ABSTRACT
A broad-specificity immunoassay was established to monitor marbofloxacin (MBF) and ofloxacin (OFX) residues in raw milk and porcine muscle samples. The main influential parameters in the assay buffer were investigated to improve the assay performance. The cross-reactivities (CRs) were lower than 0.25%, except for OFX (41.38%). The quantitative working ranges for MBF and OFX based on fortified matrices ranged from 0.11 to 30.85 ng mL−1, with a 50% inhibitory concentration of 0.76±0.12 ng mL−1 for MBF and 2.70±0.28 ng mL−1 for OFX. The detection limits of enzyme-linked immunosorbent assay (ELISA) ranged from 0.053 to 0.358 ng mL−1 in spiked samples. The mean recoveries were in the range of 80.52–95.46%, less than 12.06% of the coefficient of variation. A good correlation between the ELISA results and those from instrument methods demonstrated the practical application of the novel ELISA. Therefore, the immunoassay proposed is feasible for MBF and OFX analysis in animal-derived foods.
Introduction
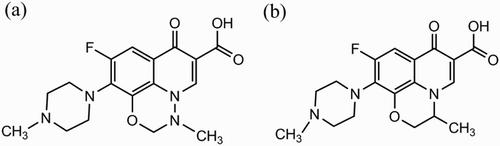
Marbofloxacin (MBF, see (a)) and ofloxacin (OFX, see (b)) are third-generation fluoroquinolone antimicrobial agents. They exhibit broad-spectrum antimicrobial activity against Gram-positive and Gram-negative pathogens and Mycoplasma spp. (Spreng, Deleforge, Thomas, Boisrame, & Drugeon, Citation1995; Thomas, Nicolas, Dizier, Mainil, & Linden, Citation2003), low toxicity, and limited development of bacterial resistance (Nie et al., Citation2016). Therefore, MBF and OFX are intensively applied for the treatment of various human and animal infectious diseases, such as urinary tract infection, respiratory tract infection, and osteomyelitis(Cotard et al., Citation1995; Potter, Illambas, Pelligand, Rycroft, & Lees, Citation2013; Sidhu, Landoni, Aliabadi, & Lees, Citation2010). However, their excessive and unreasonable use can also leave residues in foodstuffs of animal origin, which may give rise to public health concerns on toxic effects and allergic reactions (Cheng et al., Citation2008). Consequently, the development of a quantification method for the simultaneous testing of MBF and OFX residues in raw milk and porcine muscle samples is urgently needed. To minimize the health risks of consumers, the European Union set maximum residue limits (MRLs) in milk and edible animal tissues (Regulation EC No. 470/2009); for example, the MRLs of MBF in raw milk and porcine muscle are 30 and 150 μg kg−1(Taranova, Berlina, Zherdev, & Dzantiev, Citation2015), respectively. Additionally, the MRLs of OFX in raw milk and porcine muscle are 30 and 50 μg kg−1, respectively, as stipulated by Japan (Tochi et al., Citation2016).
Analytical methodologies have been established to quantify MBF and OFX in various foodstuffs, including high-performance liquid chromatography with ultraviolet (UV) detection (Gajda, Posyniak, Zmudzki, Gbylik, & Bladek, Citation2012), with fluorescence detection (Arroyo-Manzanares, Huertas-Perez, Lombardo-Agui, Gamiz-Gracia, & Garcia-Campana, Citation2015; Garcia, Solans, Aramayona, Rueda, & Bregante, Citation1999), and with tandem mass spectrometry (HPLC-MS) (Jimenez-Diaz et al., Citation2013; Khan et al., Citation2016), as well as chemiluminescence assays (Liu et al., Citation2015), and electrochemical methods (Li, Lv, Shan, & Zhang, Citation2015; Wu et al., Citation2016). However, some clear shortcomings of these formats are their high costs, expensive instruments used, and complicated pretreatments.
