ABSTRACT
Enrofloxacin (ENR) is a synthetic fluoroquinolone antibiotic used for the treatment of infection in animals. The use of ENR has been strictly regulated because of its harmful effect on consumers of animal products. In this study the generation of polyclonal antibody (pAb) against ENR, the preparation of an immunoaffinity chromatography column and its potential application to the selective extraction of ENR residues from food samples were described. The produced pAb exhibited good sensitivity to ENR with an IC50 value of 5.4 ng/mL. The cross-reactivity values of the antibody with ENR structurally related compounds were lower than 0.5%. An immunoaffinity column was constructed by coupling antibody covalently with chitosan microspheres. 0.8% NaOH/0.01 mol/L phosphate-buffered saline (PBS) (pH 7.4) and 85% methanol were selected as loading and eluting solution by careful optimization. Further characterization indicated that the binding capacity of the column for ENR was approximately 3780 ng/mL gel. The immunoaffinity columns were then challenged with ENR-fortified pork, and the recoveries of ENR were found to be in the range of 79.8–94.9%, demonstrating the feasibility of the prepared immunoaffinity column for sample clean-up in ENR residue determination.
Introduction
Enrofloxacin (ENR) is a synthetic fluoroquinolone antibiotic and has been widely used for the treatment of respiratory and gastrointestinal infections in farm animals due to its broad spectrum of antibacterial action and pharmacokinetic performance (Martinez, McDermott, & Walker, Citation2006). However, the widespread use of ENR in food producing animals has caused public concern because of the development of antimicrobial resistance. Moreover, the results of a growing number of studies have suggested that the presence of ENR residues in edible animal products may pose adverse effect to humans, such as inducing allergic hypersensitivity, reducing sperm count, deteriorating sperm motility and causing enteric dysbacteriosis (Aral, Karacal, & Bab, Citation2008; Chen, Yuan, Feng, Wei, & Hua, Citation2011). To protect food and health safety, maximum residue limits (MRLs) of ENR and its metabolite ciprofloxacin (CIP) in food producing animals have been established by the European Union (Regulations CE No. 37/2010) and the Ministry of Agriculture of China (Bulletins 2002 No. 235). The MRL values are in the range of 100–300 μg kg−1 for the sum of ENR and CIP in different tissues. Therefore, the development of effective, convenient and accurate methods for monitoring ENR residues in livestock products is of increasing interest (Tochi et al., Citation2016).
Instrumental analysis methods including liquid chromatography (González, Moreno, Small, Jones, & Bruni, Citation2006; Stoilova, Surleva, & Stoev, Citation2013; Tayeb-Cherif, Peris-Vicente, Carda-Broch, & Esteve-Romero, Citation2016), liquid chromatography-tandem mass spectrometry (Dorival-García, Junza, Zafra-Gómez, Barrón, & Navalón, Citation2016; Krebber, Hoffend, & Ruttmann, Citation2009) and capillary electrophoresis (Xu, Liu, Jia, & Shu, Citation2015; Zhou, Xing, Zhu, Tang, & Jia, Citation2008) have been widely used for screening and confirmation of quinolone residues. These techniques have been proved sensitive and accurate, but a pretreatment step is always necessary before the instrumental analysis because of the low level of the target analyte and the complexity of the real sample matrices. However, the traditional approaches used in sample pretreatment, such as Soxhlet extraction, liquid–liquid partitioning and solid phase extraction, are far from the satisfactory for analytical purpose in terms of high sensitivity, reproducibility, small amounts of extraction solvents and time saving (Şenyuva & Gilbert, Citation2010). Immunoaffinity chromatography (IAC) is a powerful method for sample clean-up, which enables selective extraction and concentration of individual compounds or classes of compounds from complex matrices in only one step (Şenyuva & Gilbert, Citation2010). The application of IAC for a variety of samples pretreatment in mycotoxins (Lattanzio, Ciasca, Powers, & Visconti, Citation2014; Pascale, Panzarini, Powers, & Visconti, Citation2014; Pavšič-Vrtač, Ojanperä, Apajalahti, Šrimpf, & Tavčar-Kalcher, Citation2014), veterinary drugs (Sun et al., Citation2016; Wang, Xu, Zhang, Wang, & Dong, Citation2011; Zhao, Lin, Song, Pan, & Wang, Citation2011) and pesticides (Esteve-Turrillas, Mercader, Agullo, Abad-Somovilla, & Abad-Fuentes, Citation2011; Xu et al., Citation2012) residues analyses have been reported. In this work, we describe the generation and application of polyclonal antibody (pAb)-based IAC as a clean-up procedure, followed by the enzyme-linked immunosorbent assay (ELISA) analysis of ENR in real food samples. The aims of this study were: (1) to prepare an immunoaffinity column by coupling anti-ENR pAb with chitosan microspheres, (2) to develop optimal chromatographic extraction conditions for ENR and (3) to evaluate the prepared immunoaffinity columns coupled with ELISA for the detection of ENR in actual food samples.
