ABSTRACT
A water-soluble expolysaccharide (EPS) was prepared from Bifidobacterium animalis RH by enzymatic hydrolysis, ethanol precipitation and Sepharose CL-6B column chromatography. Its immunomodulating activity was evaluated using murine macrophage cell line RAW 264.7. The data obtained showed that the EPS promoted proliferation and phagocytosis activity of RAW 264.7 cells. In addition, the EPS promoted RAW 264.7 cells to produce NO, IL-6, TNF-α, MCP-1 and MIP-1α in a dose-dependent manner. Further work revealed that the EPS activated RAW 264.7 cells majorly through TLR4.
1. Introduction
Prebiotics are selectively fermentable ingredients that allow specific changes in the composition and/or activity of gastrointestinal microbiota that allow benefits to the host (Gibson, Probert, Loo, Rastall, & Roberfroid, Citation2004). The food industry is interested in prebiotics for their application as functional ingredients in foods targeted toward health-conscious consumers (Hutkins et al., Citation2015). Prebiotics are health-promoting for their immunoregulative activities (Breton et al., Citation2015; Du, Liu, Zhang, & Li, Citation2013; Kim et al., Citation2013; Rajkumar et al., Citation2015; Ramnani, Costabile, Bustillo, & Gibson, Citation2015; Shimakage, Citation2014; Spencer et al., Citation2013; Yu et al., Citation2015). Exopolysacharides (EPSs) are long-chain polysaccharides consisting of branched, repeating units of sugars or sugarderivatives (Ismail & Nampoothiri, Citation2010). EPSs synthesized by biofidobacteria are potential prebiotics, which promote colonization of probiotic bacteria (German et al., Citation1999). Current researches mainly focus on the natural polysaccharides from plants, marine algae and others. Few studies have been carried out about immunoregulative activities of the EPS produced by Bifidobacteria, which are of importance in the microbial ecology of the human gut.
The strain Bifidobacterium animalis RH was isolated from the feces of centenarians in Bama, Guangxi of China, which was listed as the fifth officially certificated village of longevity by the International Society of Natural Medicine in 1991. The structure and its antioxidant activity of the EPS from B. animalis RH have been reported previously (Shang, Xu, & Li, Citation2013; Xu, Shang, & Li, Citation2011). In the present study, we investigated the immunostimulatory activity of this EPS on macrophages in vitro. Our data showed that the EPS from B. animalis RH was immunoregulative by promoting in vitro proliferation and phagocytosis activity of RAW264.7 cells.
2. Materials and methods
2.1. Bacterial strain and cell culture
B. animalis RH was originally isolated from the feces of Bama centenarians in Guangxi, China, which is a great producer of EPS. Its complete genome sequence was deposited in the GenBank database (accession number CP007755) (Liu et al., Citation2014). The strain was kept frozen at −70°C in a sterilized mixture of culture medium and glycerol (80:20, v/v) and grown at 37°C in MRS broth and MRS-Agar (de Man–Rogosa–Sharpe, Difco Laboratories; Becton, Diskinson and Co., USA) under anaerobic conditions.
Mouse RAW 264.7 macrophage cells were purchased from American Type Culture Collection (ATCC, Manassas, WA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Amresco), supplemented with 10% (v/v) fetal calf serum (Zhejiang tianhang Biological technology stock Co., Ltd.), penicillin (100 IU/mL, Gibco) and streptomycin (100 μg/mL, Gibco) at 37°C in an atmosphere of 5% CO2/95% air at constant humidity.
2.2. Isolation and purification of the EPS
The EPS was extracted from the fermentation supernatant of B. animalis RH by enzymatic hydrolysis method. In brief, the strain was cultured in skim milk at 37°C for 60 h under anaerobic conditions. Then the bacterial cells were removed by centrifugation at 8000 rpm for 10 min and the fermentation supernatant was harvested and the pH adjusted to 7.5. Subsequently, the protein and small molecules in the supernatant were hydrolyzed at 45°C for 120 min by adding 2 mg/mL compound enzyme consisting of 0.67 mg/mL neutral protease (Beijing Solarbio Science & Technology Co., Ltd) and 1.33 mg/mL trypsin (Amresco, USA). Enzymatic hydrolysis was stopped by heating in boiling water for 5 min and then supernatant was collected by centrifugation at 8000 rpm for 10 min. The crude EPS was collected from the concentrated supernatant by ethanol precipitation and centrifugation. Subsequently the crude EPS was dialyzed using dialysis bags (Mw cut-off 8000–12,000 Da) against distilled water. The EPS was purified by a Sepharose CL-6B (Pharmacia) column (1.6 cm×80 cm, Tosoh, Japan) chromatography. Finally the purity and the average molecular mass of the EPS were determined by gel permeation chromatography-multiangle laser light scattering technology (GPC/MALLS, Wyatt Technology Crop.).
2.3. Cell proliferation assay
The proliferation assay was used to evaluate the cell viability and cytotoxicity (Schilling, Miralles, & Eder, Citation2014). Cell proliferation was measured by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay (Li et al., Citation2008). RAW 264.7 macrophages were cultured in 96 serial concentrations of EPS (25, 50, 100, 200, 400 and 800 μg/mL) for 12, 24 and 48, respectively. The MTT solution (5 mg/mL, Beijing Solarbio Science & Technology Co., Ltd) was added and cells were cultured for an additional 4 h. Culture medium was then removed.and 150 μL of dimethyl sulfoxide (DMSO, Amresco) was added to each well. Absorbance at 570 nm was measured using a microplate reader (Synergy H1, BioTek Instruments, Inc.).
