ABSTRACT
A rapid, simple, and sensitive immunochromatographic strip test has been developed to detect aldicarb (ALD) in 0.01 M phosphate buffer solution (PBS) (pH 7.4) and cucumber samples. The test was developed with a sensitive monoclonal antibody specific for ALD, generated by immunizing BALB/c mice with a well-characterized ALD – keyhole limpet hemocyanin conjugate, produced in our laboratory. Under the optimized condition, the cut-off limits of test strip for ALD were found to be 25 ng/mL in 0.01 M PBS (pH 7.4) and 100 ng/mL in cucumber samples, and the highest standard relative deviation was 9.05%. Both results were obtained within 5 min. The data indicate that the method is sensitive, rapid, and specific, so this immunochromatographic test strip has utility for the rapid high-throughput screening of the pesticide, especially in food samples.
Introduction
Fresh fruits, vegetables, and pulses are very important components of a healthy diet because they contain high levels of nutrients and minerals. However, they could also contain toxic substances, such as pesticides (Knezevic & Serdar, Citation2009). As several insect pests attack the fruits and vegetables, therefore insecticides can be applied during the entire period of growth and sometimes even at the fruiting stage for better yield and higher quality. These pesticides are absorbed by the fruits and vegetables and are consumed by humans, which may be hazardous if safe waiting period is not observed. The potential toxicity of these vegetables may present a risk to consumer health. In some cases, diseases such as acute neurological toxicity, neurodevelopmental impairment, cancer, allergies, neurological disorders, and reproductive disorders may be related to pesticide exposure (Chowdhury et al., Citation2013; Mostafalou & Abdollahi, Citation2013; Qin et al., Citation2015).
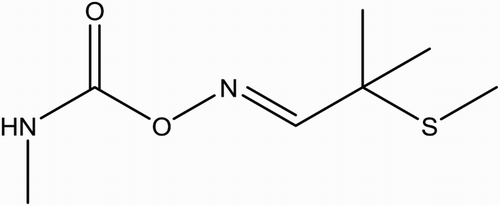
Aldicarb (ALD) (2-methyl-2-(methylthio)propionaldehyde-O-(methylcarbamoyl)oxime; (Temik) () is an oxime carbamate insecticide introduced in 1962 by the Union Carbide Corporation (USA) (Xu, Xu, & Dai, Citation2006). ALD carbamate is a systemic insecticide that has great significance in pest control and has been used widely instead of organochlorine and organophosphorus pesticides, due to its broad spectrum of biological activity. ALD was used to protect crops against a large variety of insects, mites, and nematodes. However, it has the drawback of pesticide residues which remain on fruit and vegetables, constituting a potential risk to consumers. Since the establishment of legal directives to control their levels through the Maximum Residue Levels and, on the other, there has been an ongoing search for pesticides that are less persistent and toxic to human (Conacher & Mes, Citation1993; Fernandez, Pico, & Manes, Citation2000; Sivaperumal, Anand, & Riddhi, Citation2015). During the mid of 1980s, a number of outbreaks of food poisoning were reported involving waster melons and cucumbers caused by ALD in California with estimated residue ranged between 0.0011 and 0.06 mg/kg body weight, although most were well below 0.025 mg/kg, the level of Lowest Observed Effect level for the depression of blood cholinesterase (a measurement of adverse effects from ALD) (Goldman, Beller, & Jackson, Citation1990).
Analytical methods, including gas chromatography (GC) (Song & McNair, Citation2002), GS/mass spectrometry (MS) (Carabias-Martinez, Garcia-Hermida, Rodriguez-Gonzalo, & Ruano-Miguel, Citation2005; Saito et al., Citation2008), high-performance liquid chromatography (HPLC)/MS (Kruve, Kuennapas, Herodes, & Leito, Citation2008; Nunes, Alonso, Ribeiro, & Barcelo, Citation2000; Yang, Li, Miao, Zhao, & Wu, Citation2014; Yuan, Xiong, Li, Chen, & Liu, Citation2011), liquid chromatography-tandem MS (LC-MS/MS) (Franca, Brandao, Sodre, & Caldas, Citation2015; Huang et al., Citation2009), and enzyme-linked immunosorbent assays (ELISAs) (Rodolico, Giovinazzo, & Mosconi, Citation1997) have been already used for the environmental analysis of ALD. The GC and HPLC methods have disadvantages insofar as they are complex, time consuming, and labor intensive. However, Lateral-flow immunochromatographic assays are increasingly popular as diagnostic tool for detecting residues because they are simple, rapid, specific, and sensitive. Compared with ELISAs, lateral-flow immunochromatographic assays have the advantage that all of the reagents are included in the strip and the results can be obtained within 5–10 min (Liu et al., Citation2014; Sun, Liu, Song, Kuang, & Xu, Citation2016; Zhao et al., Citation2008).