Experimental design
Chemicals and apparatus
ENR, danofloxacin (DAN), norfloxacin (NOR), Ofloxacin (OFL), CIP, N-hydroxysuccinimide (NHS), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl), N,N-Dimethylformamide (DMF), triethylamine, bovine serum albumin (BSA), complete Freund’s adjuvant (CFA) and incomplete Freund’s adjuvant (IFA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). High-performance liquid chromatography (HPLC)-grade methanol was purchased from Fisher Scientific, Inc. (Pittsburgh, PA, USA). HRP-conjugated goat anti-rabbit IgG was obtained from Shangon Biotech Co., Ltd (Shanghai, China). All other chemicals were of analytical grade and supplied by Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
The ELISA plate reader (synergy HT) was obtained from Bio Tek Instrument, Inc. (Winooski, VT, USA). A UV-Vis spectrometer (Cary 100) was purchased from Varian Medical Systems, Inc. (Palo Alto, CA, USA). An ultrapure water system was supplied by Merck Millipore Co., Ltd. (Billerica, MA, USA) and 5417R centrifuge was purchased from Eppendorf (Hamburg, Germany).
Hapten conjugation
The synthesis of the ENR–BSA conjugate was performed according to the method of Watanabe, Satake, Kido, and Tsuji (Citation2002) with modifications. A total of 20 mg of ENR, 10 mg of NHS and 12.5 mg of EDC were dissolved in 900 μL of DMF and 100 μL of triethylamine. The mixture was stirred overnight at room temperature. The solution was then added into 5 mL of BSA solution (5 mg/mL) dropwise and vortexed for 3 h at room temperature. After dialysis with phosphate-buffered saline (PBS) for 3 days, the conjugates were finally lyophilized and kept at −80°C until future use.
Antibody production and purification
The pAb was generated according to Wang, Wang, Xu, Wu, and Dong (Citation2014). A total of 1 mg of ENR–BSA conjugate was dissolved in 1 mL of 0.9% NaCl solution and emulsified with CFA (1:1, v/v). The mixture was injected intradermally at multiple sites on the back of the rabbit (about 2 kg each). Booster injections were carried out in the same procedure every two weeks, except that IFA was used instead of CFA. A week after the third booster injection, blood samples were collected from ear vein and the serum was isolated by centrifugation.
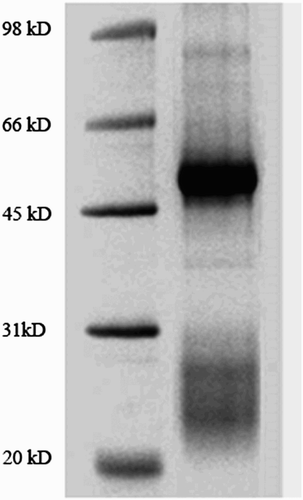
The anti-ENR pAb was purified from antiserum using a caprylic acid and ammonium sulfate precipitation method (Perosa, Carbone, Ferrone, & Dammacco, Citation1990), and further analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The immunoglobulin concentration was measured using a UV-spectrophotometer and calculated by the formula: Cprotein (mg/mL) = 1.45A280nm − 0.74A260nm.
