ABSTRACT
The use of ribavirin (RBV) as an antiviral drug for livestock has been prohibited in China, the USA, and many other countries. In this study, we developed a rapid and sensitive indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) and a gold nanoparticle immunochromatographic (ICA) strip test for detecting RBV in chicken muscles. Under the optimum assay conditions, where the assay employed phosphate-buffered saline at pH 7.4, no acetonitrile, and an ionic strength of 0.8%, the quantitative working range was 1.43–26.47 ng/ml with an IC50 of 6.15 ng/ml. The recovery rate for RBV in real samples ranged from 82.1% to 112.3%. The immunochromatographic test strip method had a visual cutoff value of 50 μg/kg. Given their high recovery rates and good sensitivity, the proposed ic-ELISA and ICA methods could be useful for the RBV analysis in chicken tissue samples.
Introduction
Ribavirin (RBV), 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Bosch, Sanchez, Rojas, & Ojeda, Citation2007; Sidwell et al., Citation1972), has a broad spectrum of antiviral activities (Sidwell et al., Citation1972; Puech, Gosselin, Balzarini, De Clercq, & Imbach, Citation1988; Yeo et al., Citation2015). At present, RBV is used widely as a medicine in humans to treat and prevent viral diseases (Manns et al., Citation2001; McHutchison et al., Citation2009; Suppiah et al., Citation2009). Prior to 2005, the Food and Drug Administration banned the use of human antiviral drugs in livestock. China also banned the use of RBV in animal feed. The abuse of RBV during the rearing of chickens may affect the quality of chicken meat and human health throughout the world. The abuse of RBV decreases the quality and flavor of chicken meat, but it can also lead to bacterial resistance and medicine residues, which may affect human health and damage the ecological balance via the food chain, thereby having adverse consequences. The accumulation of RBV in animal bodies may have side effects such as genetic toxicity, reproductive toxicity, possible carcinogenicity, and especially hemolytic anemia. In December 2012, the “fast-growing chicken” incident occurred in China, which was linked to the illegal use of RBV and other antiviral drugs in chicken feed. The abuse of RBV in chicken feed may have severe deleterious consequences, so establishing a simple, rapid, and effective method for detecting RBV residue in foodstuffs is imperative.
At present, the methods for detecting plasma and foodstuffs residues of RBV are mainly based on high-performance liquid chromatography (Loregian, Scarpa, Pagni, Parisi, & Palu, Citation2007; Morello et al., Citation2007; Paroni, Sirtori, Borghi, & Kienle, Citation1987), liquid chromatography-mass spectrometry (LC-MS), capillary electrophoresis (Breadmore, Theurillat, & Thormann, Citation2004), spectrophotometric methods (Darwish, Khedr, Askal, & Mohamed, Citation2006), solid-phase extraction methods (Morello et al., Citation2007; Svensson, Bruchfeld, Schvarcz, & Stahle, Citation2000), and UV detection (Svensson et al., Citation2000). These methods are mainly employed in the clinical analysis of RBV and the main disadvantages of these methods are their complicated operations and the sample pretreatment processes also take a long time, which result in high testing costs, long cycles, the inability to screen samples rapidly in large quantities, and the failure to satisfy requirements for rapid detection on the spot. By contrast, enzyme-linked immunosorbent assay (ELISA) and the colloidal gold strip immunoassay methods facilitate rapidity, specificity, convenience, high sensitivity, and high-purity testing of samples, where these methods are easy to operate and are suitable for the rapid analysis of a large number of samples. At present, ELISA methods and gold nanoparticle immunochromatographic (ICA) strip tests are used for detecting various drug residues in real systems.
In this study, for the first time, we prepared a sensitive monoclonal antibody (mAb) against RBV. Based on the RBV mAb, we developed indirect competitive ELISA (ic-ELISA) and gold nanoparticle ICA tests, which can be used to detect RBV residues in chicken samples.