Multi-residue immunoassays for the detection of fluoroquinolones have been reported over the past few decades (Liu et al., Citation2013; Peng et al., Citation2016; Sheng et al, Citation2011; Tao et al., Citation2013; Wang, Zhang, Ni, Zhang, & Shen, Citation2014). Liu et al. (Citation2013) prepared a broad-spectrum monoclonal antibody that recognizes 12 different fluoroquinolones, including MBF. Wang et al. (Citation2014) developed an enzyme-linked immunosorbent assay (ELISA) for enrofloxacin, with a 50% inhibitory concentration (IC50) of 0.78 ng mL−1, and showed CRs to OFX (8.23%), MBF (8.97%), and pefloxacin (7.29%). Sheng et al. (Citation2011) developed a colloidal gold-based immunochromatographic assay for the detection of several fluoroquinolone agents, with the limits of detection (LOD) for OFX, MBF, and fleroxacin in milk ranging from 3.5 to 8.9 ng mL−1. Various studies describing immunoassays for MBF or OFX only have been published. Sheng et al. (Citation2009) prepared a polyclonal antibody against MBF with an IC50 of 4.6 ng mL−1, which showed a high CR value for OFX (148%) (Sheng et al., Citation2009). Tochi et al.(Citation2016) established an indirect competitive immunoassay to screen for OFX residues in edible animal tissues.
To date, there has been no report on the simultaneous screening of MBF and OFX residues in animal-derived foods. Thus, our purpose was to generate a broad-specificity polyclonal antibody against MBF and OFX and to develop an indirect competitive ELISA (icELISA) for the simultaneous detection of these two antimicrobial agents in milk and porcine muscle samples.
Material and methods
Chemicals
MBF, difloxacin, OFX, enrofloxacin, and ciprofloxacin standards were purchased from the Tianjin Institute of Environmental Protection (Tianjin, China). Complete Freund’s adjuvant and incomplete Freund’s adjuvant were obtained from Sigma Chemical Co. (St. Louis, MO). Bovine serum albumin (BSA) and ovalbumin (OVA) were purchased from Bioengineering Co. (Shanghai, China). N-Hydroxysuccinimide; 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide; 4-morpholine ethyl sulfonic acid (MES), and 3,3′,5,5′-tetramethylbenzidine were purchased from Aladdin Reagent Co. (Shanghai, China). Goat anti-rabbit IgG (HL) horseradish peroxidase conjugate was purchased from Amyjet Scientific Co. (Wuhan, China). Other chemical reagents were obtained from Crownbo Trade Co. (Luoyang, China).
Antigen preparation
MBF was coupled to two carrier proteins containing BSA and OVA using an activated ester method for antigen preparation (MBF-BSA and MBF-OVA) (Sun, Liu, Kuang, & Xu, Citation2013). Under ice-bath conditions, the mixed solution reacted for six hours and was dialyzed using phosphate buffer solution (PBS) buffer (0.01 M PBS, pH 7.2, NaH2PO4 0.296 g, Na2HPO4·12H2O 2.9 g in 1 L water) every six hours for a total of eight times. Two conjugates were characterized with a UV–visible spectrophotometer (Bokin Instruments, Tsushima, Japan). The final conjugates were aliquoted and stored at −20°C until use.
Antibody production
Two female New Zealand white rabbits were subcutaneously injected with the MBF-BSA conjugate. The immunization procedure was performed following a previously reported method (Yan, Liu, Xu, Kuang, & Xu, Citation2015). Rabbits were bled through the ear vein 7–10 days after the fifth injection. The titer and inhibition of antisera were examined using the icELISA. The selected rabbit was bled from the heart. The resulting blood was left at 37°C for 30 min and then overnight at 4°C, followed by centrifugation at 3500 g for 15 min to obtain the antisera. The antisera were purified with an ammonium sulfate precipitation method (Song et al., Citation2010). The purified polyclonal antibody was added to the same volume of glycerol (1:1, v/v), mixed, and then stored at −20°C until use.