Indirect ELISA procedure
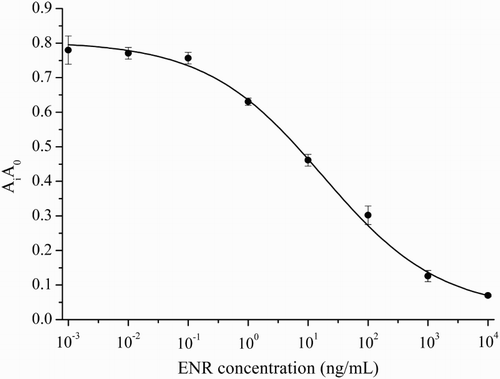
For ELISA, a polystyrene microtiter plate was coated with 100 μL of coating antigen (ENR-OVA) in 0.05 M carbonate buffer (pH 9.6) followed by incubation at 4°C overnight. Unoccupied sites were blocked with 0.2% gelatin for 2 h at 37°C. After 3 times of washing, 50 μL/well of ENR followed by 50 μL/well of pAb were added and incubated for 1 h at 37°C. After the washing procedure, 100 μL/well HRP-conjugated goat anti-rabbit IgG was added and incubated for 1 h at 37°C. The color development was initiated by adding 50 μL of the substrate (TMB/H2O2 in acetate buffer pH 5.5) for each well and incubated for 15 min at 37°C. The absorbance values were measured at 450 nm by an ELISA reader. The inhibition curve was plotted as Ai/A0 versus the ENR concentration (0.001, 0.01, 0.1, 1, 10, 100, 1000 and 10,000 ng/mL), where Ai was the absorbance of the well containing the analyte and A0 was the absorbance of the well without ENR. The IC50 value was defined as the concentration of ENR that provides a 50% decrease in Ai/A0.
Specificity of the pAb
The cross-reactivity (CR) of the obtained antibody to other four ENR structurally related compounds of DAN, NOR OFL and CIP () was estimated using the procedure as previously reported (Cooper, Caddell, Elliott, & Kennedy, Citation2004) and determined by measuring their IC50 values by the indirect competitive ELISA method. CR values were calculated according to the following formula: CR = [IC50 of ENR/IC50 of structurally related compound] × 100%.
Preparation of immunoaffinity column
The immunoaffinity column was prepared by coupling the purified antibodies with cross-linked chitosan microsepheres according to the previously described method (Wang et al., Citation2011) with modifications. In brief, 2 g of chitosan powder was dissolved in 100 mL of 1% acetic acid. The resulting chitosan solution was mixed with 100 mL of paraffin oil/carbon tetrachloride (1:1, v/v) containing 0.3% span-80. After stirring for 40 min, glutaraldehyde solution (25%, v/v) was added dropwise into the mixture to the final concentration of 1%. The cross-linking reaction was then performed by agitation at 60 oC for 3 h, with pH maintained between 7 and 9 throughout. Finally, 10 mL of 5 M NaBH4 was added into the mixture to reduce the products of the Schiff-base reaction.
The obtained chitosan microsepheres were suspended in 10 mL of 0.1 M NaHCO3 and mixed with 0.3 mg antibodies. After gentle agitation at room temperature for 3 h, epichlorohydrin was added to the above mixture to the final concentration of 20% and mechanically stirred for another 5 h. Finally, the immunoaffinity adsorbent was obtained after filtering and washing with distilled water. About 0.5 mL of immunoaffinity adsorbent was packed into a column (50 × 10 mm i.d.).
Characterization of the prepared immunoaffinity column
According to Wang et al. (Citation2011), the chromatographic performance of the column was characterized in terms of eluting condition, binding capacity, specificity, reproducibility and reusability.
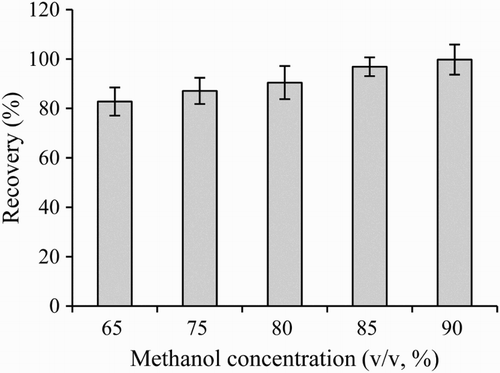
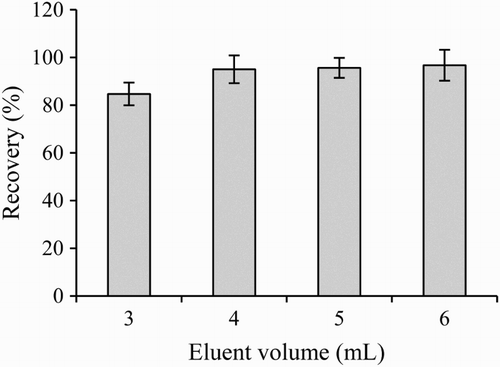
Sample loading and eluting conditions were optimized as described by Qiao, Yang, Wang, Song, and Deng (Citation2009). In brief, 150 ng of ENR was dissolved in 150 μL either pure water, 5% methanol/0.01 M PBS (pH 7.4) or 0.8% NaOH/ 0.01 mol/L PBS (pH 7.4) and applied onto the immunoaffinity columns, respectively. After removing the unspecific bound ENR using 10 mL 0.01 mol/L PBS (pH 7.4), eluting solutions (65, 70, 75, 80, 85 or 90% methanol) with different volumes (3, 4, 5 and 6 mL) were used to release the bound ENR from the column. ENR contents in effluents were measured by the ELISA method.
