ABSTRACT
The study is aimed at studying the effects of soluble and insoluble oat β-glucan on colon carcinogenesis in mice. One hundred and twenty male Kunming mice were divided into normal control (NC), model control (MC), high doses (100 mg/kg body weight) of soluble (H-SOG) and insoluble β-glucan (H-IOG), and low doses (50 mg/kg body weight) of soluble (L-SOG) and insoluble β-glucan (L-IOG) groups. The mice except those in the NC group were given subcutaneous injections of DMH to induce colon cancer. The bile acid content was significantly reduced but the colonic short-chain fatty acid content was enhanced (p < .05) in mice administered β-glucan, moreover, the tumor cells apoptosis was significantly promoted (p < .05), compared to the MC group. The effect of high doses β-glucan was better than the low doses, but there is little difference between the SOG and IOG. Results suggested that both SOG and IOG exert favorable effects in preventing colon cancer in a doses-dependent manner while the specific mechanism maybe different.
1. Introduction
Colon cancer is a world-wide health problem and the second-most dangerous type of cancer, affecting both men and women, with potential to spread to distinct parts of the body (Kuppusamy et al., Citation2014). Human colon cancer involves a range of mutational events, at least some of which are likely to result from exposure to carcinogens in the intestinal tract (Macfarlane & Macfarlane, Citation2012). Lifestyle and dietary patterns influence colon cancer risk both positively and negatively, especially diet (MacDonald & Wagner, Citation2012). Epidemiological study concluded that total dietary fiber was inversely associated with colon cancer in humans (Murphy et al., Citation2012). There are many speculations on the mechanism of dietary fiber reacting on the colon cancer, but all emphasize that dietary fiber can promote the bowel movements, decrease the staying time of stool in the intestine, change the intestinal flora and local metabolism, and increase the stool volume (Bingham, Citation1990; Kim, Citation2000). Moreover, soluble dietary fiber ferments in the large intestine and produce short-chain products: lactate, butyrate, acetate, and propionate, in which butyrate serves as an energy source for colon epithelium and protect colon from cancer and colitis (Hague, Singh, & Parasceva, Citation1997; Scott, Duncan, & Flint, Citation2008), while insoluble dietary fiber softens fecal content with the high capacity for water binding as well as shortens transit time of stool through the colon, thus adjusting intestinal function to maintain colon health (Yapo & Koffi, Citation2008).
Oats are rich in dietary fiber, and can be recommended as the best source of β-glucan (Dodevska et al., Citation2013). And β-glucan, a natural polymer comprised individual glucose molecules that are linked together by a series of β-(1-3) and β-(1-4) linkages (Queenan et al., Citation2007), is thought to be the active component for the physiological functions of oats. Oat β-glucan has important physiological benefits on the body, such as hypolipidemic, hypoglycemic, and immunomodulatory function (Shen, Cai, Dong, & Hu, Citation2011). Moreover, Oat β-glucan can promote proliferation of probiotics as a prebiotic in the intestine (Shen, Dang, Dong, & Hu, Citation2012). Early studies showed that β-glucan could promote proliferation of Bifidobacteria and Lactobacilli in the intestine, reducing the number of Escherichia coli (Dong, Zhu, Li, Shen, & Li, Citation2014; Mitsou, Panopoulou, Turunen, Spiliotis, & Kyriacou, Citation2010). The former can reduce the activity of enzymes of microbial metabolic, such as glucosidase, glucuronidase, and urease, which are inducing factors for colon cancer, transforming precarcinogens into carcinogens (Roberfroid et al., Citation2010). In addition, consumption of oat β-glucan can increase total colonic short-chain fatty acid (SCFA) concentration and proportion of butyrate in high-fat diet-induced obese mice (Dong et al., Citation2014). In summary, oat β-glucan may reduce the incidence of colon cancer through a variety of ways. A systematic and specific experimental proof of effects of oat β-glucan on colon cancer, however, remains deficient.