ELISA establishment
The icELISA was modified according to the description by Chen et al. (Citation2013). The optimum conditions were investigated using MBF as the only competitor analyte to improve immunoassay performance. The optimized concentrations of the antibody and coating antigen were tested using a two-dimensional titration. The effect of different coating buffers on ELISA performance was evaluated in carbonate buffer solution (CBS) (0.02 M CBS, pH 9.6, Na2CO3 1.59 g, NaHCO3 2.93 g, diluted with 1 L H2O), borate bicarbonate solution (0.02 M BBS, pH 8.5, Na2B4O7·10H2O 5.34 g, H3BO3 1.48 g in 1 L water), and phosphate buffer solution (PBS) buffer (0.02 M PBS, pH 7.2, NaH2PO4 0.592 g, Na2HPO4·12H2O 5.8 g in 1 L water). The concentration of NaCl (0%, 0.5%, 0.8%, 1%, 2%, and 4%, m/v) was varied in the assay buffer to investigate the effect of ionic strength on assay sensitivity. The effect of pH variation (5.3, 6.0, 7.2, and 8.0) on assay performance was also assessed. Under optimum conditions, the standard inhibition curves for MBF and OFX were plotted by a four-parameter regression equation using Origin 8.5 software (Originlab Corporation, USA). The concentration range of 20% and 80% inhibitory concentrations (IC20–80) was defined as the quantitative working concentration. Furthermore, 50% inhibitory concentrations (IC50) were calculated to determine assay sensitivity. The CR was calculated based on a previously reported equation (Chen et al., Citation2014):
Sample analysis
Raw milk and porcine muscle were purchased from the local Denis supermarket (Luoyang, China). Milk and porcine muscle samples without MBF and OFX were tested by HPLC-MS/MS. An aliquot of porcine muscle sample (2 g) was minced and ground into pulpiness, and transferred into a 50-mL polypropylene centrifugal tube. Then, 2 mL of 0.5 M ethylenediaminetetraacetic acid (pH 7.0) and 15 mL of dichloromethane were added to the tube. Porcine muscle samples were shaken in the dark for 10 min and centrifuged at 3500 g for 10 min. Furthermore, the supernatant was separated and the extraction procedure repeated twice. The combined organic phase was evaporated to dryness under vacuum at 45°C. The residue was reconstituted in PBS buffer (0.01 moL L−1, pH 7.2) containing 10% (v/v) methanol and then detected by the icELISA. Milk samples were centrifuged at 3500 g for 10 min and diluted with five fold PBS buffer (0.01 moL L−1, pH 7.2) containing 10% (v/v) methanol prior to ELISA analysis.
ELISA validation
The calibration curves of MBF and OFX based on raw milk and porcine muscle matrices were set using the aforementioned method. Then, intra-assay variation was tested on six replicates at each concentration, and inter-assay variation was measured on three consecutive days to evaluate the precision of the icELISA. The detection limit was defined as the 15% inhibitory concentration (IC15) of the assay. For the recovery test, MBF was fortified at three final concentrations of 0.1, 0.4, and 1.6 ng mL−1, and OFX was spiked at three different levels of 0.5, 2.0, and 8.0 ng mL−1, respectively.
Comparison of ELISA and HPLC-MS/MS analysis
To estimate the quantitative accuracy of the proposed icELISA, a comparison of the samples was performed using both icELISA and HPLC-MS/MS on five spiked milk samples. HPLC-MS/MS analysis was carried out on an Agilent HPLC system coupled to an Agilent 6490 triple quadrupole mass spectrometer with an electrospray ionization (ESI) source (Agilent, Palo Alto, CA, USA). Chromatographic separation was performed by injecting 10 μL on an analytical RP C18 column (5 μm, 4.6 mm × 250 mm, Thermo, USA). The column temperature was maintained at 30°C. The mobile phase consisted of 0.5% formic acid in 1 mM ammonium acetate buffer (solvent A, pH 2.5, 80% by volume) and methanol (solvent B, 20% by volume) at a constant flow rate of 0.6 mL min−1. The ESI was operated in positive ion mode and MS data acquisition was obtained using multiple reaction monitoring mode. The detailed procedure of sample preparation was described previously by Janusch et al. (Citation2014). The spiked samples were analyzed by the proposed ELISA and the modified HPLC-MS/MS five times. Linear regression curves were plotted to evaluate the correlation between the icELISA and HPLC-MS/MS.
Results and discussion
Antigen characterization
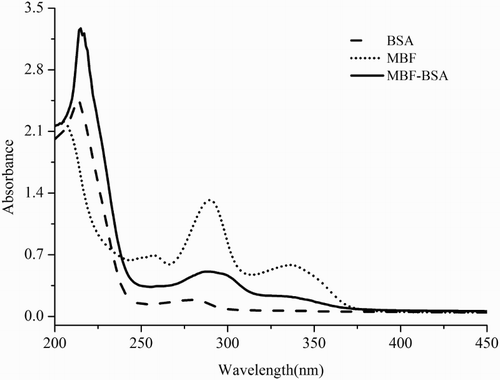
Immunogen preparation is critical for antibody production and the establishment of an immunoassay. The molecular weight of MBF is less than 1000 Da and lacks immunogenicity. To obtain an antibody, MBF needs to be conjugated to a carrier protein to elicit an immune response via a carboxyl group. According to previous reports (Sheng et al., Citation2009), when dimethylformamide (DMF) is used as the reaction solvent of MBF and carrier proteins, there is more white precipitate in the final solution. While MES was chosen as a buffer, the obtained solution was transparent. Consequently, MES was selected as the buffer of antigens synthesized. Moreover, the UV absorption spectrum of MBF showed three characteristic absorption peaks at 257, 291, and 337 nm, while carrier proteins (BSA and OVA) shared the same characteristic absorption peak at 278 nm. shows that the conjugates were successfully prepared, as indicated by the three characteristic absorption peaks at 215, 288, and 334 nm, which are known characteristic absorption peaks of MBF and carrier proteins.