The principle of immunochromatographic lateral-flow testing has broad applicability because it is rapid, simple, and effective, and to the best of our knowledge, no immunochromatographic lateral-flow test device for the detection of ALD residues has been previously reported. The aim of this study was to develop an immunochromatographic lateral-flow test strip for the detection of ALD in cucumber.
Material and methods
Chemicals and reagents
ALD was purchased from J&K Scientific (Shanghai, China). Complete Freund’s adjuvant (FCA), incomplete Freund’s adjuvant (FIA), and enzyme immunoassay-grade horseradish peroxidase (HRP)-labeled goat anti-mouse immunoglobulin were obtained from Sigma (St. Louis, MO, USA). Gelatin was obtained from Beijing Biodee Biotechnology Co., Ltd (Beijing, China). Tetramethylbenzidine and HRP were purchased from Aladdin Chemistry Co., Ltd (Shanghai, China). All reagents used for cell fusion were from Sunshine Biotechnology Co., Ltd (Nanjing, China). Ovalbumin (OVA) and keyhole limpet hemocyanin (KLH) were obtained from Solarbio Science & Technology, Co, Ltd (Beijing, China). All other reagent and chemicals were obtained from the National Pharmaceutical Group Chemical Reagent Co, Ltd (Shanghai, China).
Nitrocellulose (NC) high-flow plus membranes (Pura-bind RP) were obtained from Whatman-Xinhua Filter Paper Co., Ltd (Hangzhou, China). Glass fiber membrane (CB-SB08) used for sample pad; polyvinylchloride (PVC) backing material and absorbance pad (SX18) were supplied by Goldbio Tech Co., Ltd (Shanghai, China). A BioDot TSR 3000 Membrane Strip Reader (Gene Company Limited, Shanghai Branch, Shanghai (China)) was used to test the color intensities of colloidal gold on the test zone. The conjugated coating antigens (ALD–OVA) and specific monoclonal antibodies (anti-ALD mAb, No. 4D10) were generated in our laboratory.
All buffer solutions were prepared by using ultrapure water (Milli-Q purification system, Millipore Co., Bedford, MA, USA). The strip cutting instrument was CM 4000 (Gene, Shanghai, China). The Airjet Quanti 3000TM and Biojet Quanti 3000TM (XinqidianGene-technology Co., Ltd., Beijing, China) were used for dispensers.
Preparation and characterization of monoclonal anti-ALD antibody
ALD was conjugated to OVA or KLH using the ester method. Briefly, a mixture ALD, carboxyl-reactive carbodiimide cross linker (1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride), and N-hydroxysuccinimide were added to N,N-dimetyl formamide and incubated for 24 h (solution 1). KLH or OVA was mixed with 0.01 M phosphate buffer solution (PBS) (solution 2). Solution 1 was slowly added to solution 2 with stirring and the mixture was stirred continuously for 8 h at room temperature. The conjugates were dialyzed against 0.01 M PBS for 3 d and subsequently distilled water for 3 d. Ultraviolet absorption was used to check the conjugation of protein and ALD.
Female BALB/c mice (6–8 weeks old) were used to produce of monoclonal antibody (mAb). The mice were immunized subcutaneously with the ALD-KLH conjugate. The first immunization using FCA and FIA was used in the subsequent boost injection. The mice were immunized every 3 weeks with 100 µg for the first immunization and 50 µg for the remainder (two to five times). The antibodies I the blood samples from immunized mice were measured with an ELISA and the mouse with the highest titer was scarified and its splenocytes were fused with Sp 2/0 murine myeloma cells, and the hybridomas were then screened with an indirect ELISA. The selected hybridoma cells were expanded and injected into BALB/c mice to produce the mAb (Deng et al., Citation2012; Suryoprabowo, Liu, Peng, Kuang, & Xu, Citation2014). The ascites was harvested and purified with the caprylic acid-ammonium sulfate precipitation method (Kuang et al., Citation2013). The purified antibody solution was stored at –20°C until further use.
