flavonoids (TF), and superoxide dismutase (Dong, Lei, Ai, & Wang, Citation2012). Among those biological actions, immune response plays an important role in protecting the body against the diseases (Shin et al., Citation2010). Our previous studies showed the fresh C. militaris extracts (FCM) was stronger than dry C. militaris extracts (DCM) on enhancing immune activation (Zhu, Pan, Liang, Wu, & Yang, Citation2013), indicating that FCM is an excellent health food resource in treating immunodeficiency diseases.
The conventional processing techniques for fresh health food resource include microwave drying, infrared drying, freezing drying, and spray drying. However, these techniques were proved to affect the quality of the final products due to the failure of fresh medicine sterilization, and the thermal degradation of thermo-sensitive active compounds (Zhou & Li, Citation2012). Thus, suitable processing techniques are essential for FCM.
The high-pressure processing (HPP), a non-thermal food processing technique, could effectively eliminate the spoilage and pathogenic microorganisms in foods by increasing the permeability or rupturing the cell membrane with no medicinal ingredients changing or losing (Oey, Lille, Van Loey, & Hendrickx, Citation2008). Moreover, HPP disrupts the cell wall and releases cytoplasm containing the bioactive components when the high pressure released suddenly (Seo et al., Citation2011). Therefore, we assume the HPP may have wide application prospect in fresh health food processing. However, previous studies showed that HPP decreased the cytotoxicity of Berberis koreana (Jin et al., Citation2008), but increased the cytotoxicity of the fruits of Rubus coreanus Miquel (Seo et al., Citation2011), which indicates the application of HPP on the fresh herb medicines processing should be extremely cautious. Moreover, there were lots of reports on the effects of HPP on antioxidant activities, while little information about the effects of HPP on the immunomodulatory activity of fresh health food resource is available. So, researches on the immune activation and toxicity of fresh health food treated with HPP are necessary.
In this study, the fresh CM was treated with the optimal HPP at 400 MPa for 9 min at ambient temperature with the ratio of water to raw material 1 ml/g, which can eliminate thoroughly the spoilage and pathogenic microorganisms in fresh C. militaris extracts (data not shown), and successive assays were conducted to evaluate the immune activation and toxicity of fresh C. militaris extracts. The details are reported in the current study.
Materials and methods
Materials
The fresh body of CM was produced by Institute of Natural Medicine, Hefei University of Technology. Fetal bovine serum (FBS), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), RPMI 1640, concanavalin A (ConA), Dulbecco’s modified eagle’s medium (DMEM), Dimethyl sulfoxide (DMSO), human embryonic kidney cell (HEK293), and the assay kits for interleukin-2 (IL-2), interleukin-4 (IL-4), and interferon-γ (IFN-γ) were obtained from Sangon Biotech (Shanghai, China). Cyclophosphamide (Cy), cordycepin (3′-deoxyadenosine), and other reagents were purchased from Sinopharma Cychemical Reagent Co., Ltd (Shanghai, China).
Sample preparation
Fresh CM (1000 g) was added into onefold water and homogenized at 4000 rmp for 15 min, and subsequently divided into two parts equally. One part was treated at 400 MPa for 9 min at ambient temperature. The crude extracts were filtered and concentrated in a rotary evaporator, then lyophilized to obtain the HPP-treated fresh C. militaris extracts, which was designed as HFCM. The other part was treated by ultrasonic with the optimal extraction conditions as follows: Ultrasonic power 680 W, extraction time 4.5 min, ratio of water to raw material 1 ml/g. After filtration and lyophilization, the obtained fresh C. militaris extracts were designated as UFCM.
Animals and experimental design
Male kunmin mice (8-week-old) purchased from Experimental Animal Center of Anhui Medical University, Animal Production License No.: SCXK (Wang) 2011–002, were housed in wire cages (60 cm × 100 cm) in an air-conditioned room at 22 ± 2°C with a 12 h light–dark cycle. All procedures including mice care were approved by the Institute of Ethics Committee of the Chinese Academy of Agricultural Sciences. After adapting to the environment for one week, the mice were randomly divided into 15 groups (10 mice each). Three groups of healthy mice, used as normal and positive groups, were treated once a day with physiological saline solution, HFCM, and UFCM (200 mg/kg/d BW), respectively. The other 12 groups of mice were intraperitoneally injected with 80 mg/kg/d Cy from day 1–3 to establish the immunodeficiency model of mice. From day 4 to 18, one model groups were treated with physiological saline solution, three model groups were treated with HFCM (50, 100, and 200 mg/kg/d BW) by gastric gavage, and three model groups were treated with UFCM (50, 100, and 200 mg/kg/d BW). The other groups were administered by CMP and TF isolated from HFCM and UFCM with the dose proportional to the contents in UFCM.