2.4. Phagocytic assay
The phagocytic ability of macrophage was measured by neutral red uptake assay (Chen, Zhang, Shen, & Wang, Citation2010). RAW 264.7 cells were cultured with EPS (25, 50, 100, 200, 400 and 800 μg/mL) or lipopolysaccharide (LPS) (10 μg/mL) at 37°C, 5% CO2 for 12, 24 and 48 h, respectively. 100 μL/well of 0.075% neutral red (Beijing Solarbio Science & Technology Co., Ltd.) was added and incubated for 2 h at 37°C. Culture medium was discarded and cells were washed with phosphate-buffered saline (PBS) three times to remove the neutral red that was not phagocytized by macrophage cells. Then, cell lysis buffer (ethanol and 0.01% acetic acid at the ratio of 1:1, 100 μL/well) was added and cultured for 4 h. The absorbance at 540 nm was measured by a microplate reader (Synergy H1, BioTek Instruments, Inc.).
2.5. Nitric oxide (NO) assay
Nitrite accumulation in the medium was used as an indicator of NO production and colorimetric assays with Griess reagent were used to measure NO production (Green et al., Citation1982; Mazzone, Rigato, & Tiribelli, Citation2010). RAW 264.7 macrophages were treated with LPS (10 μg/mL) or EPS (25, 50, 100, 200, 400 and 800 μg/mL) for 12, 24 and 48 h. Fifty-microliter mediums were incubated with equal volumes of modified Griess reagent (1% sulfanilamide, 0.1% napthylethylenedia-mine dihydrochloride and 2.5% H3PO4, Beyotime) at room temperature for 15 min. Nitrite production was determined by measuring the absorbance at 540 nm with a standard curve generated by NaNO2.
2.6. Cellular reactive oxygen species (ROS) assay
After RAW 264.7 macrophages were cultured with LPS (10 μg/mL) or EPS (25, 50, 100, 200, 400 and 800 μg/mL) for 24 h, cells were collected, washed with DMEM and stained with 10 μM of 2′,7′-dichlorfluorescein-diacetate (Beyotime) at 37°C, 5% CO2 for 20 min. Cells were washed again with PBS twice and the cell suspension was loaded onto 96-well white ELISA plates at 100 μL/well. The fluorescence intensities were measured at 488 nm excitation wavelength and 525 nm emission wavelength using a multimode microplate reader (Synergy H1, BioTek Instruments, Inc.).
2.7. Chemokines assay
After RAW 264.7 cells were cultured with EPS (0, 50, 100, 200, 400 and 800 μg/mL) or LPS (10 μg/mL) for 24 h at 37°C, culture medium was collected. Chemokines MCP-1 (CCL-2) and MIP-1α (CCL-3) in medium were determined by assay kits (Beyotime) according to the manufacturer’s instructions.
2.8. Quantitative PCR analysis of IL-6, TNF-α, iNOS mRNA
RAW 264.7 cells were cultured with EPS (0, 50, 100, 200, 400 and 800 μg/mL) or LPS (10 μg/mL) at 37°C for 24 h. Total RNA from cells was isolated using TRIzol Regeant (Invitrogen, USA). cDNA was synthesized using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Germany). qPCR was performed using the SYBR premix ExTaq II (TaKaRa, Japan) and the IQ5 real-time PCR detection system (Bio-Rad, Hercules, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The relative expression of specific genes was calculated by using the 2−ΔΔCT method according to Livak and Schmittgen (Citation2001). The primer sequences used were as follows: IL-6, forward, 5′-ATCATTGATGCCCCAGGACA-3′, reverse, 5′-CCAGGGTGTAAGCCAGAAGA-3′; TNF-α, forward, 5′-AGCACAGAAAGCATGATCCG-3′, reverse, 5′-CCTGATGAGAGGGAGGCCATT-3′; iNOS, forward, 5′-CACCTTGGAGTTCACCCAGT-3′, reverse, 5′-ACCACTCGTACTTGGGATGC-3′; and GAPDH, forward, 5′-AACAGCAACTCCCACTCTTC-3′, reverse, 5′-CCTGTTGCTGTAGCCGTATT-3′.
2.9. Receptor assay
RAW 264.7 cells were cultured with EPS (0, 25, 50, 100 and 200 μg/mL) at 37°C for 24 h. Total RNA of RAW 264.7 cells were isolated using TRIzol Regeant (Invitrogen, USA) and cDNA was synthesized using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Germany). Then the expression of TLR2, TLR4 and CR3 were analyzed using qRT-PCR. The primer sequences used were as follows: TLR2, sense, 5′-CACCACTGCCCGTAGATGAAG-3′, antisense, 5′-AGGGTACAGTCGTCGAACTCT-3′; TLR4, sense, 5′-GCCTTTCAGGGAATTAAGCTCC-3′, antisense, 5′-GATCAACCGATGGACGTGAAA-3′; CR3, sense, 5′-CCATGACCTTCCAAGAGAATGC-3′, antisense, 5′-ACCGGCTTGTGCTGTAGTC-3′; and β-actin, sense. 5′-AGCCATGTACGTAGCCATCC-3′, antisense, 5′-CTCTCAGCTGTGGTGGTGAA-3′. The amount of each respective amplification product was determined relative to the house-keeping gene β-actin.