Materials and methods
The reagents RBV, tributylamine, isobutylchloroformate, succinic anhydride, Tween 20, and 3,3,5,5′-tetramethylbenzidine (TMB) were obtained from J&K Scientific (Shanghai, China). Bovine serum albumin (BSA), ovalbumin (OVA), Freund's complete and incomplete adjuvants, and polyethylene glycol solution (Hybri-Max, 50% (w/v)) were purchased from Sigma Chemical Co. (Shanghai, China). The Sp2/0-Ag14 murine myeloma cell line was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). A horseradish peroxidase (HRP)-goat anti-mouse IgG conjugate was purchased from Kangcheng Bioengineering Co. (Shanghai, China). Chicken samples were obtained from a local supermarket (Wuxi, China). All other chemicals and solvents were analytical-grade reagents without further purification.
Synthesis of the RBV derivative
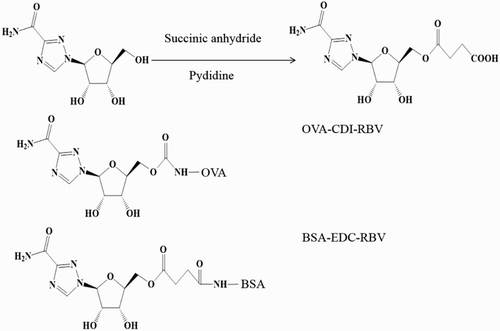
A specific hapten was synthesized. First, RBV 100 mg (0.41 mmol) and 50 mg (0.5 mmol) succinic anhydride were dissolved in 10 ml pyridine in a 50 ml round-bottomed flask, which was equipped with a mechanical stirrer and a condenser. The reaction was refluxed with vigorous stirring for 12 h in a water bath at 60°C. The pyridine was removed using a nitrogen gas appliance, and the solid residue was dissolved in 10 ml of deionized water. The pH of the solution was adjusted to ca 2.0 using 1 mol/l HCl and a cloudy white precipitate was formed. The solid RBV hapten was filtered and washed using deionized water, before drying at 37°C for 2 h.
Immunogen and coating of the antigen preparation
Two steps were performed, as described in previous studies (Chen, Huang, Li, Kong, & Huai, Citation2014; Suryoprabowo, Liu, Peng, Kuang, & Xu, Citation2014). First, the RBV derivative (10 mg, 0.041 mmol) was dissolved in 2 ml of 0.1 M (pH 5.5) 2-[N-morpholino] ethanesulfonic acid to provide an acidic reaction environment. After the RBV hapten was dissolved, 11.09 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and 6.68 mg of N-hydroxysuccinimide were added, followed by stirring for 6 h. Next, 25 mg of BSA was dissolved in 0.01 M (pH 9.6) carbonate–bicarbonate buffer at a concentration of 5 mg/ml. The RBV hapten solution was added dropwise into the BSA solution. The reaction continued for 24 h at 4°C and the mixture was then dialyzed with phosphate-buffered saline (PBS) (0.01 M, pH 7.4) for 2 days at 4°C.
The coating antigen comprised OVA, which was linked with RBV via the N,N′-carbonyldiimidazole (CDI) method and used as the heterogeneous coating antigen. RBV (3.3 mg, 0.013 mmol) was dissolved in anhydrous N,N-dimethylformamide, before CDI (11.2 mg, 0.067 mmol) was added and stirred for 1 h at 24°C. Next, 10 mg of OVA was dissolved in 2 ml 0.01 M (pH 9.6) carbonate–bicarbonate buffer at a concentration of 5 mg/mL and the RBV solution was poured slowly into the OVA solution. The reaction was continued for 12 h at 4°C and then dialyzed with PBS (0.01 M pH 7.4) for 48 h at 4°C. The protein concentrations of the conjugates were measured using the Bradford method. After characterization by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the RBV immunogen antigen and RBV coating antigen were stored at –20°C until use.
Immunization and generation of the mAb
The immunization program was performed as described previously (Lin et al., Citation2011). Eight-week-old female BALB/c mice were immunized with the RBV antigen solution (conjugates of the hapten with BSA) and Freund's complete adjuvant at a dosage of 0.2 ml per mouse (containing 100 mg antigen). Immunization was performed at 3-week intervals with 0.2 ml per mouse (containing 80 mg antigen) and Freund's incomplete adjuvant. Seven days after the final immunization, antisera from mice were tested using ic-ELISA. The mouse with the highest titer and lowest inhibition was selected for cell fusion. The cell fusion and cell screening processes were as described previously (Liu, Yan, Zhang, Kuang, & Xu, Citation2014; Sun & Zhuang, Citation2015). Ascites were collected and then purified with caprylic acid and ammonium sulfate precipitation (Guo et al., Citation2015; Kuang et al., Citation2013; Song et al., Citation2010). The mAb concentrations were measured using the Bradford method.