Different organs index
Twenty-four hours after the final administration, the excised spleen and thymus were immediately weighed for calculating the indexes of spleen and thymus: index (mg/g) = (weight of thymus or spleen)/body weight (Wang et al., Citation2012).
Phagocytic index
The function of the macrophage cells was assessed via a carbon clearance test performed on three mice from each group as described by Wang et al. (Citation2012). Each mice was injected with India ink (fourfold diluted with 0.8% NaCl solution) at 0.05 ml/10 g BW by the tail vein. Blood samples were collected from the retinal venous plexuses for phagocytic index α assay at 2 min (t 1) and 10 min (t 2) using a heparinized syringe with a 23-gauge needle. A volume of 20 μl blood was mixed with 2 ml 0.1% Na2CO3. The phagocytic index α = K 1/3 × A/(B + C), K = (lgOD1 − lgOD2)/(t 2 − t 1), where A, B, and C represent the body, liver, and spleen weight, respectively. OD1 is for t 1 and OD2 for t 2.
Lymphocyte proliferation assay
Spleen cells proliferation was assessed using MTT method as described by Yuan, Song, Li, Li, and Dai (Citation2006) with slight modification. Homogenized spleens were filtered through a 40 μm nylon cell strainer and centrifuged at 200 g for 10 min to obtain spleen cells, which were then washed twice with hyperosmotic haemolysis to remove the red cells. The purified cells were re-suspended to a density of 5 × 106 cells/ml with RPMI 1640 medium containing 10% FBS, ConA (5.0 μg/ml), 100 μg/ml streptomycin, and 100 U/ml penicillin, and seeded on a 96-well plate. After culturing for 72 h at 37°C in 5% CO2 atmosphere, 10 μl MTT (5 mg/ml) was added into each well and incubated for 4.5 h. The plate was centrifuged at 200 g for 15 min to discard the supernate, and the precipitates were suspended by DMSO (100 μl/well) and shaken to dissolve formazan crystals (the metabolite of MTT). The cell proliferation was determined at 570 nm and expressed with optical density.
Cytokines assay
Seven mice from each group were anesthetized with pentobarbital sodium salt (65 mg/kg BW) and sacrificed by decapitation. Blood samples were further collected immediately and centrifuged at 4000 g for 10 min to obtain the serums required in IL-2 and IFN-γ assay, which were determined by enzyme-linked immunosorbent assay.
Polysaccharide determination and isolation
The content of polysaccharide in HFCM and UFCM was measured according to the phenol-sulfuric acid method (Yang, Zhu, Xu, Wu, & Bian, Citation2008). In brief, 1 ml of HFCM or UFCM (1 mg/ml) solution and 1 ml of phenol solution (5%) were mixed. A measure of 2.5 ml of sulfuric acid (ρ = 1.84 g/ml) was added into the mixture and shaken for 30 min. The absorbance was measured at 490 nm. A calibration curve with glucose standard was produced using six concentrations (10–100 µg/ml).
The CMP was isolated according to the method used by Zhu et al. (Citation2013) with minor modifications. HFCM (100 g) and UFCM (100 g) were defatted with 1000 ml ether, respectively, and then stirred with 1000 ml aqueous (80% alcohol) for 24 h. The mixes were filtrated, and the residues were ultrasound-assisted extracted twice for 2 h with 500 ml water at room temperature, and the combined filtrates were concentrated to a final volume of approximately 100 ml. These concentrates were deproteinized by adding 100 ml chloroform/isopentanol (4:1, v/v). After filtrating, the filtrates were added into four volumes of absolute ethanol (24 h) and centrifuged to obtain the polysaccharide samples, which were washed three times with acetone, then filtrated and freeze-dried to obtain the CMP samples and designated as FCMP and UCMP, respectively.