Nowadays, most of the proposed protective mechanisms of oat products consumption are likely to be relevant with soluble oat β-glucan (SOG), and few reports are available on the functional properties of water-insoluble oat β-glucan (IOG). We previously concluded that IOG was more effective on weight loss while SOG might play a more important role in improving serum lipids in high-fat diet-induced obese mice (Dong et al., Citation2014). In this paper, we divide the oat β-glucan into soluble and insoluble β-glucan to study their effects on colon carcinogenesis induced by 1,2-dimethylhydrazine (DMH) in mice to provide experimental evidence for understanding the action and mechanism of oat β-glucan and dietary fiber preventing colon cancer and also supply theoretical basis for the development of oat β-glucan health products.
2. Materials and methods
2.1. Chemicals
Pancreatin (≥1.0 units/mg), α-amylase (≥10 uints/mg), and DMH were purchased from Sigma Chemical Company (St. Louis, MO, USA). Serum diamine oxidase assay kit and Annexin V/PI apoptosis detection kit were obtained from Nanjing Jiancheng Reagent Company (Nanjing, China). Other chemicals were of analytical-reagent grade.
2.2. SOG and IOG
SOG and IOG used in this experiment were extracted from oat bran (Crops Research Institute in Severe Cold Region, Shanxi Academy of Agricultural Sciences) according to the method of Johansson, Tuomainen, Ylinen, Ekholm, and Virkki (Citation2004) with minor modifications. Commercial oat bran was comminuted, and the powder that passed through a 0.3 mm screen was then defatted with ether. The polysaccharides were suspended in water with a solid–liquid ratio of 1:20 (w/v) at 80°C and starch was hydrolyzed using thermostable α-amylase. The insoluble fraction was separated by centrifugation. Pancreatin was added into the supernatant to degrade proteins. The water-soluble β-glucan was precipitated by 70% ethanol, separated by centrifugation and dissolved in water at 80°C. The solution was concentrated by rotary evaporator (RE-52AA, Shanhai Yarong Lab Instrument Development Co., Ltd., Shanghai, China) and then freeze dried. Water-insoluble β-glucan was obtained from the insoluble fractions collected before. It was extracted by Ba(OH)2 solution (adjusting the initial pH value to 9).
The total β-glucan contents of SOG and IOG samples were measured using reagent kits obtained from Megazyme International Ireland Ltd. (Bray, Ireland) according to the manufacturer's instructions, which gave values of 80.19 and 81.60%, respectively.
2.3. Animals and diets
A total of 120 male Kunming (KM) mice (6 weeks of age, weighing 25 ± 2 g) were purchased from the Laboratory Animal Center of Henan Province (Zhengzhou, China) and maintained under controlled temperature (23 ± 1°C), humidity (50 ± 5%) and air flow conditions with a fixed 12-h light/dark cycle (light 7:00 am to 7:00 pm). During 1-week acclimation, the mice were fed commercial pellet diet (Laboratory Animal Center of Henan Province). Then, the mice were randomly divided into six groups (n = 20): the normal control (NC) group, the model control (MC) group, the high-dose (100 mg/kg body weight) groups of soluble β-glucan (H-SOG) and insoluble β-glucan (H-IOG), and low-dose (50 mg/kg body weight) groups of soluble β-glucan (L-SOG) and insoluble β-glucan (L-IOG). The mice except those in the NC group were given weekly subcutaneous injection of DMH (20 mg/kg body weight in 0.9% NaCl solution) for 11 weeks (Devasena, Rajasekaran, Gunasekara, Viswanathan, & Menon, Citation2003). SOG and IOG groups were administered soluble and insoluble oat β-glucan (dissolved in distilled water) by intragastric gavage throughout the experiment. Simultaneously, NC and MC groups were given a daily placebo (normal saline). During the experimental period of 18 weeks, mice in all groups were fed with the same basal diet and had free access to water. The experiments were conducted in accordance with the ethical norms approved by Animal Care and Use Committee, Henan Province Bureau of Quality Supervision and Testing of experimental animals (SCXK No. 2010-0002).