Evaluation of the mAb by ic-ELISA
The ic-ELISA procedure was developed using the checkerboard method to optimize the concentration of the coating antigen, RBV hapten–OVA (1–0.01 μg/ml). The procedure was performed as described previously (Liu, Kuang, Peng, Wang, & Xu, Citation2014; Sun & Zhuang, Citation2015) but with some modifications. A 96-well microplate was coated with 100 μl/well of the RBV coating antigen diluted with PBS (0.01 M) and incubated for 2 h at 37°C. The plates were washed three times with PBS-Tween 20 (PBST) and blocking buffer was then added, followed by blocking overnight at 4°C. The plates were then washed once to remove the blocking buffer. Fifty microliters of RBV standard at different concentrations in PBS and 50 μl RBV antibody were added to the wells of the plate sequentially, and then incubated in the dark for 30 min at 37°C. After three washes, 100 μl of HRP-goat anti-mouse IgG conjugate (diluted 1:3000) was added to each well and incubated for 30 min at 37°C. After four washes, the plates were developed with 100 ml per well of TMB substrate solution for 15 min at 37°C. Next, 2 M sulfuric acid was added at 50 ml per well to stop the color development. The optical density (OD) of the plates was read at 450 nm and the data were analyzed using a four-parameter logistic equation. The standard curve was generated using the OD data obtained with different RBV concentrations. From the sigmoidal curve, the half inhibition concentration (IC50) value of the mAb was evaluated, which characterized the sensitivity of the mAb.
Cross-reactivity
Amantadine hydrochloride, rimantadine hydrochloride, trifluorothymidine, RBV impurity A, and RBV impurity C were used to determine any cross-reactivity (CR) in the ic-ELISA. The CR was calculated using the following equation (Kuang et al., Citation2010): CR (%) = (IC50 of RBV/IC50 of the related compound) × 100%.
Sample preparation
Chicken muscles that lacked RBV were used to determine the recovery rates of the tests. The chicken muscles were analyzed by LC-MS and no residual RBV was detected. The samples (1.0 ± 0.01 g) were weighed and spiked with RBV, before adding 5 ml of trichloroacetic acid/acetonitrile (1:100 v/v) and shaking vigorously for 10 min. Each sample was centrifuged at 2400 g for 10 min and the supernatant was placed in a polyethylene tube, before drying using a nitrogen gas appliance. The residue was dissolved in 1 ml PBS (pH 7.4), which was then used in the ic-ELISA and ICA tests. Each test was repeated three times.
Synthesis of gold nanoparticles labeled with the mAb
First, the sodium citrate reduction method was used to prepare gold nanoparticles as described previously (Chen, Liu, Kuang, Song, & Xu, Citation2013; Yang et al., Citation2015). The formation of colloidal gold particles was confirmed by transmission electron microscopy. Second, the prepared nanoparticles were labeled with the purified RBV mAb, where 1 ml of borate buffer (pH 8.0) containing 0.2 mg of anti-RBV mAb was added to 20 ml of the prepared nanoparticle solution. The mixed solution was incubated for 2 h. The gold nanoparticles labeled with the mAb were centrifuged at 6400 g for 30 min. The sediment was then dissolved in 10 ml of PBS containing 0.2% BSA.
Strip preparation
The test and control lines were drawn on a nitrocellulose membrane (1 μl/cm) using the coating antigen and goat anti-mouse IgG, where the distance between the two lines was 0.5 cm (Kuang et al., Citation2013; Xing et al., Citation2014). We optimized the concentration of goat anti-mouse IgG used for the test line, the concentration of the coating antigen for the control line, and the reaction time. For detection, 80 μl standard solutions or samples were added to gold-labeled RBV mAb and PBST. The results could be observed with the naked eye.