Cordycepin determination
Cordycepin and adenosine in HFCM and UFCM were determined by HPLC as described (Guo, Zhu, Zhang, & Zhang, Citation1998) with minor modifications. The HPLC analyses were conducted with a Waters HPLC system consisting of a pump (A00515), a UV detector (2487), a C18 column (Pinnaclell 5 μm, 250 × 4.6 mm), an autosampling valve (7725i) equipped with a sample loop of 20 μl used for sample injection, and Waters Empower software for data analysis. Ethanenitrile and ultra-high-purify water (5/95, v/v) was used as mobile phase. The flow was set at 1 ml/min and column temperature kept at 35°C. A calibration curve with cordycepin or adenosine as standards (1, 2, 5, 10, 20, and 50 mg/l) was detected at 260 nm. Results are expressed as mg of cordycepin or adenosine equivalents/g dry weight (mg cordycepin/g or adenosine/g DW).
Total flavonoid determination and isolation
Total flavonoid content was done as previously described by Abeysinghe et al. (Citation2007), with some modifications. In brief, 0.05 ml of HFCM or UFCM (1 mg/ml), 1.35 ml of distilled water, 0.05 ml of NaNO2 (5%), 0.05 ml of AlCl3 (10%) were mixed and reacted for 10 min at room temperature in the darkness. Absorbance was measured at 420 nm. A calibration curve with rutin as standards (0, 20, 40, 60, 80, and 100 mg/l) was produced. The result of TFs was expressed as mg of rutin equivalent/g dry weight (mg RE/g extract).
The isolation of TF was done as described by Zhu et al. (Citation2013). HFCM (100 g) and UFCM (100 g) were added into 85% ethanol solvent (1:20, w/v) and stirred for 3 h, and then ultrasound-assisted extracted for 30 min. The extracts were filtered (pore size, 0.45 pm). The residues were re-extracted twice with the same volume of ethanol solvent (85%). The filtrates were collected and concentrated under reduced pressure in a rotary evaporator to yield dried crude extracts, which were defatted with petroleum ether and ethyl acetate, then filtrated and freeze-dried to obtain the TF samples, and designated as FTF and UTF, respectively.
Cell viability assay
Cell viability was measured by Cell Counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto, Japan) as described by Park, Choi, and Park (Citation2012). The HEK293 was added into a full DMEM medium containing FBS (10%), streptomycin (100 μg/ml), and penicillin (100 U/ml), with a final density of 1 × 104 cells/ml. The cells suspension was added into a 96-well plate and incubated for 24 h with different concentrations of HFCM or UFCM (0.1, 0.2, 0.3, and 0.4 mg/ml per well) at 37°C, followed by 10 μl of medium containing CCK-8 to each well and another incubation for 2 h. The absorbance was read at 450 nm. Cell viability was expressed as the percentage of cell death.
Hepatic and kidney function assay
The hepatic and kidney function assay was measured according to Seo et al. (Citation2011).The mice from normal and positive control groups were sacrificed by decapitation, and the blood samples were combined immediately in centrifuge tube, which were centrifuged at 4000 g for 10 min to afford the serums required in hepatic function and kidney function assay. The levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, creatinine, and serum urea nitrogen (BUN) were detected using the kits from Bioassay Systems (Hayward, CA). All assays were carried out according to the manufacturer's instructions.
Statistical analysis
Results are expressed as means ± standard error (SE). Statistical analysis was performed for the experiments conducted at least triplicate using one-way analysis of variance (ANOVA) and Dunnett test, by using SPSS, version 18 (SPSS Inc., Chicago, IL, USA). Results with P < 0.05 were considered to be statistically significant.