Development of lateral-flow test device
Preparation of colloidal gold particles
All the solvents were prepared with ultrapure water then filtered through a transfer membrane (0.22 µm). Chlorauric acid (25 mL of a 0.1 g/L) solution was heated to boiling under constant stirring, and then 1% w/v sodium citrate tribasic dihydrate solution (1.0 mL) was added. The mixture was stirred for 30 min. the color of the solution turned wine-red, it was cooled to room temperature, and stored at 4°C. A transmission Electron Microscopy (TEM) analysis showed that the gold nanoparticles had a nearly uniform particle size of 15 nm.
Preparation of colloidal gold-labeled mAb
The half-maximal inhibitory concentration (IC50) of the anti-ALD mAb (no. 4D10) was 20 ng/mL was used. Adjusted pH 7.0 of 10 mL colloidal gold solution with 0.1 M K2CO3. Briefly, the mAb (0.40 mL) was added to the solution dropwise, and 35 min later, 10% (w/v) bovine serum albumin (BSA) (1 mL) was added, and the mixture was stirred for 2 h. The product was centrifuged for 45 min in 7000 rpm to remove the gold aggregates. The solution was separated into two layers, the lower layer (red gold-labeled mAb) was collected and washed with 0.02 M phosphate buffer containing 5% sucrose, 1% BSA, and 0.5% polyethylene glycol (PEG) 6000 (pH 7.4). The conjugation products were reconstituted to 1 mL in gold-labeling resuspension buffer (0.02 M PBS, 5% sucrose, 2% sorbitol, 1% mannitol, 0.1% PEG, 0.1% tween, and 0.04% NaN3) and stored at 4°C until use.
Preparation of NC capture membranes
The coating antigen (ALD–OVA) () and goat anti-mouse IgG antibody were used as the capture reagent in the control line on the test strip. The antigen and goat anti-mouse IgG coatings were sprayed onto the NC membrane at 1 µL/cm using a dispenser to produce the test line and a control line on the strip. The capture and control reagent was sprayed onto the glass fiber membrane to prepare the conjugate pad, which was dried at 37°C for 2 h. The NC membrane coated with capture reagents was pastes onto the center of the plastic backing plate (PVC) and the conjugate pad, sample pad, and absorbent pad were laminated and pasted onto the back plate. Finally, the plate was cut into 2.8 mm wide strips with a strip cutter.
Test procedure and principle
Before the test, 50 µL of gold-labeled mAb is mixed with 150 µL of sample solution, allow it to react for about 5 min, than added to sample pad. The solution migrates to the absorbent pad. The test results are obtained within 5 min. If the ALD is present in the sample, it competes with the ALD–OVA conjugates embedded in the test line for the finite amount of anti-ALD mAb. When a sufficient amount of ALD is present, the free ALD binds with all the labeled mAb, preventing the mAb binding to the ALD–OVA in the test line. Therefore, the more ALD that is present, the weaker color of the test line will appear. If ALD does not exist, the limited amount of colloidal gold-labeled mAb is trapped by the immobilized ALD–OVA conjugate, and a clearly visible red test line appears.
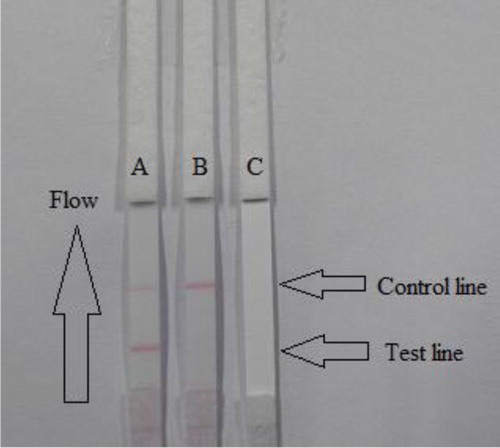
The flow must reach the control line, which contains goat anti-mouse IgG antibody, and also produces an indicator reaction. Therefore, the control line must always appear in a successful test, whereas the test line will only appear when the sample is negative ((A)). The appearance of only the control line is a positive result ((B)), whereas if neither the control line nor the test line does not appear ((C)), the test procedure is incorrect or the strip is invalid, indicating that the test should be repeated with a new strip.