In order to further determine the receptor of the EPS, RAW 264.7 cells were treated with TLR2 antibody (10 μg/mL), TLR4 antibody (10 μg/mL) and CR3 antibody (10 μg/mL) (all of them were purchased from eBioscience) for 1 h to blockade the matched receptors. Then above cells were cultured with EPS (200 μg/mL) at 37°C for 24 h and the cells treated with EPS in absence of antibody and the cells without any treatment were used as control groups. Then the production of NO and TNF-α were determined with Griess reagent and ELISA, respectively.
2.10. Statistical analysis
Statistical significance was evaluated by one-way analysis of variance to determine the difference between groups (GraphPad Prism 5.0, SanDiego, CA, USA). Values of p < .05 were regarded as statistically significant. Each experiment was repeated at least three times per group. Data were expressed as mean ± SEM.
3. Results and discussion
3.1. Purity and molecular mass determination of the EPS
Crude EPS was purified by gel chromatography on a Sepharose CL-6B column, which was eluted with 0.05 M NaCl. As shown in (a), the sample generated one single elution peak affording EPS. The EPS solution was freeze dried to fine white powder shown in (b). The purity and average molecular mass of EPSs were determined using GPC/MALLS (the second peak in (c)). As shown in , the purity of the EPSs was 97.045% and the average molecular mass was 5.946 × 104 D. Previously, EPSs were mostly isolated by chemical method, of which the activity of polysaccharide could be inhibited by chemical regents and residual chemical regents may result in potential safety hazard (Dabour & LaPointe, Citation2005; Paulo et al., Citation2012). In the present study, the EPS was isolated by enzymatic hydrolysis to avoid the above problems. Compared with chemical method in our previous research (Xu et al., Citation2011), EPSs isolated by enzymatic hydrolysis exhibited similar molecular mass but higher purity. The structure of the EPSs was reported in previous study (Shang et al., Citation2013).
Figure 1. Elution profile (a), photograph (b) and GPC/MALLS (c) of EPS. The EPS was eluted on Sepharose CL-6B column (1.6 cm × 80 cm) with 0.05 M NaCl at a flow rate of 0.8 mL/min (a). The EPS solution was freeze dried to powder (b) for storage and its purity and the average molecular mas were determined by GPC/MALLS (red curve, green curve and blue curve represent the laser value, the ultraviolet value and refractive index, respectively).
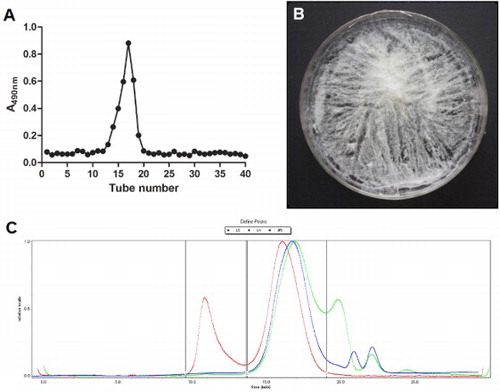
Table 1. Molecular weight and purity of EPS.
3.2. EPSs promoted proliferation and phagocytic activity of RAW 264.7 cells
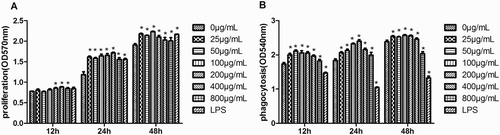
Macrophages play an important role in initiating the adaptive immune response and phagocytosis is a crucial defense mechanism, which protects against pathogen invasions (Miyata & van Eeden, Citation2011). To investigate the effect of EPSs on the proliferation and phagocytic activity of macrophages, RAW 264.7 cells were treated with EPSs (25, 50, 100, 200, 400 and 800 μg/mL). As shown in (a), EPSs promoted the proliferation of RAW264.7 cells in a time-dependent but not dose-independent manner. The phagocytosis assay showed that EPSs significantly increased neutral red uptake after cells were treated with EPSs for 12 and 24 h treatment ((b)). In contrast, LPSs inhibited phagocytosis activity of RAW 264.7 cells. Therefore, EPSs promoted the proliferation and enhanced phagocytic activity of macrophages.
Figure 2. Effects of the EPS on proliferation (a) and phagocytic (b) activity of RAW 264.7 cells. Cells were pretreated with EPS (0, 25, 50, 100, 200, 400 and 800 μg/mL) or LPS (10 μg/mL) for 12, 24 and 48 h, respectively. Then the proliferation (a) and phagocytic (b) activity were determined by measuring optical densities. Values are mean ± SEM of three independent experiments. *p < .05, **p < .01 compared with untreated group(0 μg/mL).