2.4. Measurement of fecal bile acid contents
The feces samples were collected at week 6, week 12, and week 18 (day before the mice were sacrificed), stored at 4°C. Bile acids in the freeze-dried feces were measured by the method of Porter et al. (Citation2003) using a spectrophotometer (UV-1600, Shanhai MAPADA Lab Instrument Development Co., Ltd., Shanghai, China).
2.5. SCFA assays in colonic contents
At the end of the 18-week experimental period, the mice were autopsied and the colonic contents were collected and then stored at −70°C prior to analysis. The measurement of the SCFA contents was conducted by the previous method (Shen et al., Citation2012).
2.6. Tumor incidence
Colons were excised from treated mice at the end of the experiment, and then were blotted dry and opened longitudinally, with the inner surface examined for visible macroscopic lesions. Tumor incidence (percentage of mice with tumors) was determined for the colons.
2.7. Analysis of colon tumor cell apoptosis
We took the colon paracancerous tissues after the mice were sacrificed at the end of the experiment and fixed them in 70% ethanol, flowed by adding appropriate amount of phosphate buffer saline (PBS) and over 300 mesh sieve with rubbing method the next day. Then, they were rinsed three times by PBS and centrifuged. We mixed the supernatant with a small amount of PBS to form the single cell suspension of colonic epithelial cells. The final concentration was no less than 1 × 106 cells/mL.
Phosphatidylserine exposure on the outer leaflet of the plasma membrane was detected using the Annexin V-FITC/PI Apoptosis Detection Kit (Merck, Shanghai Yu Bo Biological Technology Co., Ltd.) according to the manufacturer's instructions. In brief, cells were rinsed with ice-cold PBS and then re-suspended in 500 µL of binding buffer. After full suspension, we added 5 µL of Annexin V-FITC and 10 µL of propidium iodide (PI). The cells were incubated for 5 min at 4°C and then immediately analyzed on a FACSC-LSR (Becton, Dickinson and Company) equipped with Cell Quest (Becton, Dickinson and Company) software. And the data were measured by the First Affiliated Hospital of Henan Medical University.
2.8. Histopathological study
After the mice were sacrificed, we took 1 cm of distal colon segments, fixed in formalin solution. The tissues were handled with tissue processors (BT-300A Dehydrator, CS-V Spreading machine and ZHB1-ZT-HB Baking machine from JinHua YiDi Medical Appliance Co., Ltd., HESTION Embedded machine from TexLab Precision Instruments Co., Ltd., YD-1508R Slicer from Shanghai Leica instruments Co., Ltd.) and then stained with hematoxylin–eosin, as Verma and Shukla (Citation2013) described. Histomorphometric analysis was performed by a light microscopy (OlympusBX51 Microscopy from Japan) and the tissue sections were observed at a 10 × 20 magnification.
2.9. Statistical analysis
All results are expressed as the mean ± standard deviation (). The results obtained were subjected to the one-way analysis of variance, flowed by least significant difference method to compare the significant difference between groups using the SPSS 10.0 software (SPSS, Inc., Chicago, IL, USA) at p < .05.
3. Results and discussion
3.1. Effects of SOG and IOG on fecal bile acid contents
Many experimental and epidemiological data have shown that bile acid is an important factor for colon cancer (Degirolamo, Modica, Palasciano, & Moschetta, Citation2011), whose risk is increased by hepatobiliary secretion of bile acids. The fecal bile acid contents in the MC group kept increasing within the experimental period, as shown in . This is consistent with the results of Chirta, Sabitha, Nalini, and Menon (Citation1994) found that during DMH treatment, there is an increased level of cholesterol in intestine and colon, which causes the increased formation and decreased absorption of bile acids. In Week 18, the bile acid contents in the H-SOG, L-SOG, H-IOG, and L-IOG groups were significantly decreased (p < .05) compared to the MC group but still could not achieve the NC group's level. As to decrease the bile acids, the effect of high-dose β-glucan was better than low-dose β-glucan (p < .05), while there was not significant difference between the SOG and IOG (same doses) (p > .05).
Table 1. Fecal bile acid contents in mice (mg/g feces).