Under the optimum eluting condition, a total of 2 μg of ENR dissolved in 5 mL of 0.8% NaOH/ 0.01 mol/L PBS (pH 7.4) was continuously loaded (1 mL for each) on the immunoaffinity column (0.5 mL). The ENR content of the flow-through was determined by ELISA to calculate the column binding capacity.
Application of immunoaffinity column for real samples clean-up
To assess the prepared immunoaffinity column, pork meat obtained from local supermarket was fortified with ENR at the final concentration of 10, 25 and 50 ng/g. A total of 5 g homogenized muscle tissues were mixed with 5 mL 0.01 mol/L PBS (pH 7.4) in polypropylene centrifuge tubes followed by vortexing for 5 min (Sun et al., Citation2016). After centrifugation at 9000 rpm for 15 min, the supernatants were collected, and the precipitate was pre-extracted with 5 mL 0.01 mol/L PBS (pH 7.4). All the supernatant fractions were pooled and then loaded on the immunoaffinity column. Under the optimum conditions, the eluate was collected, and ENR content was analyzed by ELISA to estimate the recovery rate in the IAC clean-up step.
Results and discussion
Synthesis and identification of ENR–BSA conjugates
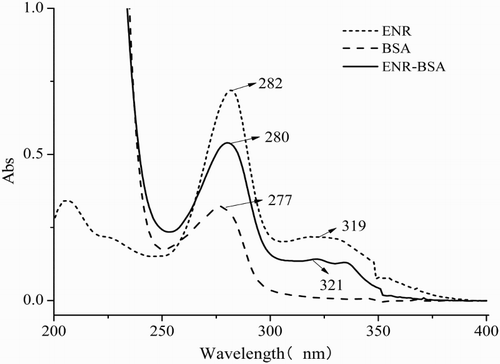
ENR is a small organic molecule with a carboxylic functional group, therefore the hapten was first activated by EDC and then coupled with BSA to form hapten–protein conjugate before animal immunization. To identify whether ENR was attached to carrier protein successfully, the ultraviolet spectra of BSA, ENR, and ENR–BSA conjugates were compared (). It can be observed that there was one absorption peak for BSA at 277 nm, two for ENR at 282 and 319 nm, and two others for ENR–BSA conjugate at 280, and 321 nm, suggesting that the artificial antigen was successfully synthesized and can be used for the subsequent antibody generation.
Generation and characterization of pAb
New Zealand rabbits were immunized with ENR–BSA conjugate. Crude antiserum was collected after the third injection, and the caprylic acid and saturated (NH4)2SO4 precipitation method was employed for antibody purification due to its simplicity and avoiding loss of antibody activity. The electrophoresis analysis indicated that a highly purified pAb was obtained (). To characterize the produced pAb, the titer, the IC50 value and the CR of the antibody were assessed. The titer of the anti-ENR antibody was over 2.56 × 105. As shown in , the antibody shows excellent sensitivity, with an IC50 value of 5.4 ng/mL. The specificity of antibody was evaluated by the comparison of the IC50 of the competitor with that of ENR. IC50 and CR values for each compound are given in and showed that the obtained antibody exhibits fairly good specificity, with negligible CR (lower than 0.5%) to DAN, NOR, OFL and CIP. In this study, the immunogen was synthesized by the linkage of carboxylic acid group of ENR with the amino group of the carrier protein (BSA). In this linkage, it is deduced that the ethyl group located at the 4′ position of the piperazinyl ring is the furthest group from the linking point, and plays an important role in epitope determination (Liu et al., Citation2009; Sheng et al., Citation2009). Of the fluoroquinolones shown in , only ENR has an ethyl group linked at the 4′ position of the piperazinyl moiety through a nitrogen atom, which may explain the low CR of the obtained anti-ENR antibody toward other fluoroquinolones.