Antibody production and characterization
The antisera obtained from the ear veins of rabbits after the fifth boosting immunization were tested by the icELISA. Polyantibody2#, which had a higher titer (1:48,000) and better sensitivity, was selected for subsequent experiments. Furthermore, the specificity of the polyclonal antibody was investigated by monitoring its four structural analogs. The CR results are presented in . The obtained antibody exhibited 41.38% CR for OFX based on the high similarity between the structures of MBF and OFX. Meanwhile, the polyclonal antibody showed less than 0.25% CR for the other three analogs. These results prove that the prepared antibody has good recognition to MBF and OFX. Hence, the icELISA developed can be applied for simultaneous screening of MBF and OFX residues in edible animal tissues.
Table 1. Specificity of the MBF antibody.
ELISA establishment
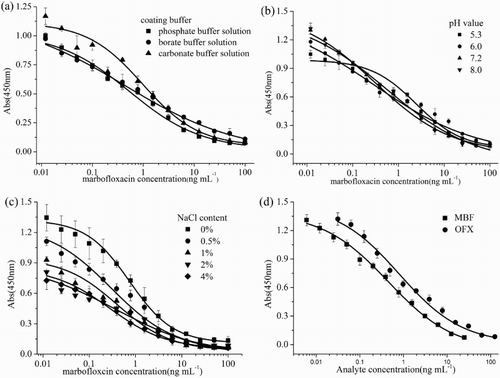
Based on the checkerboard results, the optimal concentration of the coating antigen was 0.25 μg mL−1, and the optimal antisera concentration was 0.5 μg mL−1. Among the three coating buffers ((a)), the changes in IC50 values (1.08, 1.16, and 1.22 ng mL−1) were not obvious, and the maximum optical density (ODmax) was the highest when CBS buffer was used. Thus, CBS buffer was chosen as the coating buffer for the subsequent experiment. (b) shows a sharp decrease in ODmax when the pH varied from 5.3 to 8.0. The IC50 values ranged from 0.503 to 0.432 ng mL−1 when the pH of the buffer changed from acidic to alkaline, which demonstrates the robustness of the ELISA. Considering the ODmax and the sensitivity, the subsequent test was performed in an assay buffer of pH 7.2. As observed in (c), the ODmax value was 1.39 in the absence of NaCl in the assay buffer. With the increasing concentration of NaCl in the buffer, the ODmax values decreased. These results indicate that the ELISA became less stable with increasing ionic strength. The NaCl concentration also affected the sensitivity of the assay; the IC50 values first decreased and then increased. The IC50 value was the lowest (0.39 ng mL−1) at 0.5% NaCl and the ODmax value (1.32) was also higher. Thus, a NaCl concentration of 0.5% in PBS buffer (0.01 M, pH 7.2) was the preferred assay buffer. Based on the aforementioned cross-reaction results, (d) plots two typical inhibition curves for MBF and OFX under optimum conditions; the detection range of the standard curves in PBS buffer (0.01 M, pH 7.2) was 0.04 to 10.03 ng mL−1, and the IC50 value of the ELISA for MBF was 0.36 ± 0.012 ng mL−1 and that for OFX was 0.87 ± 0.056 ng mL−1.
Sample analysis
To estimate the validity of the assay, we investigated the LOD, accuracy, and precision of the icELISA. On the basis of the calibration standard curves, the LOD of the obtained icELISA in milk and porcine muscle was 0.053 and 0.085 μg kg−1 for MBF, and 0.27 and 0.36 μg kg−1 for OFX, respectively.