Determination of performance
Sensitivity of the test strip
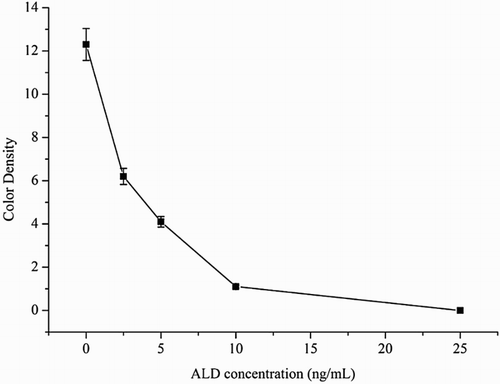
The sensitivity of the test strip was determined by testing ALD samples. ALD standard was diluted at concentration 0, 2.5, 5, 10, and 25 ng/mL in 0.01 M PBS (pH 7.4) and the detection limit was determined. The sample solution (100 µL) solution was mixed with 50 µL gold-labeled mAb, allowed to react for 5 min and added sample pad. After 5 min, the test strip reader recorded the color intensities of the different strips. The lowest detection limit (LDL) with naked eyes was defined at the amount of ALD that produced a color reaction on the strip that clearly visible differed in intensity from the result of 0 ng/mL ALD. Six replicate samples for each concentration were analyzed on the same day.
Detection of the ALD in cucumber samples
The detection of ALD in cucumber samples were determined by using cucumber samples that bought in local market. The samples (40 g) were crushed, and only the liquid from the cucumber was tested. The samples to be analyzed were spiked with the ALD standard solution (10 µg/mL, prepared with 0.01 M PBS, pH 7.4). The ALD concentrations were 10, 20, 50, 100 ng/mL. An un-spiked (blank) sample was used as the control. Six replicates of each concentration were analyzed with the test strips.
Result and discussion
Optimization of the strip test
To function as the reaction matrix in an immunochromatographic system, the materials must be hydrophilic and have consistent flow characteristics. NC, used as a base material, is hydrophobic but can be rendered hydrophilic by the addition of rewetting agents during the membrane production process. These rewetting agents are surfactants. One of the main effects to be considered in developing these assays is the choice of surfactant reagents because the correct reagent is required for the success of the test. In this study, suspension buffer (0.02 M PBS, 5% sucrose, 2% sorbitol, 1% mannitol, 0.1% PEG, 0.1% tween, and 0.04% NaN3) was added to 13 kinds of reagent (polyvinylpyrrolidone (PVP), PEG, polyvinyl alcohol (PVA) BSA, casein, sucrose, trehalose, sorbitol, mannitol, tween-20, brij-35, triton X-100, and Rhodasurf® On-870 (an ethoxylated oleyl alcohol)). As shown in , reagents PVP, PEG, and BSA produced the best and most stable color reactions, and were used for the subsequent experiments.
Figure 4. Result of using 14 kinds of reagent. 1 = suspension buffer, 2 = PVP, 3 = PEF, 4 = PVA, 5 = BSA, 6 = Casein, 7 = Sucrose, 8 = Trehalose, 9 = Sorbitol, 10 = Mannitol, 11 = tween-20, 12 = Brij-35, 13 = Triton X-100, and 14 = On-870.