Assay of RBV in chicken samples using the strip test
In this assay, the sample solution was mixed with labeled mAb and reacted for 5 min before adding to the sample strip. The effect of capillary action allowed the labeled mAb and sample solution to migrate on the nitrocellulose membrane. If the sample lacked RBV, the labeled mAb was not captured by the coating antigen, and thus a visible red test line was formed. A red control line formed when the goat anti-mouse IgG captured the labeled mAb. When RBV was present in the sample, the competitive effect meant that the labeled mAb conjugated with RBV but not with the coating antigen, so the test line was colorless. The color of the test line was weaker when the amount of RBV in the sample was higher.
Results and discussion
Synthesis of the RBV hapten and conjugated antigen
For small molecules, hapten synthesis is the first and most important step in the development of immunoassays (Kim, Lee, Chung, & Lee, Citation2003; Liu, Yan et al., Citation2014). The procedure employed to synthesize the hapten and hapten–protein conjugates is shown in . We synthesized the hapten from the 5-hydroxyl of RBV using the succinic anhydride method, which can retain the overall structure of RBV. In addition, the succinic anhydride method was used to modify the hydroxyl group so that it could conjugate with the protein more efficiently. Therefore, we designed a novel specific hapten.
The RBV derivative was connected with BSA as the complete antigen using the EDC method. The heterologous coating antigen comprised OVA linked with RBV via the CDI method. The complete antigen and coating antigen were confirmed by SDS-PAGE, as shown in .
Figure 2. SDS-PAGE of carrier proteins and conjugates. Lane 1: BSA; lane 2: 5-RBV-BSA; lane 3: OVA; and lane 4: RBV-OVA.
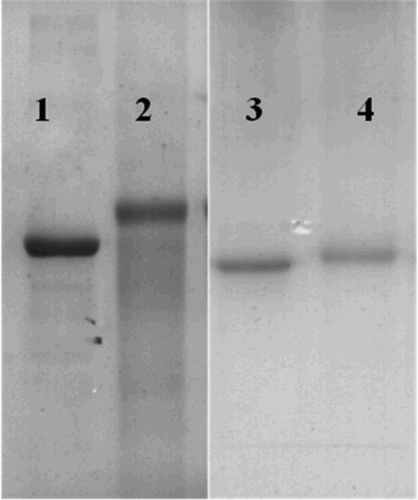
The protein molecule transfer speed during electrophoresis depends on the molecular weight of the protein, where the direction of electrophoresis is from the bottom up. shows that the transfer speed of the hapten was slower than that of BSA, which indicated that the immunogenic molecule was larger than BSA. Thus, after coupling with OVA, the complete antigen and coating antigen were conjugated successfully.
Optimization and specificity of ic-ELISA
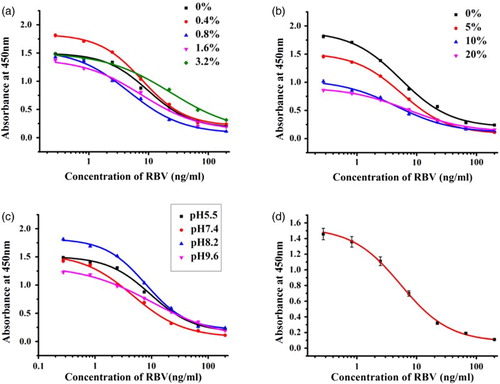
Different concentrations of NaCl (0%, 0.4%, 0.8%, 1.6%, or 3.2%) were added to the assay buffer to evaluate the effects of ionic strength. The results shown in (a) demonstrate that the concentration of NaCl had little effect on the maximum OD values, but the IC50 was the lowest when the concentration of NaCl was 0.8%; therefore, the NaCl concentration was set at 0.8% in subsequent assays. Acetonitrile was added to the standard dilute solution to evaluate the antibody. The IC50 values were similar, but the maximum OD values changed greatly with the acetonitrile content (0–20%, v/v), as shown in (b). Different pH conditions were also tested, as shown in (c). The condition is considered to be most suitable when the Amax/IC50 ratio (Amax = 1.603; IC50 = 6.15 ng/ml) is the highest (Kuang et al., Citation2010). According to (c), the highest Amax/IC50 ratio occurred at a pH of 7.4. Therefore, based on the results, the best conditions for RBV in the ic-ELISA comprised a NaCl concentration of 1.6%, no acetonitrile, and a pH of 7.4. The standard curve obtained in these conditions is shown in (d), where the IC50 value was 6.15 ng/ml and the quantitative working range was 1.43–26.47 ng/ml (IC20–IC80).