The milk and porcine muscle samples were selected to perform the spiked test. The recovery rates were calculated according to the calibration standard curves. As shown in , the mean recovery values ranged from 80.52% to 95.46% in milk and porcine muscle samples, respectively. The intra-assay coefficients of variation were in the range of 4.54–7.06%, and the inter-assay coefficients of variation were in the range of 8.43–12.06%. Thus, the established icELISA is feasible for the determination of MBF and OFX residues in raw milk and porcine muscle samples.
Table 2. Results of the spiked test.
Comparison of the developed ELISA and HPLC-MS/MS
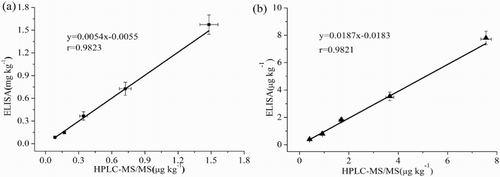
All contaminated milk and porcine muscle samples were subjected to the icELISA and HPLC-MS/MS analysis. indicates an excellent correlation r = 0.9823 for MBF and r = 0.9821 for OFX between the icELISA and HPLC-MS/MS results. These results demonstrate the accuracy and quantitative character of the newly developed ELISA. Hence, the newly developed icELISA is a reliable method for monitoring MBF and OFX residues in animal-derived foods.
Conclusion
A broad-specificity ELISA was developed for the simultaneous detection of MBF and OFX residues in milk and porcine muscle samples. The LOD was far below the MRL stipulated by the European Commission and Japan. This established ELISA has satisfactory recovery rates in the range of 80.52–95.46% and correlates well with HPLC-MS/MS. Consequently, this novel immunoassay can be used to test for MBF and OFX residues in other foods of animal origin.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Xiujin Chen began to work in the College of Food and Bioengineering, Henan University of Science and Technology (Luoyang, China) in 2003. She obtained her PhD degree in Food Nutrition and Safety from Jiangnan University (Wuxi, China) in 2013. She is currently a vice professor in food safety. Her research has concentrated on the application of immunoassays in food.
Zhaozhou Li obtained his PhD degree from Chinese Academy of Agricultural Science (Beijing, China) in 2011 and then began to work as a faculty in the College of Food and Bioengineering, Henan University of Science and Technology, Luoyang, China. His research has focused on the novel detection technology based on biomimetic antibodies.
Jinying Guo obtained his PhD degree from North West Agriculture and Forestry University in 2005 and then became as a faculty in the College of Food and Bioengineering, Henan University of Science and Technology, Luoyang, China. His research has focused on the immune activity of polysaccharides in food.
Daomin Li is a vice professor in the College of Food and Bioengineering, Henan University of Science and Technology, Luoyang, China. Her research has focused on the establishment of instrumental analysis methods for harmful substances in food.
Hongli Gao obtained her PhD from Nanjing University (Nanjing, China) in 2012 and then began to work in the College of Food and Bioengineering, Henan University of Science and Technology, Luoyang, China. Her research has concentrated on high-sensitivity electrochemistry sensors development.
Yao Wang obtained his Ph.D. from North West Agriculture and Forestry University in 2015 and then began to work as a faculty in the College of Food and Bioengineering, Henan University of Science and Technology, Luoyang, China. His research has focused on the development and application of green immunochromatographic strips.
Xu Chuanlai obtained his Ph.D. in Food Science in 2002. He is currently a full professor in Food Science and Technology of Jiangnan University, Wuxi, China. His research has focused on the food safety and rapid detection technology.