3.3. EPSs increased the production of NO but not ROS by RAW 264.7 cells
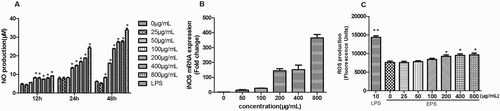
Since NO is a highly reactive free radical involved in a number of physical and pathological processes and may play an important role in the pathophysiology of various diseases and macrophage toxicity (Lakics & Vogel, Citation1998; Moncada, Palmer, & Higgs, Citation1991), we addressed whether EPSs modulate NO production from the macrophages. As shown in (a), NO production was significantly induced in macrophages by EPSs in a dose-dependent manner after cells were treated for 24 and 48 h. The effect of EPSs on iNOS expression is shown in (b); EPS induced iNOS expression dose-dependently, which was consistent with NO production ((a)). ROS assay showed that the EPSs have no significant influence on ROS production, but the LPSs increased ROS production ((c)).The balance between NO and ROS is important for the maintenance of homeostasis (Cao, Roursgaard, Danielsen, Moller, & Loft, Citation2014; Huang, Mei, & Zhang, Citation2011). These results indicated that the EPSs may promote the release of NO to decrease the production of ROS. According to the results, we speculated that the EPSs may activate macrophages but did not induce inflammation.
Figure 3. Effects of the EPS on NO production (a), iNOS mRNA expression (b) and ROS production (c) of RAW 264.7 cells. Cells were pretreated with EPS (0, 25, 50, 100, 200, 400 and 800 μg/mL) or LPS (10 μg/mL) for 12, 24 and 48 h, respectively and the NO production (a) was determined using Griess reagent. Cells were pretreated with EPS (0, 50, 100, 200, 400 and 800 μg/mL) or LPS (10 μg/mL) for 24 h and iNOS mRNA expression (D) was determined using qRT-PCR (b). Cells were pretreated with EPS (0, 25, 50, 100, 200, 400 and 800 μg/mL) or LPS (10 μg/mL) for 24 h and ROS production was determined by measuring fluorescence intensities. Values are mean ± SEM of three independent experiments. *p < .05, **p < .01 compared with the untreated group (0 μg/mL).
3.4. EPSs regulated the release of cytokines (IL-6 and TNF-α) and chemokines (MCP-1 and MIP-1α) from RAW 264.7 cells
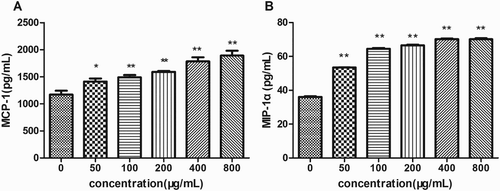
Cytokines are mainly produced by macrophages and lymphocytes mediate the unleashing of an effective immune response, link innate and adaptive immunity, and influence the macrophage’s microenvironment (Huynh, Kay, Stow, & Grinstein, Citation2007). Transcriptional levels of cytokines (IL-6 and TNF-α) were measured by qRT-PCR after 24 h of incubation with EPSs. As shown in (a), 800 μg/mL of EPSs obviously elevated IL-6 mRNA level, while no effect of EPSs at lower concentrations was observed. Interestingly, EPSs promoted transcriptional levels of TNF-α in a dose-dependent manner ((b)). Moreover, the mRNA expression of TNF-α within cells treated with 800 μg/mL of EPSs was higher than any other groups. The EPSs increased the secretion of chemokines MCP-1 and MIP-1α in a dose-dependent manner after treatment for 24 h ((a),(b)).These results suggested that the EPS was an activator of macropahges and enhanced the production of cytokines IL-6, TNF-α and chemokines.
Figure 4. Effects of EPS on IL-6 (a), and TNF-α (b) transcription of RAW 264.7 cells. Cells were incubated with EPS (0, 50, 100, 200, 400 and 800 μg/mL) for 24 h, then the mRNA expression of IL-6 and TNF-α were analyzed using real-time PCR. Values are mean ± SEM of three independent experiments.
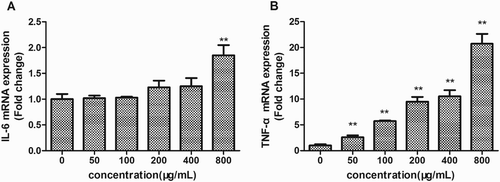
Figure 5. Effects of EPS on the expression levels of MCP-1 (a) and MIP-1α (b) of RAW 264.7 cells. Cells were incubated with EPS (0, 50, 100, 200, 400 and 800 μg/mL) for 24 h and the expression levels of MCP-1 and MIP-1α were assessed using ELISA. Values are mean ± SEM of three independent experiments. *p < .05, **p < .01 compared with the untreated group (0 μg/mL).
Macrophages are the first line of host defense against bacterial infections and cancer. Activated macrophages secrete some secondary compounds. Among them, IL-6 plays a crucial role in the host immune response, acute protein synthesis and the maintenance of homeostasis (Luig et al., Citation2015). TNF-α is a cytokine with tumor necrosis activity that is secreted mainly by macrophages and has been recognized as an important host regulatory molecule (Dimitrijević et al., Citation2014). In this study, EPSs activated RAW 264.7 cells to release proinflammatory factor IL-6 and TNF-α, which activate T cells and B cells to initiate adaptive immune response and kill tumor cells directly. Chemokines constitute a large family of structurally similar cytokines that contain a signature of conserved cysteine residues joined by disulfide bridges, which play crucial roles in cell migration (Czaja, Citation2014; Stuart & Baune, Citation2014). Furthermore, chemokines are able to control immunological cell migration in the process of inflammatory and other responses. MCP-1, a member of chemokine CC subfamily, induces monocytes, basophilic granulocytes, memory T cells and dendritic cells to the inflammatory zone and enhances the anti-tumor activity of monocytes (Wood et al., Citation2014). MIP1-α, also called CCL-3, is important for the immune response of infection and inflammation (Nagashima, Nakagawa, & Kushiro, Citation2012). It induces and activates polymorphonuclear leukocytes to acute inflammatory zone and promotes the production of proinflammatory cytokines by fibroblast and macrophages (Nagashima et al., Citation2012). In addition, MIP1-α is important in leukocytes’ migration in the process of normal repair and development (Nagashima et al., Citation2012). Our results indicated that the EPSs increased the production of MCP-1 and MIP1-α, which may induce more immunocytes to transfer to the inflammatory zone and tumor site and promote the release of proinflammatory cytokines and have a direct killing effect on tumor cells.