Figure 3. Optimization of assay buffer for ic-ELISA system and standard content in assay buffer on ic-ELISA performance. (a) Effects of NaCl content in assay buffer on ic-ELISA performance; (b) effects of acetonitrile content in assay buffer on ic-ELISA performance; (c) effects of pH about assay buffer on ic-ELISA performance; and (d) standard inhibition curve for the ic-ELISA analysis of RBV. Each point of inhibition curve represents five replicates in the analysis.
Furthermore, we determined the specificity of the RBV mAb under the optimized conditions for ic-ELISA. As given in , RBV mAb exhibited no CR with amantadine hydrochloride, rimantadine hydrochloride, trifluorothymidine, RBV impurity A, and RBV impurity C.
Table 1. CR results under the optimized ic-ELISA conditions.
Analysis of samples by ic-ELISA
Spiked chicken samples were used in this experiment. The chicken samples were spiked with different standard concentrations (1.5, 5, and 15 μg/kg) and the results are given in . The average recoveries from chicken samples using the ic-ELISA ranged from 82.1% to 112.3% for RBV. The test was repeated three times on different days and the coefficients of variation were 3.21–8.53%.
Table 2. Recovery results for chicken muscle samples.
Detection of RBV in chicken samples using test strips
The cutoff value of the ICA test was defined as the lowest RBV concentration for which the color of the test line disappeared completely (Sun et al., Citation2013). In this evaluation, we added 0, 5, 10, 25, or 50 ng/ml RBV in PBS to the strip and the results are shown in . An obvious change in the test line was observed with 10 ng/ml of RBV and the test line disappeared completely with 50 ng/ml of RBV. We also spiked chicken samples with 0, 5, 10, 25, or 50 μg/kg in PBS and applied them to the test strips, and the results are shown in the . The test line disappeared completely with 50 ng/ml. Thus, the test strip assay is suitable for the sensitive and practical detection of RBV to determine the presence of RBV residues in chicken samples on the spot tests.
Conclusions
In this study, for the first time, we synthesized an RBV immunogen antigen and heterologous coating antigen, as well as a high-quality mAb for RBV based on the immunogen. We then developed ic-ELISA and gold nanoparticle ICA tests based on the mAb raised against RBV, which we employed to detect RBV in spiked chicken samples. The IC50 for the ic-ELISA was 6.15 ng/ml and the cutoff level for the strips was 50 μg/kg using chicken samples spiked with RBV. In addition, the ic-ELISA and gold nanoparticle ICA tests exhibited good recovery rates, high sensitivity, and low detection limits, and thus they can be used to determine the presence of RBV residues in actual samples in on the spot tests.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work is financially supported by the Key Programs from MOST [grant number 2012BAK08B01, 2012BAD29B04], and grants from the Natural Science Foundation of Jiangsu Province, MOF and MOE [grant numbers BK20140003, BE2013613, and BE2013611].
Notes on contributors
Shuang Peng is a Master candidate in Food analysis. Her research interests are immunoassay developments. Presently, she is studying as a graduate in School of Food Science & Technology Jiangnan University, Wuxi, China.
Shanshan Song got her Master degree in 2012 from School of Food Science & Technology Jiangnan University, Wuxi China. Currently she works as a research assistant in Jiangnan University. Her research interests are monoclonal antibody screenings.
Liqiang Liu is a Ph.D in food analysis. Presently, he is working as an associate professor in School of Food Science & Technology Jiangnan University, Wuxi, China. His research interests are hapten design and antibody evaluations.
Hua Kuang is a Ph.D in analytical chemistry. She is working as a professor in School of Food Science & Technology Jiangnan University, Wuxi China. Her research interest is biosensor development.
Chuanlai Xu is a Ph.D in Food Science. He is working as a professor in School of Food Science & Technology Jiangnan University, Wuxi, China. His research interests are fast detection technologies.