Additional information
Funding
References
- Arroyo-Manzanares, N., Huertas-Perez, J. F., Lombardo-Agui, M., Gamiz-Gracia, L., & Garcia-Campana, A. M. (2015). A high-throughput method for the determination of quinolones in different matrices by ultra-high performance liquid chromatography with fluorescence detection. Analytical Methods, 7(1), 253–259. doi:10.1039/c4ay01940g
- Cheng, G. W., Wu, H. L., & Huang, Y. L. (2008). Simultaneous determination of malondialdehyde & ofloxacin in plasma using an isocratic high-performance liquid chromatography/fluorescence detection system. Analytica Chimica Acta, 616(2), 230–234. doi:10.1016/j.aca.2008.04.012
- Chen, X. J., Wu, H. L., Huang, Y. L., Xu, L. G., Ma, W., Liu , L. Q., & Xu , C. L. (2013). Development of an Enzyme-Linked Immunosorbent Assay for Cyhalothrin. Immunol. Invest., 42(6), 493–503. doi:10.3109/08820139.2013.797909
- Chen, X. J., Xu, L. G., Ma, W., Liu, L. Q., Kuang, H., Wang, L. B., & Xu, C. L. (2014). General immunoassay for pyrethroids based on a monoclonal antibody. Food and Agricultural Immunology, 25(3), 341–349. doi:10.1080/09540105.2013.794328
- Cotard, J. P., Gruet, P., Pechereau, D., Moreau, P., Pages, J. P., Thomas, E., & Deleforge, J. (1995). Comparative study of marbofloxacin and amoxicillin–clavulanic acid in the treatment of urinary tract infections in dogs. The Journal of Small Animal Practice, 36(8), 349–353. doi:10.1111/j.1748-5827.1995.tb02948.x
- Gajda, A., Posyniak, A., Zmudzki, J., Gbylik, M., & Bladek, T. (2012). Determination of (fluoro)quinolones in eggs by liquid chromatography with fluorescence detection and confirmation by liquid chromatography-tandem mass spectrometry. Food Chemistry, 135(2), 430–439. doi:10.1016/j.foodchem.2012.04.106
- Garcia, M. A., Solans, C., Aramayona, J. J., Rueda, S., & Bregante, M. A. (1999). Determination of marbofloxacin in plasma samples by high-performance liquid chromatography using fluorescence detection. Journal of Chromatography. B, Biomedical Sciences and Applications, 729(1–2), 157–161. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10410938 doi: 10.1016/S0378-4347(99)00144-9
- Janusch, F., Scherz, G., Mohring, S. A. I., & Hamscher, G. (2014). Determination of fluoroquinolones in chicken feces – a new liquid–liquid extraction method combined with LC-MS/MS. Environmental Toxicology and Pharmacology, 38(3), 792–799. doi:10.1016/j.etap.2014.09.011
- Jimenez-Diaz, I., Hermo, M. P., Ballesteros, O., Zafra-Gomez, A., Barron, D., Barbosa, J., & Navalon, A. (2013). Comparison of three analytical methods for the determination of quinolones in pig muscle samples. Chromatographia, 76(11–12), 707–713. doi:10.1007/s10337-013-2435-5
- Khan, F. U., Nasir, F., Iqbal, Z., Khan, I., Shahbaz, N., Hassan, M., & Ullah, F. (2016). Simultaneous determination of moxifloxacin and ofloxacin in physiological fluids using high performance liquid chromatography with ultraviolet detection. Journal of Chromatography B, 1017–1018, 120–128. doi:10.1016/j.jchromb.2016.03.002
- Li, R., Lv, S., Shan, J., & Zhang, J. (2015). A novel electrochemical method for ofloxacin determination based on interaction of ofloxacin with cupric ion. Ionics, 21(11), 3117–3124. doi:10.1007/s11581-015-1492-1
- Liu, W., Guo, Y., Li, H., Zhao, M., Lai, Z., & Li, B. (2015). A paper-based chemiluminescence device for the determination of ofloxacin. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 137, 1298–1303. doi:10.1016/j.saa.2014.09.059
- Liu, Y. Z., Zhao, G. X., Wang, P., Liu, J., Zhang, H. C., & Wang, J. P. (2013). Production of the broad specific monoclonal antibody against sarafloxacin for rapid immunoscreening of 12 fluoroquinolones in meat. Journal of Environmental Science and Health. Part B, Pesticides, Food Contaminants, and Agricultural Wastes, 48(2), 139–146. doi:10.1080/03601234.2013.727668
- Nie, J., Wang, Y. G., Gao, X. F., OuYang, X. K., Yang, L. Y., Yu, D., & Xu, H. P. (2016). Pharmacokinetic study of ofloxacin enantiomers in pagrosomus major by chiral HPLC. Biomedical Chromatography, 30(3), 426–431. doi:10.1002/bmc.3565