Table 1. IC50 and CR of anti-ENR pAb against ENR structurally related compounds.
Optimization of extraction conditions for immunoaffinity column
The extraction conditions should be optimized because these conditions have a crucial influence on the association/dissociation of analyte–antibody complex and the activity of antibody (Klinglmayr, Nöbauer, Razzazi-Fazeli, & Cichna-Markl, Citation2010). ENR is easily soluble in basic or polar organic solvents. In view of this, three different loading solutions, namely pure water, 5% methanol/0.01 mol/L PBS (pH 7.4) and 0.8% NaOH/0.01 mol/L PBS (pH 7.4) were tested to study whether the loading medium had an effect on antibody-antigen binding. It was found that when pure water or 5% methanol/PBS used as a loading solution, only approximately 78% and 85% of ENR was bound on the column, respectively; when 0.8% NaOH/0.01 mol/L PBS (pH 7.4) was used as a loading solution, almost all analyte (more than 95%) was specifically bound on the column. The reason for this may be due to the presence of 0.8% NaOH in the loading medium, which can cause a change in pH and ionic strength, and therefore influence the electrostatic interaction between antibody and antigen, resulting in binding of the analyte onto the column. Therefore, 0.8% NaOH/0.1 mol/L PBS (pH 7.4) was selected as the loading solution.
The selection of the most appropriate eluting conditions was made considering (1) the recovery of the analyte, (2) the volume needed for an acceptable recovery, (3) the potential damage of the IAC after several cycles of usage and (4) the compatibility with the immunochemical analytical method (Sanvicens, Moore, Guilbault, & Marco, Citation2006). Previous research has shown that the mixtures of organic solvents, such as methanol or acetonitrile, were suitable to remove analytes from IAC (Sun et al., Citation2016; Wang et al., Citation2011). In this study, five eluting solutions with different methanol concentrations were evaluated. As shown in , when 150 ng ENR load on the column, the ENR content found in the eluting solution was increased from 121 to 149.5 ng with the increase in methanol concentration from 65% to 90%. High concentration of methanol in the eluting solution might enhance the extraction efficiency, but would be also more harmful to the antibody, decreasing the stability of the column. At 3 mL of 85% methanol, the releasing efficiency of ENR from immunoaffinity column was about 85%, while the eluting solutions with more than 4 mL, the releasing efficiency of ENR from the immunoaffinity column was higher than 95% (). Therefore, 85% methanol was chosen as the eluting solution.
Characterization of the immunoaffinity column
Under the optimum extraction conditions, the performance of the prepared immunoaffinity column was evaluated in terms of binding capacity. The binding capacity of the column was examined by sequential application of 1 mL of ENR standard solution with a concentration of 400 ng/mL to the single column (gel volume 0.5 mL). Nearly all the ENR in the first four 1-mL solutions was retained on the column, and approximately 110 ng of ENR was observed in the effluent when the fifth 1-mL of ENR standard solution was loaded. Therefore, the binding capacity of this IAC, containing 0.5 mL of the immunoaffinity adsorbent, was estimated to be: [4 × 400 (completely retained) + 290 (partially retained)] = 1890 ng.
Application of immunoaffinity column for real samples clean-up
Finally, the prepared columns were challenged with actual food samples (pork) fortified with 10, 25 and 50 ng/g according to the MRL (100 ng/g) accepted by European Union and the Ministry of Agriculture of China. As shown in , the recoveries of ENR from the fortified samples were found to be in the range of 79.8–94.9% with RSD lower than 7.5%.
Table 2. Extraction recoveries of ENR from spiked food samples using IAC clean-up (n = 3).