References
- Bosch, M. E., Sanchez, A. J. R., Rojas, F. S., & Ojeda, C. B. (2007). Ribavirin: Analytical determinations since the origin until today. Journal of Pharmaceutical and Biomedical Analysis, 45(2), 185–193. doi: 10.1016/j.jpba.2007.06.004
- Breadmore, M. C., Theurillat, R., & Thormann, W. (2004). Determination of ribavirin in human serum and plasma by capillary electrophoresis. Electrophoresis, 25(1011), 1615–1622. doi: 10.1002/elps.200305819
- Chen, G. Y., Huang, X. H., Li, S. P., Kong, X. K., & Huai, B. B. (2014). Synthesis of a newly designed artificial antigen and preparation of a polyclonal antibody against salbutamol. Food and Agricultural Immunology, 25(3), 322–331. doi: 10.1080/09540105.2013.791970
- Chen, X. J., Liu, L. Q., Kuang, H., Song, S. S., & Xu, C. L. (2013). A strip-based immunoassay for rapid determination of fenpropathrin. Analytical Methods, 5(21), 6234–6239. doi: 10.1039/c3ay41030g
- Darwish, I. A., Khedr, A. S., Askal, H. F., & Mohamed, R. M. (2006). Application of inorganic oxidants to the spectrophotometric determination of ribavirin in bulk and capsules. Journal of AOAC International, 89(2), 341–351.
- Guo, J. N., Liu, L. Q., Xue, F., Xing, C. R., Song, S. S., Kuang, H., & Xu, C. L. (2015). Development of a monoclonal antibody-based immunochromatographic strip for cephalexin. Food and Agricultural Immunology, 26(2), 282–292. doi: 10.1080/09540105.2014.907242
- Kim, M. J., Lee, H. S., Chung, D. H., & Lee, Y. T. (2003). Synthesis of haptens of organophosphorus pesticides and development of enzyme-linked immunosorbent assays for parathion-methyl. Analytica Chimica Acta, 493(1), 47–62. doi: 10.1016/S0003-2670(03)00793-1
- Kuang, H., Xing, C. R., Hao, C. L., Liu, L. Q., Wang, L. B., & Xu, C. L. (2013). Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors (Basel), 13(4), 4214–4224. doi: 10.3390/s130404214
- Kuang, H., Xu, L. G., Cui, G., Ma, W., & Xu, C. L. (2010). Development of determination of di-n-octyl phthalate (DOP) residue by an indirect enzyme-linked immunosorbent assay. Food and Agricultural Immunology, 21(3), 265–277. doi: 10.1080/09540101003758962
- Lin, F., Song, S., Liu, L., Kuang, H., Wang, & L., Xu, C. (2011). Development of the detection of benzophenone in recycled paper packaging materials by ELISA. Food and Agricultural Immunology, 22(1), 39–46. doi: 10.1080/09540105.2010.523781
- Liu, L., Kuang, H., Peng, C., Wang, L., & Xu, C. (2014). Structure-specific hapten design for the screening of highly sensitive and specific monoclonal antibody to salbutamol. Analytical Methods, 6(12), 4228–4233.
- Liu, L., Yan, H., Zhang, X., Kuang, H., & Xu, C. (2014). Development of an anti-chlorothalonil monoclonal antibody based on a novel designed hapten. Food and Agricultural Immunology, 26(3), 410–419. doi: 10.1080/09540105.2014.938319
- Loregian, A., Scarpa, M., Pagni, S., Parisi, S., & Palu, G. (2007). Measurement of ribavirin and evaluation of its stability in human plasma by high-performance liquid chromatography with UV detection. Journal of Chromatography B: Biomedical Sciences, 856(1–2), 358–364. doi: 10.1016/j.jchromb.2007.05.039
- Manns, M., McHutchison, J., Gordon, S., Rustgi, V., Shiffman, M., Reindollar, R., … Albrecht, J. (2001). Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. The Lancet, 358(9286), 958–965. doi: 10.1016/S0140-6736(01)06102-5
- McHutchison, J., Lawitz, E., Shiffman, M., Muir, A., Galler, G., McCone, J., … Sulkowski, M. (2009). Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection (vol. 361, p. 580, 2009). New England Journal of Medicine, 361(10), 1027–1027.