3.5. EPSs activated RAW 264.7 through toll-like receptor 4
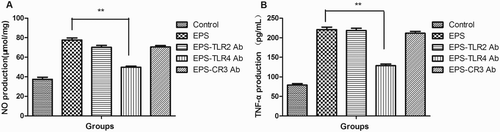
Polysaccharides cannot penetrate cells because of their large molecular mass, so the first step in the modulation of celluar events is binding to its receptors (Lull, Wichers, & Savelkoul, Citation2005). As shown in , the EPSs activated the transcriptional levels of TLR2, TLR4 and CR3 in a dose-dependent manner. When TLR4 was blocked by antibodies, the production of NO and TNF-α decreased significantly ((a),(b)). Antibodies for TLR2 and CR3 did not diminish the effect of EPS on the production of NO and TNF-α ((a),(b)). These results indicated that EPSs activated RAW 264.7 cells to stimulate immune regulation majorly through binding to TLR4.
Figure 6. mRNA transcriptional levels of TLR2, TLR4 and CR3 of RAW 264.7 cells treated with EPS (0, 25, 50, 100 and 200 μg/mL). Cells were incubated with EPS (0, 50, 100 and 200 μg/mL) for 24 h, then the mRNA expression of TLR2, TLR4 and CR3 were analyzed using real-time PCR. Values are mean ± SD of three independent experiments.
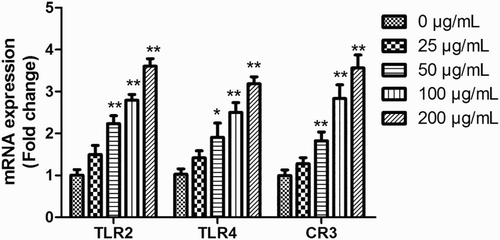
Figure 7. NO (a) and TNF-α (b) production of RAW 264.7 cells treated with EPS in the presence or absence of TLR2, TLR4 and CR3 antibodies. Cells were treated with TLR2 antibody, TLR4 antibody and CR3 antibody for 1 h and then cultured with EPS (200 μg/mL) for another 24 h. The NO production was determined using a chemical method (a) and TNF-α prodution was determined using ELISA (b).
TLR4 are expressed on multiple cells including macrophages, dentritic cells, B cells, T cells and endothelial cells. TLR4 ligands can be divided into four categories: (1) polysaccharides; (2) glycolipids, including G-bacterial LPS and lipid A; (3) DNA, specifically CpG motifs of bacterial DNA; and (4) protein, including EDA fibronectin, fibrinogen and tenascin-C. Among them, many polysaccharides could function as immunomodulators (Zhang, Qi, Guo, Zhou, & Zhang, Citation2016). TLR4 signaling plays a role in immune cell activation by polysaccharide biological response modifiers and TLR4 has been identified as an important membrane receptor of polysaccharides. It has been reported that polysaccharides from Platycodon grandiflorum and Ganoderma lucidum interact with TLR4 in macrophages (Shao, Dai, Xu, Lin, & Gao, Citation2004; Yoon et al., Citation2004). Polysaccharides from Sparassis crispa, Angelicangigas Nakai, Cordyceps militaris and Acanthopanax koreanum activate DCs and B cells through TLR4 (Han et al., Citation2001; Kim, Kim, Kang, et al., Citation2010; Kim, Kim, Ryu, et al., Citation2010; Kim et al., Citation2007). Binding of ligands to TLR4 leads to a change in the complex between the cytoplasmic region of TLR and the adaptor protein MyD88. A polysaccharide from Dictyophora indusiata could induce macrophage activation via TLR4/NF-κB signaling pathways and polysaccharide of Dendrobium huoshanense activates macrophages via its direct binding to TLR4 to trigger TLR4 signaling pathways as well. (Deng et al., Citation2016; Xie et al., Citation2016). Yu et al. (Citation2016) has reported that an EPS which was isolated and purified from an Antarctic psychrophlilic bacterium B-3 might activate RAW264.7 cells by combining with TLR4 on the cell surface and triggering activation of NF-κB signaling pathways, implying that this EPS could activate macrophages and regulate initial immune response (Yu et al., Citation2016). In the present study, EPSs activated RAW 264.7 through toll-like receptor 4. Although the protein expression of TLR4 was not shown, we determined TLR4 was the main receptor of the EPS through an antibody blocking assay. The changes of protein expression of TLR4 were usually in accordance with the mRNA expression. For example, it has been reported that LPS induced TLR4 mRNA levels analyzed by quantitative real-time PCR, and the protein expression of TLR4 was also increased analyzed by flow cytometry (Ibeagha-Awemu et al., Citation2008). In addition, some materials have the same effects on mRNA and protein expression of TLR4, such as soothing liver and invigorating spleen recipes (Gong et al., Citation2014). Furthermore, according to previous studies, we speculated that upon binding with the EPS, TLR4 might activate MyD88-dependent and TRIF-dependent signaling pathways. Although the detailed events downstream of MyD88 and TRIF differ, MAPKs and NF-κB signaling which are downstream of TLR4 might be activated to release IL-6 and TNF-α. In addition, the EPS might induce the phosphorylation of ERK, JNK and p38. The downstream signaling pathways are not well defined and further studies are need.