Conclusions
In this study, pAb against ENR was generated and then an immunoaffinity column was prepared by coupling the antibody covalently with chitosan microsepheres. The produced antibody exhibited high sensitivity and specificity toward ENR with an IC50 of 5.4 ng/mL and CR to ENR structurally related compounds lower than 0.5%. We demonstrated that the ENR can be applied in 0.8% NaOH/0.01 mol/L PBS (pH 7.4) and the bound ENR can be released quantitatively from the immunoaffinity column by 4 mL of 85% methanol. Under the optimal extraction conditions, the binding capacity of the column reached 3780 ng/mL gel. The prepared immunoaffinity column was further challenged with ENR-spiked real food samples and satisfactory recoveries ranging from 79.8% to 94.9% were achieved. From the above experimental results, it can be observed that the developed IAC method is not only simple and practical but exhibits also high selectivity, and it could be used as an alternative method for regulatory monitoring of ENR in real samples.
Acknowledgements
Gratitude is expressed to Dr. Apaliya Maurice Tibiru for critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Yadan Wang was born in 1990. In 2013, she received the B.Sc. degree in Food Science and Engineering from Henan University of Technology, China, and in 2016 the M.Sc. degree in Food Science and Engineering from Jiangsu University, China. Her research interests are majorly involved in the developing of immunology-based methods used in the detection of antibiotics or mycotoxin residues in agricultural products.
Yamei Yang was born in 1991. In 2014, she received the B.Sc. degree in Food Science and Engineering from Yangzhou University, China, and now she is completing her M.Sc. in Food Science and Engineering in Jiangsu University, China.
Rui Niu was born in 1994. In 2015, she received the B.Sc. degree in Food Quality and Safety from Jiangsu University, China, and now she is completing her M.Sc. in Food Science and Engineering in Jiangsu University, China.
Xinsheng Zhu was born in 1966. In 1987, he received his B.Sc. degree in Analytic Chemistry from Suzhou University, China. His research interests are majorly involved in the instrumental analysis-based detecting or monitoring antibiotics or mycotoxin residues in agricultural products.
Min Qiang was born in 1964. In 1985, he received his B.Sc. degree in Food Science and Engineering from Jiangsu University, China. His research interests are majorly involved in the food safety and quality management.
Gang Niu was born in 1958. In 1982, he received his B.Sc. degree in Basic Medicine from Beijing University, China. His research interests are majorly involved in the research or development of novel test kit used for clinical diagnosis.
Yun Wang was born in 1974. In 1996, he received his B.Sc. degree in Agronomy from Jiangsu Agricultural College, China, and in 2006, his Ph.D. degree in Biochemistry and Molecular Biology from Nanjing Agricultural University, China. His research interests are majorly involved in (1) developing immunology-based methods used in the detection of antibiotics or mycotoxin residues in agricultural products; (2) exploring the molecular mechanism underlying the pathogenic fungi (i.e. Penicillium expansum, Aspergillus flavus and Aspergillus ochraceus)-host plant interaction.
Additional information
Funding
References
- Aral, F., Karacal, F., & Bab, F. (2008). The effect of enrofloxacin on sperm quality in male mice. Research in Veterinary Science, 84, 95–99. PubMed PMID: 17561208. doi:10.1016/j.rvsc.2007.04.007
- Chen, T. T., Yuan, J., Feng, X., Wei, H., & Hua, W. (2011). Effects of enrofloxacin on the human intestinal microbiota in vitro. International Journal of Antimicrobial Agents, 37, 567–571. PubMed PMID: 21420834. doi:10.1016/j.ijantimicag.2011.01.013
- Cooper, K. M., Caddell, A., Elliott, C. T., & Kennedy, D. G. (2004). Production and characterization of polyclonal antibodies to a derivative of 3-amino-2-oxazolidinone, a metabolite of the nitrofuran furazolidone. Analytica Chimica Acta, 520, 79–86. doi:10.1016/j.aca.2004.05.074