- Morello, J., Rodriguez-Novoa, S., Cantillano, A., Gonzalez-Pardo, G., Jimenez, I., & Soriano, V. (2007). Measurement of ribavirin plasma concentrations by high-performance liquid chromatography using a novel solid-phase extraction method in patients treated for chronic hepatitis C. Therapeutic Drug Monitoring, 29(6), 802–806. doi: 10.1097/FTD.0b013e31815bddf3
- Paroni, R., Sirtori, C. R., Borghi, C., & Kienle, M. G. (1987). High-performance liquid chromatographic determination of ribavirin in serum and urine and of its urinary metabolite 1, 2, 4-triazole-3-carboxamide. Journal of Chromatography B: Biomedical Sciences and Applications, 420, 189–196. doi: 10.1016/0378-4347(87)80172-X
- Puech, F., Gosselin, G., Balzarini, J., De Clercq, E., & Imbach, J. L. (1988). Synthesis and biological evaluation of isomeric dinucleoside monophosphates and monomethylphosphonates of 9-beta-D-arabinofuranosyladenine and related analogs. Journal of Medicinal Chemistry, 31(10), 1897–1907. doi: 10.1021/jm00118a006
- Sidwell, R. W., Huffman, J. H., Khare, G. P., Allen, L. B., Witkowski, J. T., & Robins, R. K. (1972). Broad-spectrum antiviral activity of virazole: 1-f8-D-ribofuranosyl-1, 2, 4-triazole-3-carboxamide. Science, 177(4050), 705–706. doi: 10.1126/science.177.4050.705
- Song, S., Lin, F., Liu, L., Kuang, H., Wang, L., & Xu, C. (2010). Immunoaffinity removal and immunoassay for rhodamine B in chilli powder. International Journal of Food Science & Technology, 45(12), 2589–2595. doi: 10.1111/j.1365-2621.2010.02445.x
- Sun, R., & Zhuang, H. (2015). Development of a highly sensitive biotin–streptavidin enzyme-linked immunosorbent assay for detecting diethyl phthalate based on a specific polyclonal antibody. Food and Agricultural Immunology, 26(5), 746–760. doi: 10.1080/09540105.2015.1027666
- Sun, W., Wang, X., Wang, Y., Ju, X., Xu, L., Li, G., & Sun, Z. (2013). Application of graphene-SnO2 nanocomposite modified electrode for the sensitive electrochemical detection of dopamine. Electrochimica Acta, 87, 317–322. doi: 10.1016/j.electacta.2012.09.050
- Suppiah, V., Moldovan, M., Ahlenstiel, G., Berg, T., Weltman, M., Abate, M., … George, J. (2009). IL28B is associated with response to chronic hepatitis C interferon-α and ribavirin therapy. Nature Genetics, 41(10), 1100–1104. doi: 10.1038/ng.447
- Suryoprabowo, S., Liu, L., Peng, J., Kuang, H., & Xu, C. (2014). Development of a broad specific monoclonal antibody for fluoroquinolone analysis. Food Analytical Methods, 7(10), 2163–2168. doi: 10.1007/s12161-014-9863-1
- Svensson, J. O., Bruchfeld, A., Schvarcz, R., & Stahle, L. (2000). Determination of ribavirin in serum using highly selective solid-phase extraction and high-performance liquid chromatography. Therapeutic Drug Monitoring, 22(2), 215–218. doi: 10.1097/00007691-200004000-00013
- Xing, C. R., Kuang, H., Hao, C. L., Liu, L. Q., Wang, L. B., & Xu, C. L. (2014). A silver enhanced and sensitive strip sensor for Cadmium detection. Food and Agricultural Immunology, 25(2), 287–300. doi: 10.1080/09540105.2013.781140
- Yang, X. D., Zhang, G. P., Wang, F. Y., Wang, Y. B., Hu, X. F., Li, Q. M., … Zeng, X. Y. (2015). Development of a colloidal gold-based strip test for the detection of chlorothalonil residues in cucumber. Food and Agricultural Immunology (Ahead-of-Print), 1–9.
- Yeo, K. L., Chen, Y. L., Xu, H. Y., Dong, H. P., Wang, Q. Y., Yokokawa, F., & Shi, P. Y. (2015). Synergistic suppression of dengue virus replication using a combination of nucleoside analogs and nucleoside synthesis inhibitors. Antimicrobial Agents and Chemotherapy, 59(4), 2086–2093. doi: 10.1128/AAC.04779-14