Bacterially modified bile acids are known colon tumor promoters, and certain colonic bacteria are able to degrade bile acids and degraded bile acids are cocarcinogens in animal models (Hill, Citation1975). Thus increased fecal bile acids in DMH-treated mice may explain its positive correlation with colon cancer. β-Glucan can modulate intestinal microflora and bacterial metabolites in rats (Shen et al., Citation2012). So reduced fecal bile acids on oat β-glucan supplementation may be due to the modulation of the growth of one or more bacterial species of the colon by β-glucan, thus preventing the conversion of primary bile acids to secondary bile acids.
3.2. Effects of SOG and IOG on SCFA contents
As shown in , the SCFA contents of H-SOG and H-IOG group were significantly higher than all the other groups (p < .05) and the butyrate contents were 55.56 and 62.96% higher than the MC group, respectively, while there was no significant difference (p > .05) between them. However, the SCFA contents of the L-SOG and L-IOG groups had no significant difference (p > .05) compared to the NC and MC groups.
Table 2. The changes in SCFA contents in colon (mmol/g colonic contents).
Studies showed that SCFAs fibrous foods produced by the bacterial fermentation in the colon had anticancer effects (Kaczmarczyk, Miller, & Freund, Citation2012). β-Glucan can produce SCFA by anaerobic bacteria in the colon, which will decline the pH value in the intestine of the mice and maintain an acidic environment, providing a good living conditions for some microorganisms, such as Bifidobacteria and Lactobacillus. Previous study (Shen et al., Citation2012) has concluded that oat β-glucan administration increased the population of Lactobacillus and Bifidobacterium, whereas decreased the number of Enterobacteriaceae, and induced a significant decrease in β-glucuronidase activity in rats. Bifidobacteria can reduce the activity of β-glucuronidase, nitroreductase, azoreductase in the intestine, which can transform the precarcinogens into carcinogens (Ahmed, Segal, & Hasan, Citation2000; Roberfroid et al., Citation2010). And β-glucuronidase is considered to be the key enzyme promoting DMH finally to turn into carcinogens in the colon (Manoj, Thampi, Leelamma, & Menon, Citation2001).
3.3. Tumor incidence
shows the incidence of colonic neoplasms in control and experimental mice. DMH-induced colon tumor development with a tumor incidence of 100%. However, the incidence was reduced to 33% in H-SOG and H-IOG groups, and 67% in L-SOG and L-IOG groups. In the mice of the NC group, no tumor was noticed.
Table 3. Incidence of colonic neoplasms.
3.4. Apoptosis in colon tumor cells of mice
Programmed cell death (apoptosis) is essential for normal development and tissue homeostasis in a healthy body, and its aberrant regulation contributes to multiple diseases including cancer, autoimmunity, and diabetes (Danial, Citation2007). shows the colon tumor cells apoptosis by Annexin V and PI double staining. Since in this experiment, the samples were fixed in 70% alcohol, there were almost no normal living cells. The fourth quadrant (lower right) cells for PI-negative and Annexin V-positive are apoptotic cells. As can be seen from , the colon cells apoptosis of the MC group was significantly decreased compared to all the other groups (p < .05). H-SOG and H-IOG groups are 34.27%, 28.67% higher than that of the NC group. No significant difference between the NC and low-dose groups was observed (p > .05). The apoptosis of high-dose groups was significantly higher than the low doses (p < .05), while there was no obvious difference between the SOG and IOG (same doses) groups (p > .05).
Figure 1. Apoptosis detected by flow cytometry with Annexin V-FITC conjugated with PI staining. The first and second quadrants (upper right and upper left) of PI+ cells were necrotic cells and the third quadrant (lower left) for the PI–/AV– cells are normal cells. The numbers indicate apoptotic cells (AV–/PI+, lower right quadrant) in percent of total gated cells (mean ± SD, n = 3). a, b, c, d, e, and f represent the NC, MC, H-SOG, L-SOG, H-IOG, and L-IOG groups, respectively. A representative experiment taken from three similar repeats is shown.