- Dorival-García, N., Junza, A., Zafra-Gómez, A., Barrón, D., & Navalón, A. (2016). Simultaneous determination of quinolone and β-lactam residues in raw cow milk samples using ultrasound-assisted extraction and dispersive-SPE prior to UHPLC−MS/MS analysis. Food Control, 60, 382–393. doi:10.1016/j.foodcont.2015.08.008
- Esteve-Turrillas, F. A., Mercader, J. V., Agullo, C., Abad-Somovilla, A., & Abad-Fuentes, A. (2011). Development of immunoaffinity columns for pyraclostrobin extraction from fruit juices and analysis by liquid chromatography with UV detection. Journal of Chromatography A, 1218, 4902–4909. PubMed PMID: 21477805. doi:10.1016/j.chroma.2011.03.022
- González, C., Moreno, L., Small, J., Jones, D. G., & Bruni, S. F. S. (2006). A liquid chromatographic method, with fluorometric detection, for the determination of enrofloxacin and ciprofloxacin in plasma and endometrial tissue of mares. Analytica Chimica Acta, 560, 227–234. doi:10.1016/j.aca.2005.12.040
- Klinglmayr, C., Nöbauer, K., Razzazi-Fazeli, E., & Cichna-Markl, M. (2010). Determination of deoxynivalenol in organic and conventional food and feed by sol-gel immunoaffinity chromatography and HPLC-UV detection. Journal of Chromatography B, 878, 187–193. PubMed PMID: 19736050. doi:10.1016/j.jchromb.2009.08.016
- Krebber, R., Hoffend, F. J., & Ruttmann, F. (2009). Simple and rapid determination of enrofloxacin and ciprofloxacin in edible tissues by turbulent flow chromatography/tandem mass spectrometry (TFC–MS/MS). Analytica Chimica Acta, 637, 208–213. doi:10.1016/j.aca.2008.11.006
- Lattanzio, V. M. T., Ciasca, B., Powers, S., & Visconti, A. (2014). Improved method for the simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in cereals and derived products by liquid chromatography-tandem mass spectrometry after multi-toxin immunoaffinity clean up. Journal of Chromatography B, 1354, 139–143. PubMed PMID: 24969088. doi:10.1016/j.chroma.2014.05.069
- Liu, Z., Lu, S., Zhao, C., Ding, K., Cao, Z., Zhan, J., … Xi, R. (2009). Preparation of anti-danofloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for detection of danofloxacin residue in chicken liver. Journal of the Science of Food and Agriculture, 89, 1115–1121. doi:10.1002/jsfa.3514
- Martinez, M., McDermott, P., & Walker, R. (2006). Pharmacology of the fluoroquinolones: A perspective for the use in domestic animals. The Veterinary Journal, 172, 10–28. PubMed PMID: 16154368. doi:10.1016/j.tvjl.2005.07.010
- Pascale, M., Panzarini, G., Powers, S., & Visconti, A. (2014). Determination of deoxynivalenol and nivalenol in wheat by ultra-performance liquid chromatography/photodiode-array detector and immunoaffinity column cleanup. Food Analytical Methods, 7, 555–562. doi:10.1007/s12161-013-9653-1
- Pavšič-Vrtač, K., Ojanperä, S., Apajalahti, J., Šrimpf, K., & Tavčar-Kalcher, G. (2014). Analytical procedures for the determination of aflatoxin B1 in eggs of laying hens using immunoaffinity columns and liquid chromatography with post-column derivatisation and fluorescence detection. Food Analytical Methods, 7, 1917–1924. doi:10.1007/s12161-014-9836-4
- Perosa, F., Carbone, R., Ferrone, S., & Dammacco, F. (1990). Purification of human immunoglobulins by sequential precipitation with caprylic acid and ammonium sulphate. Journal of Immunological Methods, 128, 9–16. PubMed PMID: 2324507. doi: 10.1016/0022-1759(90)90458-8
- Qiao, Y., Yang, H., Wang, B., Song, J., & Deng, A. (2009). Preparation and characterization of an immunoaffinity chromatography column for the selective extraction of trace contraceptive drug levonorgestrel from water samples. Talanta, 80, 98–103. PubMed PMID: 19782197. doi:10.1016/j.talanta.2009.06.029
- Sanvicens, N., Moore, E. J., Guilbault, G. G., & Marco, M. P. (2006). Determination of haloanisols in white wine by immunosorbent solid-phase extraction followed by enzyme-linked immunosorbent assay. Journal of Agricultural and Food Chemistry, 54, 9176–9183. PubMed PMID: 17117807. doi:10.1021/jf0612373