- Peng, C. F., Pan, N., Xie, Z. J., Liu, L. Q., Xiang, J., & Liu, C. L. (2016). Determination of bisphenol a by a gold nanoflower enhanced enzyme-linked immunosorbent assay. Analytical Letters, 49(10), 1492–1501. doi: 10.1080/00032719.2015.1113420
- Potter, T., Illambas, J., Pelligand, L., Rycroft, A., & Lees, P. (2013). Pharmacokinetic and pharmacodynamic integration and modelling of marbofloxacin in calves for Mannheimia haemolytica and Pasteurella multocida. The Veterinary Journal, 195(1), 53–58. doi:10.1039/c5ay01261a
- Sheng, W., Li, Y. Z., Xu, X., Yuan, M., & Wang, S. (2011). Enzyme-linked immunosorbent assay and colloidal gold-based immunochromatographic assay for several (fluoro)quinolones in milk. Microchimica Acta, 173(3–4), 307–316. doi:10.1007/s00604-011-0560-0
- Sheng, W., Xia, X. F., Wei, K. Y., Li, J., Li, Q. X., & Xu, T. (2009). Determination of marbofloxacin residues in beef and pork with an enzyme-linked immunosorbent assay. Journal of Agricultural and Food Chemistry, 57(13), 5971–5975. doi:10.1021/jf900940n
- Sidhu, P. K., Landoni, M. F., Aliabadi, F. S., & Lees, P. (2010). Pharmacokinetic and pharmacodynamic modelling of marbofloxacin administered alone and in combination with tolfenamic acid in goats. The Veterinary Journal, 184(2), 219–229. doi:10.1016/j.tvjl.2009.02.009
- Song, S. S., Lin, F., Liu, L. Q., Kuang, H., Wang, L. B., & Xu, C. L. (2010). Immunoaffinity removal and immunoassay for rhodamine B in chilli powder. International Journal of Food Science & Technology, 45(12), 2589–2595. doi:10.1111/j.1365-2621.2010.02445.x
- Spreng, M., Deleforge, J., Thomas, V., Boisrame, B., & Drugeon, H. (1995). Antibacterial activity of marbofloxacin: A new fluoroquinolone for veterinary use against canine and feline isolates. Journal of Veterinary Pharmacology and Therapeutics, 18(4), 284–289. doi:10.1111/j.1365-2885.1995.tb00592.x
- Sun, F. X., Liu, L. Q., Kuang, H., & Xu, C. L. (2013). Development of ELISA for melamine detection in milk powder. Food and Agricultural Immunology, 24(1), 79–86. doi:10.1080/09540105.2011.641170
- Tao, X. Q., Chen, M., Jiang, H. Y., Shen, J. Z., Wang, Z. H., Wang, X., & Wen, K. (2013). Chemiluminescence competitive indirect enzyme immunoassay for 20 fluoroquinolone residues in fish and shrimp based on a single-chain variable fragment. Analytical and Bioanalytical Chemistry, 405(23), 7477–7484. doi:10.1007/s00216-013-7174-9
- Taranova, N., Berlina, A., Zherdev, A., & Dzantiev, B. (2015). Traffic light immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosensors and Bioelectronics, 63, 255–261. doi:10.1016/j.bios.2014.07.049
- Thomas, A., Nicolas, C., Dizier, I., Mainil, J., & Linden, A. (2003). Antibiotic susceptibilities of recent isolates of Mycoplasma bovis in Belgium. The Veterinary Record, 153(14), 428–431. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14582732 doi: 10.1136/vr.153.14.428
- Tochi, B. N., Peng, J., Song, S., Liu, L., Kuang, H., & Xu, C. (2016). Production and application of a monoclonal antibody (mAb) against ofloxacin in milk, chicken and pork. Food and Agricultural Immunology, 27(5), 643–656. doi:10.1080/09540105.2016.1148125
- Wang, Z., Zhang, H., Ni, H., Zhang, S., & Shen, J. (2014). Development of a highly sensitive and specific immunoassay for enrofloxacin based on heterologous coating haptens. Analytica Chimica Acta, 820, 152–158. doi:10.1016/j.aca.2014.02.043
- Wu, F., Xu, F., Chen, L., Jiang, B., Sun, W., & Wei, X. (2016). Cuprous oxide/nitrogen-doped graphene nanocomposites as electrochemical sensors for ofloxacin determination. Chemical Research in Chinese Universities, 32(3), 468–473. doi:10.1007/s40242-016-5367-4
- Yan, H. J., Liu, L. Q., Xu, N. F., Kuang, H., & Xu, C. L. (2015). Development of an immunoassay for carbendazim based on a class-selective monoclonal antibody. Food and Agricultural Immunology, 26(5), 659–670. doi:10.1080/09540105.2015.1007446