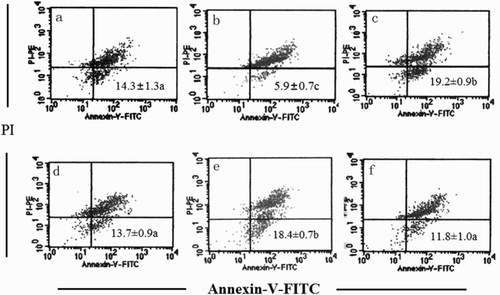
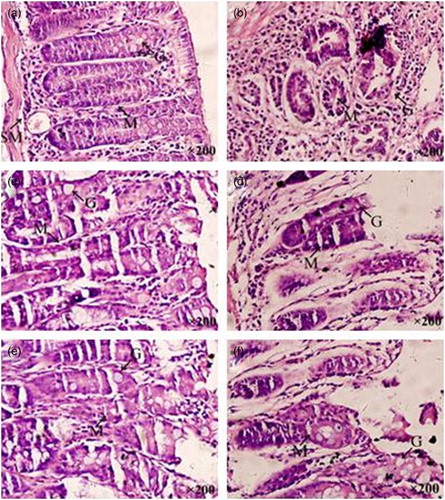
Figure 2. Photomicrograph of the colon of the NC (a), MC (b), H-SOG (c), L-SOG (d), H-IOG (e), and L-IOG (f) groups at week 18. Experimental groups exhibited minor dysplastic changes and injuries with varying degrees compared with the MC group. The H-SOG and H-IOG groups were almost the same as and similar to the NC group. Goblet cells (G), lymphocytic infiltration in mucosa (M), and edema in submucosa (SM) (H&E 200×).
Bifidobacteria can induce the tumor cells apoptosis and inhibit tumor growth (Ullman & Itzkowitz, Citation2011). Moreover, the butyric acid in SCFAs has the effect of promoting apoptosis and also provided energy for colon cells and protected intestinal tract (Ou, DeLany, Zhang, Sharma, & O'Keefe, Citation2012). This maybe one of the reasons that apoptosis in animals administrated oat β-glucan increased. Whether the oat β-glucan modifies the interrelated enzymes or even genes, thus increasing the apoptosis is worth being studied (Cabello, Bair, Bause, & Wondrak, Citation2009).
3.5. Histopathological studies
The colon segments of control mice showed closely packed normal mucus glands, goblet cells, with fewer lymphocytic clusters compared to severe dysplasia, increased lymphocytic clusters, reduced mucin-producing goblet cells and edema in submucosa of DMH-treated mice ((a) and 2(b)). Interestingly, animals belonging to H-SOG and H-IOG groups showed minor dysplastic changes but had a degree of injury compared with the control mice ((a), 2(c), and 2(e)). However, animals administered low doses of oat β-glucan had somewhat more serious injuries than the high doses, and there was no obvious difference between the SOG and IOG (same doses) groups ((c)–(f)).
4. Conclusion
Both the soluble and insoluble oat β-glucan have the function to reduce fecal bile acid levels, increase levels of SCFA in the intestinal contents and facilitate paracancerous apoptosis in mice and thereby prevent the DMH-induced colon injury and cancer risk, and the effect of the high-dose group is better than the low-dose group. However, the causes of oat β-glucan preventing the early colon carcinogenesis in mice are complex, multifaceted, and its specific mechanism still needs further study. In addition, the difference in mechanism of SOG and IOG is also worth being studied.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmed, R., Segal, I., & Hasan, H. (2000). Fermentation of dietary starch in humans. The American Journal of Gastroenterology, 95(4), 1017–1020. doi: 10.1111/j.1572-0241.2000.01848.x
- Bingham, S. A. (1990). Mechanisms and experimental and epidemiological evidence relating dietary fibre (non-starch polysaccharides) and starch to protection against large bowel cancer. Proceedings of The Nutrition Society, 49(2), 153–171. doi: 10.1079/PNS19900021
- Cabello, C. M., Bair, W. B., Bause, A. S., & Wondrak, G. T. (2009). Antimelanoma activity of the redox dye DCPIP (2,6-dichlorophenolindophenol) is antagonized by NQO1. Biochemical Pharmacology, 78(4), 344–354. doi: 10.1016/j.bcp.2009.04.016
- Chirta, S., Sabitha, K., Nalini, N., & Menon, V. P. (1994). Influence of red chilli on lipids and bile acids in different tissues in experimental colon cancer. Indian Journal of Experimental Biology, 32(11), 793–796.