Results
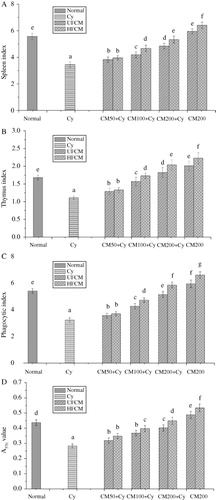
Effect of HFCM on the organ index, macrophage, and spleen lymphocyte activity
The spleen and thymus are important immune organs, and the macrophages and spleen lymphocytes cell are important immune cells. As can be seen in , the spleen and thymus index, the macrophage phagocytic index, and the spleen lymphocyte proliferation increased by 14–59%, 19–91%, 15–96%, 23–82% in HFCM groups, and increased by 11–44%, 16–62%, 10–74%, 12–68% in UFCM groups, compared to model group. At the same dose, the spleen and thymus index, the macrophage phagocytic index, and the spleen lymphocyte proliferation in HFCM groups (100, 200 mg/kg BW) were higher than UFCM groups by 11.45–12.43%, 11.52–13.95%, 8.92–11.05%, 8.98–9.23%, respectively, and ANOVA showed significant difference. Compared to normal group, the spleen index, thymus index, macrophage phagocytic index, and spleen lymphocyte proliferation in HFCM positive control groups were increased by 14.87%, 32.39%, 22.1%, and 19.2%, which were higher than UFCM positive control groups by 8.55%, 10.95%, 11.06%, and 10.18%, respectively. The results indicated that HFCM is stronger than UFCM on enhancing the immune function of immune organs and immune cells in immunosuppressed mice.
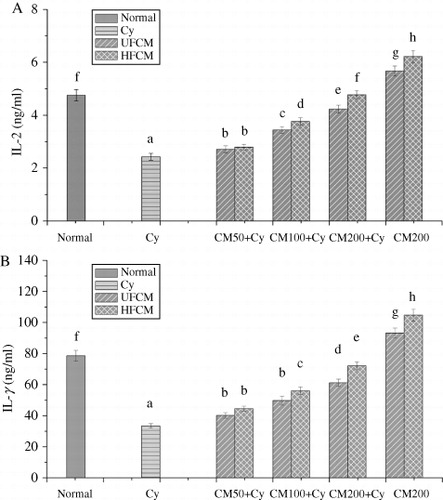
Effect of HFCM on the levels of IL-2 and IFN-γ
The IL-2 and IFN-γ are important immune factors. The results were shown in , the levels of IL-2 and IFN-γ were enhanced by 14.81–96.3%, 33.14–115.64% in HFCM groups, and by 11.52–74.07%, 20.28–83.05 in UFCM groups, compared to model group. At the same dose, the levels of IL-2 and IFN-γ in HFCM groups (100, 200 mg/kg BW) were 9.3–12.66% and 12.69–17.83% higher than that in UFCM groups, respectively. also showed that the levels of IL-2 and IFN-γ in HFCM positive control groups were increased by 30.09% and 32.38%, which were higher than that of UFCM positive control groups by 9.7% and 11.54%, respectively. The results indicated that the increasing ability of HFCM on the levels of IL-2 and IFN-γ was stronger than UFCM.
Contents of the polysaccharides, cordycepin, TFs in HFCM
The polysaccharides (CMP), cordycepin, and TF are the main components of C. militaris. To clarify the relationship between the main components and the higher immune activation of HFCM, the contents of CMP, cordycepin, and TF in HFCM and UFCM were determined. As shown in , the levels of CMP, cordycepin, and TF in HFCM were 21.97%, 21.5%, and 20.74% higher than that in UFCM, respectively, indicating that the stronger immune activation of HFCM may partly benefit from the higher content of CMP, cordycepin, and TF, compared to UFCM.
Table 1. The levels of CMP, cordycepin, and TP and TF in HFCM and UFCM.
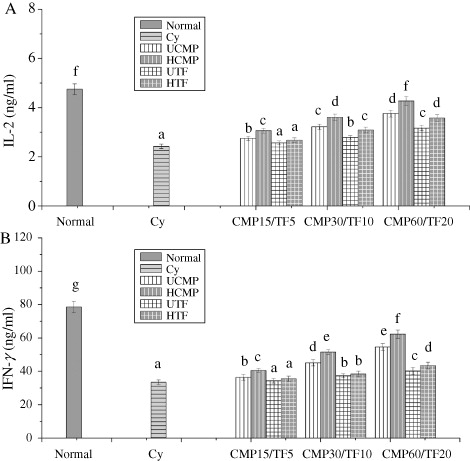
Effect of CMP and TF from HFCM and UFCM on mice immune organs and immune cells
shows that the weight of spleen and thymus, the phagocytic activity of macrophage, and the spleen lymphocyte proliferation were improved by the UCMP, FCMP, UTF, and FTF in a dose-dependent manner, suggesting that the CMP and TF in FCM and DCM improve the immune function of immune organs and immune cells. also shows that the value of immune organs and immune cells in the FCMP and FTF groups were significantly higher than those in UCMP and UTF groups, indicating that the immune activation of FCMP and FTF was stronger than UCMP and UTF.