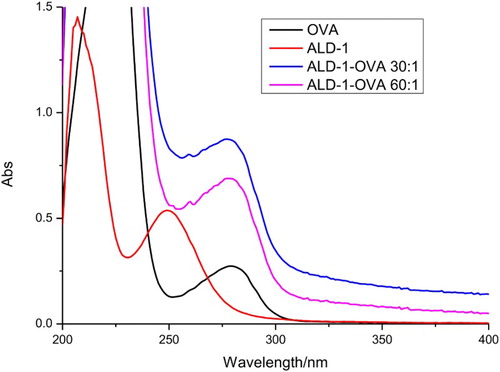
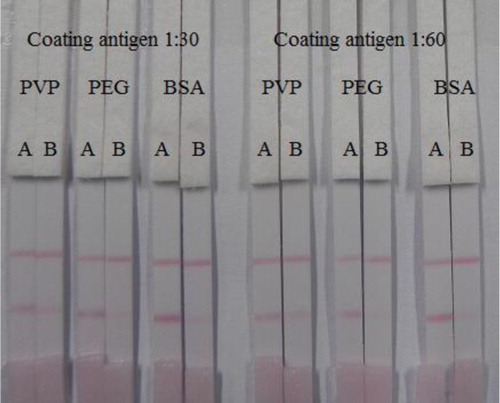
The coating antigens were synthesized in our laboratory. ALD was conjugated to OVA at different ratio (OVA: ALD; 30:1 or 60:1). The ultraviolet absorption spectra () showed that conjugation of ALD and OVA was successful. The optimization procedures, using two different kind coating antigen ratios (1:30 and 1:60) and three kind of reagent solutions (PVP, PEG, and BSA), are shown in . The result shows that there is significant difference in the color intensities produced when ALD was conjugated with OVA in ratio 1:30 and 1:60. The color intensity of the test line when the ALD–OVA 1:60 coating antigen was weaker than when ALD–OVA 1:30 was used. Significant differences were also produced when different reagent solutions were. The reagent solution with containing BSA produces a deeper color than using the other reagent solutions. However, ALD–OVA 1:30 with the reagent solution containing BSA did not differ significantly from ALD–OVA 1:60 in the same solution when detecting 5 ng/mL ALD, whereas in detecting the negative samples (0 ng/mL ALD), ALD–OVA 1:30 produced a deeper color than ALD–OVA 1:60 (0 ng/mL). Therefore, ALD–OVA in ratio of 1:30 and reagent solution containing BSA were selected to optimize the assay specificity.
Figure 5. Result of optimization by using two kind of coating antigen ratio (1:30 and 1:60) and three kind of reagent solution (PVP, PEG, and BSA). A = 0 ng/mL and B = 5 ng/mL.
The sensitivity of the assay was investigated with a series ALD standards diluted in using 0.01 M PBS (pH 7.4). The LDL with naked eyes was obtained at the amount of ALD producing a significant different in color intensity of the test strip in comparison with negative control (no ALD added). shows that the signal color on the test lines changes from strong (0 ng/mL) to weak and finally disappeared completely at 25 ng/mL ALD. In quantitative assay under the optimized detection conditions, a scanning reader was used to measure the intensity of the signal on the test zone and the optical response curves are shown in .
Determination of ALD in cucumber samples
In this study, the matrix effect was determined for cucumber samples. One of the major advantages by an immunochromatographic strip assay is that it is rapid and easy to use. ALD was detected in the liquid from 40 g of crushed cucumber bought at a local market. The liquid was spiked with the ALD standard solution (10 µg/mL, prepared with 0.01 M PBS pH 7.4) at final ALD concentrations of 10, 20, 50, and 100 ng/mL. Six replicates were determined for each concentration. The spiked series was prepared and analyzed with the optimized test strips. The results are summarized in . The color intensity decreased as the ALD concentration increased. The signal color on the test lines changed from strong to weak with increasing ALD, and disappeared completely at 100 ng/mL.
Figure 8. Results of ALD detection with colloidal gold immunochromatographic strip assay spiked in cucumber (n = 6). 1 = 0ppb, 2 = 10ppb, 3 = 20ppb, 4 = 50ppb, and 5 = 100ppb. C, control line; T, test line.

To determine the accuracy of the test, cucumber samples containing 10 or 20 ng/mL of ALD were tested. Six replicates were carried out for the test with a single batch of test strips and the result was measured using the test strip reader. The highest standard relative deviation was 9.05% ().
Table 1. Analysis of cucumber samples spiked with ALD.
Conclusions
A simple and rapid analytical method for simultaneous determination of ALB pesticide was developed by lateral-flow immunochromatography assay. Our results demonstrate that quantitative and semi-quantitative utility of the immunochromatography strip assay, with cut-off limits for ALD with the semi-quantitative test strip as low as 25 ng/mL in 0.01 M PBS (pH 7.4) and 100 ng/mL in cucumber samples. In summary, the immunochromatography strip assay represents a sensitive, simple, and rapid method for detecting ALD in 0.01 M PBS (pH 7.4) and cucumber samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Liqiang Liu got his Ph.D. in Food science in 2014 from Jiangnan University, Wuxi, China, and then became a faculty in College of Food Science and Technology of Jiangnan University. His research interests are immunochromatographic strip design and application.