- Danial, N. N. (2007). BCL-2 family proteins: Critical checkpoints of apoptotic cell death. Clinical Cancer Research, 13(24), 7254–7263. doi: 10.1158/1078-0432.CCR-07-1598
- Degirolamo, C., Modica, S., Palasciano, G., & Moschetta, A. (2011). Bile acids and colon cancer: Solving the puzzle with nuclear receptors. Trends in Molecular Medicine, 17(10), 564–572. doi: 10.1016/j.molmed.2011.05.010
- Devasena, T., Rajasekaran, K. N., Gunasekara, G., Viswanathan, P., & Menon, V. P. (2003). Anticarcinogenic effect of bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione a curcumin analog on DMH-induced colon cancer model. Pharmacological Research, 47(2), 133–140. doi: 10.1016/S1043-6618(02)00283-9
- Dodevska, M. S., Djordjevic, B. I., Sobajic, S. S., Miletic, I. D., Djordjevic, P. B., & Dimitrijevic-Sreckovic, V. S. (2013). Characterisation of dietary fibre components in cereals and legumes used in Serbian diet. Food Chemistry, 141(3), 1624–1629. doi: 10.1016/j.foodchem.2013.05.078
- Dong, J. L., Zhu, Y. Y., Li, L., Shen, R. L., & Li, H. (2014). Effect of oat soluble and insoluble β-glucan on lipid metabolism and intestinal lactobacillus in high-fat dietinduced obese mice. Journal of Food and Nutrition Research, 2(8), 510–516. doi: 10.12691/jfnr-2-8-13
- Hague, A., Singh, B., & Parasceva, C. (1997). Butyrate acts as a survival factor for colonic epithelial cells: Further fuel for the in vivo versus in vitro debate. Gastroenterology, 112, 1036–1040. doi: 10.1053/gast.1997.v112.agast971036
- Hill, M. J. (1975). The role of colon anaerobes in the metabolism of bile acids and steroids and its relation to colon cancer. Cancer, 36(6), 2387–2400.
- Johansson, L., Tuomainen, P., Ylinen, M., Ekholm, P., & Virkki, L. (2004). Structural analysis of water-soluble and-insoluble β-glucans of whole-grain oats and barley. Carbohydrate Polymers, 58(3), 267–274. doi: 10.1016/j.carbpol.2004.06.041
- Kaczmarczyk, M. M., Miller, M. J., & Freund, G. G. (2012). The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism, 61(8), 1058–1066. doi: 10.1016/j.metabol.2012.01.017
- Kim, Y. I. (2000). AGA technical review: Impact of dietary fiber on colon cancer occurrence. Gastroenterology, 118(6), 1235–1257. doi: 10.1016/S0016-5085(00)70377-5
- Kuppusamy, P., Yusoff, M. M., Maniam, G. P., Ichwan, S. J. A., Soundharrajan, I., & Govindan, N. (2014). Nutraceuticals as potential therapeutic agents for colon cancer: A review. Acta Pharmaceutica Sinica B, 4(3), 173–181. doi: 10.1016/j.apsb.2014.04.002
- MacDonald, R. S., & Wagner, K. (2012). Influence of dietary phytochemicals and microbiota on colon cancer risk. Journal of Agricultural and Food Chemistry, 60(27), 6728–6735. doi: 10.1021/jf204230r
- Macfarlane, G. T., & Macfarlane, S. (2012). Bacteria, colonic fermentation, and gastrointestinal health. Journal of AOAC International, 95(1), 50–60. doi: 10.5740/jaoacint.SGE_Macfarlane
- Manoj, G., Thampi, B. S., Leelamma, S., & Menon, P. V. (2001). Effect of dietary fiber on the activity of intestinal and fecal beta-glucuronidase activity during 1,2-dimethylhydrazine induced colon cancinogenesis. Plant Foods for Human Nutrition, 56(1), 13–21. doi: 10.1023/A:1008188009174