Table 2. Effect of HFCM and UFCM on organ index, phagocytic index, and spleen lymphocyte proliferation in mice.
Effect of CMP and TF from HFCM on the levels of IL-2 and IFN-γ
shows that the levels of IL-2 and IFN-γ had at least a 16.05%, 8.73% increase in CMP groups, and 10.69%, 14.83% in TF group, and ANOVA showed significant difference, indicating that the CMP and TF from HFCM and UFCM have immune activation. Moreover, levels of IL-2 and IFN-γ in HCMP groups had at least 9.22%, 11.44% higher than that in UCMP. At 10 mg/kg BW and levels of IL-2 and IFN-γ in HTF groups were higher than UTF groups by 9.67%, 13.99%, indicating that the increasing ability of HCMP and HTF on IL-2 and IFN-γ levels was also stronger than UCMP and UTF.
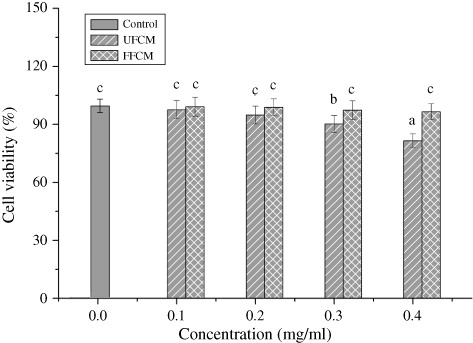
Effect of HFCM on the HEK 293 cell viability and hepatic and kidney function
The human kidney 293 cell (HEK239 cells) is usually used for evaluation of in vitro cytotoxicity. As shown in , in the range of 0–0.4 mg/ml, HFCM exhibited no inhibition on the viability of HEK239 cells, while the cell viability decreased to 81.54% in UFCM groups, suggesting that some toxic substances exist in fresh C. militaris were extracted by ultrasonic extraction process, while the data showed that the toxic substances might be decreased by HPP.
The hepatic and kidney function were shown in . Although a significant difference was found in the level of ALT, AST, urea, creatinine, and albumin among the mice from normal and positive control groups, all the values were well within the normal range, suggesting that the HFCM has no toxicity in vivo.
Discussions
In our previous studies, we found the fresh C. militaris was significantly stronger than its dry product for enhancing the immunity in immunosuppressed mice within the dosage of 50–200 mg/kg/d BW, and no obvious toxic effect was observed (Zhu et al., Citation2013), which suggests that fresh C. militaris has a great potential in preventing or treating immunodeficiency disease. Therefore, it is required to develop an appropriate processing method for fresh C. militaris. Among the multiple processing methods reported in food and medicine processing field, the HPP, a food processing technology, has a unique superiority in inactivating pathogenic microorganisms and enzymes in foods without reducing its original flavor, color, and quality (Seo et al., Citation2011). However, recent studies revealed that HPP also change some components in foods, which indicate that the pharmacological activities and toxicities should be detected when the HPP is used for processing fresh health food. Thus, the present study was designed to evaluate the immune activation and toxicities of fresh C. militaris extracts by HPP with the comparison of ultrasonic processing, which has an advantage over other methods in extraction (Ashokkumar et al., Citation2009), with the dosage of 50, 100, and 200 mg/kg/d BW. Our results show that the immune activation of fresh C. militaris extracts by HPP is remarkably stronger than that by ultrasonic.