- Şenyuva, H. Z., & Gilbert, J. (2010). Immunoaffinity column clean-up techniques in food analysis: A review. Journal of Chromatography B, 878, 115–132. PubMed PMID: 19525155. doi:10.1016/j.jchromb.2009.05.042
- Sheng, W., Xia, X., Wei, K., Li, J., Li, Q. X., & Xu, T. (2009). Determination of marbofloxacin residues in beef and pork with an enzyme-linked immunosorbent assay. Journal of Agricultural and Food Chemistry, 57, 5971–5975. PubMed PMID: 19522498. doi:10.1021/jf900940n
- Stoilova, N. A., Surleva, A. R., & Stoev, G. (2013). Simultaneous determination of nine quinolones in food by liquid chromatography with fluorescence detection. Food Analytical Methods, 6, 803–813. doi:10.1007/s12161-012-9488-1
- Sun, L., Mei, L., Yang, H., Zhao, K., Li, J., Jiang, D., … Deng, A. (2016). Development and application of immunoaffinity column for the simultaneous determination of norfloxacin, pefloxacin, lomefloxacin, and enrofloxacin in swine and chicken meat samples. Food Analytical Methods, 9, 342–352. doi:10.1007/s12161-015-0192-9
- Tayeb-Cherif, K., Peris-Vicente, J., Carda-Broch, S., & Esteve-Romero, J. (2016). Use of micellar liquid chromatography to analyze oxolinic acid, flumequine, marbofloxacin and enrofloxacin in honey and validation according to the 2002/657/EC decision. Food Chemistry, 202, 316–323. PubMed PMID: 26920300. doi:10.1016/j.foodchem.2016.02.007
- Tochi, B. N., Khaemba, G., Isanga, J., Mukunzi, D., Liu, L., Peng, J., … Chuanlai, X. (2016). Monoclonal antibody for the development of specific immunoassays to detect enrofloxacin in foods of animal origin. Food and Agricultural Immunology, 27, 435–448. doi:10.1080/09540105.2015.1089844
- Wang, Y., Wang, E., Xu, Y., Wu, J., & Dong, Y. (2014). Improved preparation of a chitosan-based immunoaffinity column using antibody against methandrostenolone as ligand. Food and Agricultural Immunology, 25, 149–159. doi:10.1080/09540105.2012.753514
- Wang, Y., Xu, Y., Zhang, X., Wang, E., & Dong, Y. (2011). Development and characterization of a chitosan-supported immunoaffinity chromatography column for the selective extraction of methandrostenolone from food and feed samples. International Journal of Biological Macromolecules, 49, 428–432. PubMed PMID: 21664371. doi:10.1016/j.ijbiomac.2011.05.027
- Watanabe, H., Satake, A., Kido, Y., & Tsuji, A. (2002). Monoclonal-based enzyme-linked immunosorbent assay and immunochromatographic assay for enrofloxacin in biological matrices. Analyst, 127, 98–103. PubMed PMID: 11827405. doi:10.1039/b109427k
- Xu, X., Liu, L., Jia, Z., & Shu, Y. (2015). Determination of enrofloxacin and ciprofloxacin in foods of animal origin by capillary electrophoresis with field amplified sample stacking–sweeping technique. Food Chemistry, 176, 219–225. PubMed PMID: 25624227. doi:10.1016/j.foodchem.2014.12.054
- Xu, Z. L., Deng, H., Lei, H. T., Jiang, Y. M., Campbell, K., Shen, Y. D., … Sun, Y. M. (2012). Development of a broad-specificity monoclonal antibody-based immunoaffinity chromatography cleanup for organophosphorus pesticide determination in environmental samples. Journal of Agricultural and Food Chemistry, 60, 5847–5852. PubMed PMID: 22612520. doi:10.1021/jf300896z
- Zhao, X. T., Lin, Q. B., Song, H., Pan, Y. L., & Wang, X. (2011). Development of an immunoaffinity chromatography purification and ultra performance liquid chromatography tandem mass spectrometry method for determination of 12 sulfonamides in beef and milk. Journal of Agricultural and Food Chemistry, 59, 9800–9805. PubMed PMID: 21848254. doi:10.1021/jf202705d
- Zhou, X., Xing, D., Zhu, D., Tang, Y., & Jia, L. (2008). Development and application of a capillary electrophoresis–electrochemiluminescent method for the analysis of enrofloxacin and its metabolite ciprofloxacin in milk. Talanta, 75, 1300–1306. PubMed PMID: 18585216. doi:10.1016/j.talanta.2008.01.040