- Mitsou, E. K., Panopoulou, N., Turunen, K., Spiliotis, V., & Kyriacou, A. (2010). Prebiotic potential of barley derived β-glucan at low intake levels: A randomised, double-blinded, placebo-controlled clinical Study. Food Research International, 43(4), 1086–1092. doi: 10.1016/j.foodres.2010.01.020
- Murphy, N., Norat, T., Ferrari, P., Jenab, M., Bueno-De-Mesquita, B., Skeie, G., … Riboli, E. (2012). Dietary fibre intake and risks of cancers of the colon and rectum in the european prospective investigation into cancer and nutrition (EPIC). PLoS ONE, 7(6), e39361. doi: 10.1371/journal.pone.0039361
- Ou, J. H., DeLany, J. P., Zhang, M., Sharma, S., & O'Keefe, S. J. D. (2012). Association between low colonic short-chain fatty acids and high bile acids in high colon cancer risk populations. Nutrition and Cancer, 64(1), 34–40. doi: 10.1080/01635581.2012.630164
- Porter, J. L., Fordtran, J. S., SantaAna, C. A., Emmett, M., Hagey, L. R., MacDonald, E. A., … Hofmann, A. F. (2003). Accurate enzymatic measurement of fecal bile acids in patients with malabsorption. Journal of Laboratory and Clinical Medicine, 141(6), 411–418. doi: 10.1016/S0022-2143(03)00040-4
- Queenan, K. M., Stewart, M. L., Smith, K. N., Thomas, W., Fulcher, R. G., & Slavin, J. L. (2007). Concentrated oat β-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial. Nutrition Journal, 6(1), 6. doi: 10.1186/1475-2891-6-6
- Roberfroid, M., Gibson, G. R., Hoyles, L., Mccartney, A. L., Rastall, R., Rowland, I., … Meheust, A. (2010). Prebiotic effects: Metabolic and health benefits. British Journal of Nutrition, 104(S2), S1–S63. doi: 10.1017/S0007114510003363
- Scott, K. P., Duncan, S. H., & Flint, H. J. (2008). Dietary fibre and the gut microbiota. Food and Nutrition Bulletin, 33, 201–211. doi: 10.1111/j.1467-3010.2008.00706.x
- Shen, R. L., Cai, F. L., Dong, J. L., & Hu, X. Z. (2011). Hypoglycemic effects and biochemical mechanisms of oat products on streptozotocin-induced diabetic mice. Journal of Agricultural and Food Chemistry, 59(16), 8895–8900. doi: 10.1021/jf200678q
- Shen, R. L., Dang, X. Y., Dong, J. L., & Hu, X. Z. (2012). Effects of oat β-glucan and barley β-glucan on fecal characteristics, intestinal microflora, and intestinal bacterial metabolites in rats. Journal of Agricultural and Food Chemistry, 60(45), 11301–11308. doi: 10.1021/jf302824h
- Ullman, T. A., & Itzkowitz, S. H. (2011). Intestinal inflammation and cancer. Gastroenterology, 140(6), 1807–1816.e1. doi: 10.1053/j.gastro.2011.01.057
- Verma, A., & Shukla, G. (2013). Administration of prebiotic inulin suppresses 1,2 dimethylhydrazine dihydrochloride induced procarcinogenic biomarkers fecal enzymes and preneoplastic lesions in early colon carcinogenesis in Sprague Dawley rats. Journal of Functional Foods, 5(2), 991–996. doi: 10.1016/j.jff.2013.02.006
- Yapo, B. M., & Koffi, K. L. (2008). Dietary fiber components in yellow passion fruit rind – a potential fiber source. Journal of Agricultural and Food Chemistry, 56(14), 5880–5883. doi: 10.1021/jf073247p