Due to that the CMP, cordycepin, and TF from FCM can enhance the immune organs index, promote the spleen lymphocyte proliferation, and macrophage function, and increase the levels of IL-1, IL-2, TNF-γ, and TNF-β in vivo and in vitro (Chen, Zhang, Shen, & Wang, Citation2010; Middleton, Kandaswami, & Theoharides, Citation2000; Seo et al., Citation2011; Zhou et al., Citation2008; Zhu et al., Citation2013), the contents and immune activation of CMP, cordycepin and TF of HFCM and UFCM were determined to clarify the mechanism. We found a higher content of CMP, cordycepin, and TF in HFCM, compared to UFCM, and the immune activation of HCMP and HTF were higher than UCMP and UTF. Obviously, the stronger immune activation of HFCM is benefited from the higher contents of HCMP, cordycepin, HTF and the stronger immune activation of HCMP and HTF. This may be that the HPP enhances the dissolution rate of CMP, cordycepin, and TF by strengthening mass transfer, damaging cell walls, facilitating solvent access to the cell, and destroying the ionic bonds and hydrophobic interaction of macromolecules (Montero, Fernández-Díaz, & Gómez-Guillén, Citation2002; Rendueles et al., Citation2011), which result in the increasing of the digestibility and bioavailability of nutrient (Heremans, Ledward, Johnston, Earnshaw, & Hasting, Citation1995). It should be noted that, due to cordycepin is small molecule, the property of which was not affected by HPP (Rendueles et al., Citation2011), the immune activation of cordycepin was not analyzed in the present study. Altogether, our results suggested that the immune activation of fresh C. militaris extracts was enhanced by HPP.
Considering the cytotoxicity of different species vary after being treated by HPP (Jin et al., Citation2008; Seo et al., Citation2011), more confirmative studies should be essential to clarify the toxicity of fresh C. militaris extracts by HPP. Thus, the HEK239 cells and the mice from the positive control groups were used to evaluate potential toxicity of HFCM with the comparison of UFCM. We found UFCM has low toxicity in HEK239 cell at 0.3 mg/ml, while HFCM showed no toxicity at 0.4 mg/ml, indicating that the toxic residues in FCM were eliminated by HPP. This result is similar to Jin et al. (Citation2008). Moreover, the value of hepatic and kidney function was all well within the normal range, suggesting that the HFCM should be safely to body. These findings further illustrate that HPP has a wide application prospect in processing fresh health food.
Conclusion
In current study, the immune activation and toxicity of fresh C. militaris extracts by HPP (HFCM) were evaluated with the comparison of the extracts by ultrasonic (UFCM). The results indicated that the fresh C. militaris extracts by HPP exhibited stronger promotability on immune activation than the extracts by ultrasonic, whereas showed no toxicity. The results were closely related to the higher content of CMP, cordycepin, and TF in HFCM, and the stronger immune activation of CMP and TF from HFCM, compared to those from UFCM. From the results we can draw the conclusion that HFCM is better immune activation than UFCM. Our findings also illustrate that ultrahigh pressure processing has a bright prospect for fresh health food.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abeysinghe, D., Li, X., Sun, C., Zhang, W., Zhou, C., & Chen K. (2007). Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chemistry, 104, 1338–1344. doi:10.1016/j.foodchem.2007.01.047
- Ashokkumar, M., Bhaskaracharya, R., Kentish, S., Lee, J., Palmer, M., & Zisu, B. (2009). The ultrasonic processing of dairy products – An overview. Dairy Science & Technology, 90, 147–168.
- Chen, W., Zhang, W., Shen, W., & Wang, K. (2010). Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cellular Immunology, 262(1), 69–74. doi:10.1016/j.cellimm.2010.01.001
- Dong, J. Z., Lei, C., Ai, X. R., & Wang, Y. (2012). Selenium enrichment on Cordyceps militaris link and analysis on its main active components. Applied Biochemistry and Biotechnology, 166, 1215–1224. doi:10.1007/s12010-011-9506-6
- Guo, C., Zhu, J., Zhang, C., & Zhang, L. (1998). Determination of adenosine and 3′-deoxyadenosine in Cordyceps militaris (L.) Link. by HPLC. Zhongguo Zhong Yao Za Zhi, 23, 236–237.
- Heremans, K. (1995). High pressure effects on biomolecules. In D. A. Ledward, D. E. Johnston, R. G. Earnshaw, & A. P. M. Hasting (Eds.), High pressure processing of foods (pp. 81–97). Nottingham: Nottingham University Press.