4. Conclusion
In the present study, the EPS with high purity of 97.045% was isolated from B. animalis RH using enzymatic hydrolysis. The EPS promoted RAW 264.7 macrophages proliferation and phagocytosis. It also stimulated RAW 264.7 cells to produce NO, IL-6, TNF-α, MCP-1 and MIP-1α. The EPS activates RAW 264.7 macrophages primarily by binding to TLR4. Therefore, the EPS from B. animalis RH has the potential to be prebiotic for functional foods.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Lei Liu is the Ph.D. candidate at the College of Food Science and Nutritional Engineering, China Agricultural University. She mainly focuses on the mechanisms and applications of probiotics and their active metabolites.
Hui Li is the master at the College of Food Science and Nutritional Engineering, China Agricultural University. She mainly focuses on anti-inflammatory effects of probiotics.
Ri-Hua Xu is associate professor at the School of Life Sciences, Inner Mongolia University. She mainly focuses on functions of active metabolites of probiotics.
Ping-Lan Li is the Professor and Ph.D. supervisor at the College of Food Science and Nutritional Engineering, China Agricultural University. She mainly focuses on the mechanisms and applications of probiotics and their active metabolites.
References
- Breton, J., Plé, C., Guerin-Deremaux, L., Pot, B., Lefranc-Millot, C., Wils, D., & Foligné, B. (2015). Intrinsic immunomodulatory effects of low-digestible carbohydrates selectively extend their anti-inflammatory prebiotic potentials. Biomed Research International, 2015, 1–13. doi: 10.1155/2015/162398
- Cao, Y., Roursgaard, M., Danielsen, P. H., Moller, P., & Loft, S. (2014). Carbon black nanoparticles promote endothelial activation and lipid accumulation in macrophages independently of intracellular ROS production. PLoS ONE, 9(9), e106711. doi: 10.1371/journal.pone.0106711
- Chen, W., Zhang, W., Shen, W., & Wang, K. (2010). Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cellular Immunology, 262(1), 69–74. doi: 10.1016/j.cellimm.2010.01.001
- Czaja, A. J. (2014). Review article: Chemokines as orchestrators of autoimmune hepatitis and potential therapeutic targets. Alimenary Pharmacology & Therapeutics, 40(3), 261–279. doi: 10.1111/apt.12825
- Dabour, N., & LaPointe, G. (2005). Identification and molecular characterization of the chromosomal exopolysaccharide biosynthesis gene cluster from Lactococcus lactis subsp. cremoris SMQ-461. Applied and Environmental Microbiology, 71(11), 7414–7425. doi: 10.1128/AEM.71.11.7414-7425.2005
- Deng, C., Shang, J., Fu, H., Chen, J., Liu, H., & Chen, J. (2016). Mechanism of the immunostimulatory activity by a polysaccharide from Dictyophora indusiata. International Journal of Biological Macromolecules, 91, 752–759. doi: 10.1016/j.ijbiomac.2016.06.024
- Dimitrijević, M., Stanojević, S., Vujić, V., Aleksić, I., Pilipović, I., & Leposavić, G. (2014). Aging oppositely affects TNF-alpha and IL-10 production by macrophages from different rat strains. Biogerontology, 15(5), 475–486. doi: 10.1007/s10522-014-9513-4
- Du, Z., Liu, H., Zhang, Z., & Li, P. (2013). Antioxidant and anti-inflammatory activities of Radix Isatidis polysaccharide in murine alveolar macrophages. International Journal of Biological Macromolecules, 58, 329–335. doi: 10.1016/j.ijbiomac.2013.04.037
- German, B., Schiffrin, E. J., Reniero, R., Mollet, B., Pfeifer, A., & Neeser, J. R. (1999). The development of functional foods: Lessons from the gut. Trends in Biotechnology, 17(12), 492–499. doi: 10.1016/S0167-7799(99)01380-3
- Gibson, G. R., Probert, H. M., Loo, J. V., Rastall, R. A., & Roberfroid, M. B. (2004). Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutrition Research Reviews, 17(2), 259–275. doi: 10.1079/NRR200479
- Gong, X. W., Yang, Q. H., Yan, H. Z., Zhang, Y. P., Liang, Y. J., Liu, Y. Z., … Li, Y. Y. (2014). Effects of soothing liver and invigorating spleen recipes on LPS-induced hepatocytes injury of rats and TLR4/p38MAPK signal pathway. Zhongguo Zhong Yao Za Zhi, 39(20), 4027–4033.