Steven Suryoprabowo was born in Indonesia and got his bachelor’s in Pelita Harapan University (Indonesia) then he got his master’s degree in food science (2014) from Jiangnan University, Wuxi, China. His research interests are monoclonal antibodies development and immunochromatographic strip test and applications.
Qiankun Zheng graduated from Nanjing Agricultural University in 1996. Currently, he works as a senior engineer in Delicious Food Company, China. He is good at food quality control and assurance.
Shanshan Song got her master’s degree in Food science in 2012 from Jiangnan University, Wuxi, China, and then became a research assistant in College of Food Science and Technology of Jiangnan University. Her research interests are monoclonal antibody development.
Hua Kuang got her Ph.D. from China Agricultural University in 2009 and then began to work as a faculty in College of Food Science and Technology of Jiangnan University. She is currently a full professor in food safety. Her research interests are biosensor development.
Additional information
Funding
References
- Carabias-Martinez, R., Garcia-Hermida, U., Rodriguez-Gonzalo, E., & Ruano-Miguel, L. (2005). Behaviour of carbamate pesticides in gas chromatography and their determination with solid-phase extraction and solid-phase microextraction as preconcentration steps. Journal of Separation Science, 28(16), 2130–2138. doi: 10.1002/jssc.200400047
- Chowdhury, M. A. Z., Fakhruddin, A. N. M., Islam, M. N., Moniruzzaman, M., Gan, S. H., & Alam, M. K. (2013). Detection of the residues of nineteen pesticides in fresh vegetable samples using gas chromatography-mass spectrometry. Food Control, 34(2), 457–465. doi: 10.1016/j.foodcont.2013.05.006
- Conacher, H. B. S., & Mes, J. (1993). Assessment of human exposure to chemical contaminants in foods. Food Additives and Contaminants, 10(1), 5–15. doi: 10.1080/02652039309374125
- Deng, X., Liu, L., Ma, W., Xu, C., Wang, L., & Kuang, H. (2012). Development and validation of a sandwich ELISA for quantification of peanut agglutinin (PNA) in foods. Food and Agricultural Immunology, 23(3), 265–272. doi: 10.1080/09540105.2011.617358
- Fernandez, M., Pico, Y., & Manes, J. (2000). Determination of carbamate residues in fruits and vegetables by matrix solid-phase dispersion and liquid chromatography-mass spectrometry. Journal of Chromatography A, 871(1–2), 43–56. doi: 10.1016/S0021-9673(99)00907-3
- Franca, J. D. A., Brandao, M., Sodre, F. F., & Caldas, E. D. (2015). Simultaneous determination of prescription drugs, cocaine, aldicarb and metabolites in larvae from decomposed corpses by LC-MS-MS after solid-liquid extraction with low temperature partitioning. Forensic Toxicology, 33(1), 93–103. doi: 10.1007/s11419-014-0255-4
- Goldman, L. R., Beller, M., & Jackson, R. J. (1990). Aldicarb food poisonings in California, 1985–1988: Toxicity estimates for humans. Archives of Environmental Health: An International Journal, 45(3), 141–147. doi: 10.1080/00039896.1990.9936707
- Huang, Z., Zhang, Y., Wang, L., Ding, L., Wang, M., Yan, H., … Zhu, S. (2009). Simultaneous determination of 103 pesticide residues in tea samples by LC-MS/MS. Journal of Separation Science, 32(9), 1294–1301. doi: 10.1002/jssc.200800605
- Knezevic, Z., & Serdar, M. (2009). Screening of fresh fruit and vegetables for pesticide residues on Croatian market. Food Control, 20(4), 419–422. doi: 10.1016/j.foodcont.2008.07.014
- Kruve, A., Kuennapas, A., Herodes, K., & Leito, I. (2008). Matrix effects in pesticide multi-residue analysis by liquid chromatography-mass spectrometry. Journal of Chromatography A, 1187(1–2), 58–66. doi: 10.1016/j.chroma.2008.01.077