- Jin, L., Han, J. G., Ha, J. H., Jeong, H. S, Kwon, M. C., & Ahn, J. H. (2008). Effect of immune activity on Berberis koreana Palibin by ultra high pressure low temperature process. Korean Journal of Medical Crop Science, 16, 439–445.
- Middleton, E. J., Kandaswami, C., & Theoharides, T. C. (2000). The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacological Reviews, 52, 673–751.
- Montero, P., Fernández-Díaz, M. D., & Gómez-Guillén, M. C. (2002). Characterization of gelatin gels induced by high pressure. Food Hydrocolloids, 16, 197–205. doi:10.1016/S0268-005X(01)00083-2
- Oey, I., Lille, M., Van Loey, A., & Hendrickx, M. (2008). Effect of high-pressure processing on colour, texture and flavour of fruit- and vegetable-based food products: A review. Trends in Food Science and Technology, 19, 320–328. doi:10.1016/j.tifs.2008.04.001
- Park, D. K., Choi, W. S., & Park, H.-J. (2012). Antiallergic activity of novel isoflavone methyl-glycosides from Cordyceps militaris grown on germinated soybeans in antigen-stimulated mast cells. Journal of Agricultural and Food Chemistry, 60, 2309–2315. doi:10.1021/jf205199j
- Rendueles, E., Omer, M. K., Alvseike, O., Alonso-Calleja, C., Capita, R., & Prieto, M. (2011). Microbiological food safety assessment of high hydrostatic pressure processing: A review. LWT, 44, 1251–1260.
- Seo, Y. C., Choi, W. Y., Kim, J. S., Yoon, C. S., Lim, H. W., Cho, J. S., … Lee, H. Y. (2011). Effect of ultra high pressure processing on immuno-modulatory activities of the fruits of Rubus coreanus Miquel. Innovative Food Science and Emerging Technologies, 12, 207–215. doi:10.1016/j.ifset.2011.04.002
- Shin, S., Kwon, J., Lee, S., Kong, H., Lee, S., Lee, C.-K., … Kim, K. (2010). Immunostimulatory effects of Cordyceps militaris on macrophages through the enhanced production of cytokines via the activation of NF-κ B. Immune Network, 10(2), 55–63. doi:10.4110/in.2010.10.2.55
- Wang, M., Meng, X. Y., Yang, R. L., Qin, T., Wang, X. Y., Zhang, K. Y., … Xue, F. Q. (2012). Cordyceps militaris polysaccharides can enhance the immunity and antioxidation activity in immunosuppressed mice. Carbohydrate Polymers, 89, 461–466. doi:10.1016/j.carbpol.2012.03.029
- Won, S.-Y., & Park, E.-H. (2005). Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. Journal of Ethnopharmacology, 96, 555–561. doi:10.1016/j.jep.2004.10.009
- Yang, H., Zhu, D. J., Xu, D. B., Wu, J., & Bian, X. Y. (2008). A study on Cordyceps militaris polysaccharide purification, composition and activity analysis. African Journal of Biotechnology, 7, 4004–4009.
- Yu, H. M., Wang, B.-S., Huang, S. C., & Duh, P.-D. (2006). Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. Journal of Agricultural and Food Chemistry, 54, 3132–3138. doi:10.1021/jf053111w
- Yuan, H., Song, J., Li, X., Li, N., & Dai, J. (2006). Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides. Cancer Letters, 243, 228–234. doi:10.1016/j.canlet.2005.11.032
- Zhou, H. D., & Li, L. (2012). Research of clinical applications and pharmacological effects of Chinese medicine fresh drug: an overview. Chinese Journal of Ethnomedicine and Ethnopharmacy, 1, 43–45.
- Zhou, X., Luo, L., Dressel, W., Shadier, G., Krumbiegel, D., Schmidtke, P., … Meyer, C. U. (2008). Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis. The American Journal of Chinese Medicine, 36, 967–980. doi:10.1142/S0192415X08006387
- Zhu, S. J., Pan, J., Liang, J., Wu, Z. Y., & Yang, J. J. (2013). Comparisons on enhancing the immunity of fresh and dry Cordyceps militaris in vivo and in vitro. Journal of Ethnopharmacology, 149, 713–719. doi:10.1016/j.jep.2013.07.037