- Green, L. C., Wagner, D. A., Glogowski, J., Skipper, P. L., Wishnok, J. S., & Tannenbaum, S. R. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry, 126(1), 131–138. doi: 10.1016/0003-2697(82)90118-X
- Han, S. B., Park, S. H., Lee, K. H., Lee, C. W., Lee, S. H., Kim, H. C., … Kim, H. M. (2001). Polysaccharide isolated from the radix of Platycodon grandiflorum selectively activates B cells and macrophages but not T cells. International Immunopharmacology, 1(11), 1969–1978. doi: 10.1016/S1567-5769(01)00124-2
- Huang, M., Mei, X., & Zhang, S. (2011). Mechanism of nitric oxide production in macrophages treated with medicinal mushroom extracts (review). International Journal of Medicinal Mushrooms, 13(1), 1–6. doi: 10.1615/IntJMedMushr.v13.i1.10
- Hutkins, R. W., Krumbeck, J. A., Bindels, L. B., Cani, P. D., Fahey, G. Jr., Goh, Y. J., … Sanders, M. E. (2015). Prebiotics: Why definitions matter. Current Opinion in Biotechnology, 37, 1–7. doi: 10.1016/j.copbio.2015.09.001
- Huynh, K. K., Kay, J. G., Stow, J. L., & Grinstein, S. (2007). Fusion, fission, and secretion during phagocytosis. Physiology (Bethesda), 22, 366–372. doi: 10.1152/physiol.00028.2007
- Ibeagha-Awemu, E. M., Lee, J. W., Ibeagha, A. E., Bannerman, D. D., Paape, M. J., & Zhao, X. (2008). Bacterial lipopolysaccharide induces increased expression of toll-like receptor (TLR) 4 and downstream TLR signaling molecules in bovine mammary epithelial cells. Veterinary Research, 39(2), 11. doi: 10.1051/vetres:2007047
- Ismail, B., & Nampoothiri, K. M. (2010). Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Archives of Microbiology, 192(12), 1049–1057. doi: 10.1007/s00203-010-0636-y
- Kim, B. H., Choi, J. S., Yi, E. H., Lee, J. K., Won, C., Ye, S. K., & Kim, M. H. (2013). Relative antioxidant activities of quercetin and its structurally related substances and their effects on NF-kappaB/CRE/AP-1 signaling in murine macrophages. Molecules and Cells, 35(5), 410–420. doi: 10.1007/s10059-013-0031-z
- Kim, H. S., Kim, J. Y., Kang, J. S., Kim, H. M., Kim, Y. O., Hong, I. P., … Han, S. B. (2010). Cordlan polysaccharide isolated from mushroom Cordyceps militaris induces dendritic cell maturation through toll-like receptor 4 signalings. Food and Chemical Toxicology, 48(7), 1926–1933. doi: 10.1016/j.fct.2010.04.036
- Kim, H. S., Kim, J. Y., Ryu, H. S., Park, H. G., Kim, Y. O., Kang, J. S., … Han, S. B. (2010). Induction of dendritic cell maturation by β-glucan isolated from Sparassis crispa. International Immunopharmacology, 10(10), 1284–1294. doi: 10.1016/j.intimp.2010.07.012
- Kim, J. Y., Yoon, Y. D., Ahn, J. M., Kang, J. S., Park, S. K., Lee, K., … Han, S. B. (2007). Angelan isolated from Angelica gigas Nakai induces dendritic cell maturation through toll-like receptor 4. International Immunopharmacology, 7(1), 78–87. doi: 10.1016/j.intimp.2006.08.017
- Lakics, V., & Vogel, S. N. (1998). Lipopolysaccharide and ceramide use divergent signaling pathways to induce cell death in murine macrophages. Journal of Immunology, 161(5), 2490–2500.
- Li, X., Jiao, L. L., Zhang, X., Tian, W. M., Chen, S., & Zhang, L. P. (2008). Anti-tumor and immunomodulating activities of proteoglycans from mycelium of Phellinus nigricans and culture medium. International Immunopharmacology, 8(6), 909–915. doi: 10.1016/j.intimp.2008.02.008
- Liu, L., Qin, Y., Wang, Y., Li, H., Shang, N., & Li, P. (2014). Complete genome sequences of Bifidobacterium animalis RH, a probiotic bacterium producing exopolysaccharides. Journal of Biotechnology, 189, 86–87. doi: 10.1016/j.jbiotec.2014.08.041
- Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods, 25(4), 402–408. doi: 10.1006/meth.2001.1262
- Luig, M., Kluger, M. A., Goerke, B., Meyer, M., Nosko, A., Yan, I., … Steinmetz, O. M. (2015). Inflammation-induced IL-6 functions as a natural brake on macrophages and limits GN. Journal of the American Society of Nephrology, 26(7), 1597–1607. doi: 10.1681/ASN.2014060620
- Lull, C., Wichers, H. J., & Savelkoul, H. F. (2005). Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators of Inflammnation, 2005(2), 63–80. doi: 10.1155/MI.2005.63
- Mazzone, G. L., Rigato, I., & Tiribelli, C. (2010). Unconjugated bilirubin modulates nitric oxide production via iNOS regulation. Bioscience Trends, 4(5), 244–248.
- Miyata, R., & van Eeden, S. F. (2011). The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicology Applied Pharmacology, 257(2), 209–226. doi: 10.1016/j.taap.2011.09.007
- Moncada, S., Palmer, R. M., & Higgs, E. A. (1991). Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacological Reviews, 43(2), 109–142.