- Kuang, H., Xing, C., Hao, C., Liu, L., Wang, L., & Xu, C. (2013). Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors, 13(4), 4214–4224. doi: 10.3390/s130404214
- Liu, L. Q., Luo, L. J., Suryoprabowo, S., Peng, J., Kuang, H., & Xu, C. L. (2014). Development of an immunochromatographic strip test for rapid detection of ciprofloxacin in milk samples. Sensors, 14(9), 16785–16798. doi: 10.3390/s140917621
- Mostafalou, S., & Abdollahi, M. (2013). Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicology and Applied Pharmacology, 268(2), 157–177. doi: 10.1016/j.taap.2013.01.025
- Nunes, G. S., Alonso, R. M., Ribeiro, M. L., & Barcelo, D. (2000). Determination of aldicarb, aldicarb sulfoxide and aldicarb sulfone in some fruits and vegetables using high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. Journal of Chromatography A, 888(1–2), 113–120. doi: 10.1016/S0021-9673(00)00553-7
- Qin, G., Li, Y., Chen, Y., Sun, Q., Zuo, B., He, F., … Ding, G. (2015). Pesticide residues determination in China vegetables in 2010–2013 applying gas chromatography with mass spectrometry. Food Research International, 72, 161–167. doi: 10.1016/j.foodres.2015.03.036
- Rodolico, S., Giovinazzo, R., & Mosconi, M. (1997). Comparison between ELISAs and traditional analytical methods to determine pesticide pollution in water. Bulletin of Environmental Contamination and Toxicology, 58(4), 644–650. doi: 10.1007/s001289900382
- Saito, M., Kozutsumi, D., Kawasaki, M., Kanbashi, M., Nakamura, R., Sato, Y., & Endo, M. (2008). Multiresidue method for pesticides and veterinary drugs in bovine milk using GC/MS and LC/MS/MS. Journal of the Food Hygienic Society of Japan, 49(3), 228–238. doi: 10.3358/shokueishi.49.228
- Sivaperumal, P., Anand, P., & Riddhi, L. (2015). Rapid determination of pesticide residues in fruits and vegetables, using ultra-high-performance liquid chromatography/time-of-flight mass spectrometry. Food Chemistry, 168, 356–365. doi: 10.1016/j.foodchem.2014.07.072
- Song, M. L., & McNair, H. M. (2002). Fast gas chromatography analysis of N-carbamates with cold on-column injection. Journal of Chromatographic Science, 40(7), 321–325. doi: 10.1093/chromsci/40.7.321
- Sun, C., Liu, L. Q., Song, S. S., Kuang, H., & Xu, C. L. (2016). Development of a highly sensitive ELISA and immunochromatographic strip to detect pentachlorophenol. Food and Agricultural Immunology, 27(5), 689–699. doi: 10.1080/09540105.2016.1148668
- Suryoprabowo, S., Liu, L. Q., Peng, J., Kuang, H., & Xu, C. L. (2014). Development of a broad specific monoclonal antibody for fluoroquinolone analysis. Food Analytical Methods, 7(10), 2163–2168. doi: 10.1007/s12161-014-9863-1
- Xu, J. A., Xu, Y. A., & Dai, S. U. (2006). Effect of surfactants on desorption of aldicarb from spiked soil. Chemosphere, 62(10), 1630–1635. doi: 10.1016/j.chemosphere.2005.06.040
- Yang, X., Li, P., Miao, H., Zhao, Y., & Wu, Y. (2014). Determination of N-methyl carbamate pesticides in diet samples by high performance liquid chromatography-linear ion trap mass spectrometry with gel permeation chromatography cleanup. Chinese Journal of Chromatography, 32(5), 499–505. doi: 10.3724/SP.J.1123.2013.12041
- Yuan, Y., Xiong, W., Li, Y., Chen, J., & Liu, Z. (2011). Determination of 4 carbamate pesticides residues in cucumber by high performance liquid chromatography-tandem mass spectrometry. Guangdong Agricultural Sciences, 38(24), 84–86, 98.
- Zhao, Y., Zhang, G., Liu, Q., Teng, M., Yang, J., & Wang, J. (2008). Development of a lateral flow colloidal gold immunoassay strip for the rapid detection of enrofloxacin residues. Journal of Agricultural and Food Chemistry, 56(24), 12138–12142. doi: 10.1021/jf802648z