- Nagashima, H., Nakagawa, H., & Kushiro, M. (2012). Opposite effects of two trichothecene mycotoxins, deoxynivalenol and nivalenol, on the levels of macrophage inflammatory protein (MIP)-1α and MIP-1β in HL60 cells. Environmental Toxicology Pharmacology, 34(3), 1014–1017. doi: 10.1016/j.etap.2012.07.008
- Paulo, E. M., Boffo, E. F., Branco, A., Valente, A. M., Melo, I. S., Ferreira, A. G., … Assis, S. A. (2012). Production, extraction and characterization of exopolysaccharides produced by the native Leuconostoc pseudomesenteroides R2 strain. Anaisda Academia Brasileirade Ciências, 84(2), 495–508. doi: 10.1590/S0001-37652012000200018
- Rajkumar, H., Kumar, M., Das, N., Kumar, S. N., Challa, H. R., & Nagpal, R. (2015). Effect of probiotic Lactobacillus salivarius UBL S22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers: A randomized controlled single-blind pilot study. Journal of Cardiovascular Pharmacology and Therapeutics, 20(3), 289–298. doi: 10.1177/1074248414555004
- Ramnani, P., Costabile, A., Bustillo, A. G., & Gibson, G. R. (2015). A randomised, double- blind, cross-over study investigating the prebiotic effect of agave fructans in healthy human subjects. Journal of Nutritional Science, 4, e10. doi: 10.1017/jns.2014.68
- Schilling, T., Miralles, F., & Eder, C. (2014). TRPM7 regulates proliferation and polarisation of macrophages. Journal of Cell Science, 127(21), 4561–4566. doi: 10.1242/jcs.151068
- Shang, N., Xu, R., & Li, P. (2013). Structure characterization of an exopolysaccharide produced by Bifidobacterium animalis RH. Carbohydrate Polymers, 91(1), 128–134. doi: 10.1016/j.carbpol.2012.08.012
- Shao, B. M., Dai, H., Xu, W., Lin, Z. B., & Gao, X. M. (2004). Immune receptors for polysaccharides from Ganoderma lucidum. Biochemcal and Biophysical Research Communications, 323(1), 133–141. doi: 10.1016/j.bbrc.2004.08.069
- Shimakage, M. (2014). Significant role of macrophages in human cancers associated with Epstein-Barr virus (review). Oncology Reports, 32(5), 1763–1771.
- Spencer, C. T., Abate, G., Sakala, I. G., Xia, M., Truscott, S. M., Eickhoff, C. S., … Hoft, D. F. (2013). Granzyme A produced by gamma(9)delta(2) T cells induces human macrophages to inhibit growth of an intracellular pathogen. PLoS Pathogens, 9(1), e1003119. doi: 10.1371/journal.ppat.1003119
- Stuart, M. J., & Baune, B. T. (2014). Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: A systematic review of biomarker studies. Neuroscience and Biobehavioral Reviews, 42, 93–115. doi: 10.1016/j.neubiorev.2014.02.001
- Wood, S., Jayaraman, V., Huelsmann, E. J., Bonish, B., Burgad, D., Sivaramakrishnan, G., … Shafikhani, S. H. (2014). Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLos ONE, 9(3), e91574. doi: 10.1371/journal.pone.0091574
- Xie, S. Z., Hao, R., Zha, X. Q., Pan, L. H., Liu, J., & Luo, J. P. (2016). Polysaccharide of Dendrobium huoshanense activates macrophages via toll-like receptor 4-mediated signaling pathways. Carbohydrate Polymers, 146, 292–300. doi: 10.1016/j.carbpol.2016.03.059
- Xu, R., Shang, N., & Li, P. (2011). In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe, 17(5), 226–231. doi: 10.1016/j.anaerobe.2011.07.010
- Yoon, Y. D., Kang, J. S., Han, S. B., Park, S. K., Lee, H. S., Kang, J. S., & Kim, H. M. (2004). Activation of mitogen-activated protein kinases and AP-1 by polysaccharide isolated from the radix of Platycodon grandiflorum in RAW 264.7 cells. International Immunopharmacology, 4(12), 1477–1487. doi: 10.1016/j.intimp.2004.06.012
- Yu, L., Sun, G., Wei, J., Wang, Y., Du, C., & Li, J. (2016). Activation of macrophages by an exopolysaccharide isolated from Antarctic Psychrobacter sp. B-3. Chinese Journal of Oceanology and Limnology, 34(5), 1064–1071. doi: 10.1007/s00343-016-4393-x
- Yu, X., Yin, J., Li, L., Luan, C., Zhang, J., Zhao, C., & Li, S. (2015). Prebiotic potential of xylooligosaccharides derived from corn cobs and their in vitro antioxidant activity when combined with Lactobacillus. Journal of Microbiology and Biotechnology, 25(7), 1084–1092. doi: 10.4014/jmb.1501.01022
- Zhang, X., Qi, C., Guo, Y., Zhou, W., & Zhang, Y. (2016). Toll-like receptor 4-related immunostimulatory polysaccharides: Primary structure, activity relationships, and possible interaction models. Carbohydrate Polymers, 149, 186–206. doi: 10.1016/j.carbpol.2016.04.097
