ABSTRACT
The misuse and illegal use of fluoroquinolones (FQs) in animal-based food products have drawn considerable attention in several countries. As a result, there has been an increased demand for efficient detection methods of FQs in food products. In this study, we developed an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) and immunochromatographic strip device based on a monoclonal antibody against lomefloxacin (LFLX), a second generation FQ. Ic-ELISA had an IC50 value of 0.19 ng/mL with a limit of detection of 0.04 ng/mL in 0.01 M phosphate-buffered saline (PBS). The intra- and inter-assay recovery rates of LFLX in bovine milk samples were 98.02–107.40% and 100.65–107.82%. The immunochromatographic assay of LFLX in PBS and spiked bovine milk samples had visual cutoff values at 1 and 5.0 ng/mL, respectively, with significant cross-reactivity with norfloxacin and enoxacin. The developed ic-ELISA and strip method may assist in the detection of FQs in foods.
Introduction
Fluoroquinolones (FQs) are a group of synthetic antibiotics that are used for the prevention and treatment of various infection diseases in clinical medicine and animal husbandry (Park, Kim, Han, & Lee, Citation2013; Tochi et al., Citation2016). FQs are subdivided into four different generations. The second generation of FQs, which includes lomefloxacin (LFLX), norfloxacin (NRFLX), enoxacin (ENX), ofloxacacin and ciprofloxacin, has a broad-spectrum antimicrobial activity against gram positive and negative bacteria (Asif, Citation2013; King, Malone, & Lilley, Citation2000).
FQs are associated with an increased risk of tendinitis and tendon rupture (Xu, Liu, Guo, & Cui, Citation2015), and LFLX causes phototoxicity and adverse effects in the central nervous system (Adachi et al., Citation2015; Rubinstein, Citation2001). Therefore, the risks of antimicrobial agents continue to be complex and controversial issues. The European Union and regulatory authorities worldwide have established maximum residue limits (MRLs) for antibiotic residues in animal-based food products. The MRL of quinolones is 10–1500 µg kg−1 (Cao et al., Citation2011; Gery et al., Citation2002; Liu, Luo, et al., Citation2014; Suryoprabowo, Liu, Peng, Kuang, & Xu, Citation2014). On 26 July 2016, the FDA established that FQ labels should contain safety information.
The misuse and illegal use of FQs in animal-based food products have drawn considerable attention in several countries. As a result, there has been an increased demand for efficient detection methods of FQs in food products. Several types of analytical methods have been developed for the detection of FQs including UPLC/MS/MS, which detects LFLX oxidation byproducts (Hubicka et al., Citation2013), RP-HPLC, HPLC, microbiological methods for LFLX detection in biological fluids (Chang, Wang, & Tsai, Citation2010; El-Shanawany, El-Adl, Abdel-Aziz, Hashem, & Sebaiy, Citation2011; Wu et al., Citation2014), spectrophotometric methods for aqueous LFLX samples, and Smart polymer aqueous two-phase flotation system for LFLX detection in food samples (Jain, Citation2012; Lu et al., Citation2016). Most of these methods are expensive, time-consuming, and require skilled personnel. Therefore, it is important to develop rapid and highly sensitive methods, which are simple, inexpensive, and easy to operate. The enzyme-linked immunosorbent assay (ELISA) is used in clinics and laboratories to determine proteins, hormones, enzymes, microbes, and drug residues (Feng et al., Citation2013; Hu et al., Citation2015; Watabe et al., Citation2016).
In this study, we developed a monoclonal antibody (mAb) against LFLX based on a novel and specific LFXL-EDEA-BSA immunogen. The mAb was used to develop sensitive immunoassay methods (ic-ELISA and immunostrip) for the determination of LFLX, NRFLX, and ENX residues in bovine milk samples. These methods were validated by evaluating their recovery, sensitivity, selectivity, and precision rates. To the best of our knowledge, this sensitive immunostrip device for LFLX residue detection has not been previously reported.
Materials and methods
Chemicals and instruments
LFLX hydrochloride was purchased from Shanghai HOPE Industry Co., Ltd. (Shanghai-PRC). Bovine serum albumin (BSA) and ovalbumin (OVA), Freund’s complete adjuvant (FCA) and Freund’s incomplete adjuvant (FIA), 3,3′,5,5′-tetramethylbenzidine (TMB), polyethylene glycol 1500 (PEG 1500), and horseradish peroxidase (HRP)-labeled goat anti-mouse IgG were obtained from Sigma-Aldrich (St. Louis, MO, USA). Carbodiimide (EDC), N-hydroxysuccinimide (NHS), Tween 20, and [2,2′-(Ethylenedioxy)bis(ethylamine)] (EDEA) were supplied by J&K (Shanghai, China). Gelatin was acquired from Beijing Biodee Biotechnology Co., Ltd (Beijing, China). Female six-week old BABL/c mice were obtained from the Shanghai Laboratory Animal Centre (Shanghai, China). All other reagents and chemicals were obtained from the National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China).
Agilent Cary 60 UV-Visible Spectrophotometer was obtained from Crawford Scientific Ltd (Lanarkshire, UK), Eon Microplate Spectrophotometer was from BioTek Instruments Company and a high-speed refrigerated centrifuge (Neofuge 15R) was obtained from Shanghai Lishen Scientific Equipment Co., Ltd (Shanghai, China). A nitrocellulose (NC) high-flow-plus membrane (Pura-bind RP) was obtained from Whatman-Xinhua Filter Paper Co. (Hangzhou, China). The sample pad (CB-SB08), poly-vinyl chloride backing card, and absorption pad (SX18) were supplied by Goldbio Tech Co. (Shanghai, China). Biostrip dispenser and cutter were from Xinqidian Gene-Technology Co. Ltd (Beijing, China).
The buffers and solutions used in this study consisted of an assay buffer (0.01 M phosphate-buffered saline (PBS) containing 8.0 g NaCl, 0.2 g KCl, 0.2 g KH2PO4, and 2.9 g Na2HPO4·12H2O in 1 L distilled water, pH 7.4); a washing buffer (PBS containing 0.05% Tween 20, v/v); a coating solution consisting of 50 mM carbonate buffer (CBS; 1.59 g Na2CO3 and 2.93 g NaHCO3, pH 9.6); a blocking buffer consisting of CBS with 0.2% (w/v, g/l) gelatin; an antibody dilution solution consisting of 0.01 M PBS with 0.1% (w/v, g/l) gelatin and 0.05% (v/v) Tween 20; solution A (9.33 g citric acid, 36.8 g Na2HPO4, and 180 μL of 30% H2O2 per 1 L water); solution B (0.06%, v/v, TMB in glycol); and a stop solution consisting of 2 M H2SO4.
Antigen design and synthesis
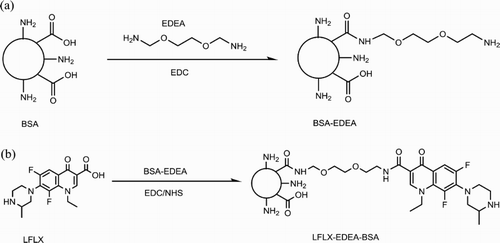
Cationization of BSA with EDEA [2,2′-(Ethylenedioxy)bis(ethylamine)]: the cationized form of BSA (cBSA) is a positively charged protein with high immunogenicity. The cationization of BSA was performed by a modified method (Cao et al., Citation2011; Helm & Fricker, Citation2015) and consisted of the amidation of the BSA carboxylic groups with EDEA by carbodiimide chemistry. Briefly, we added BSA (200 mg) to a solution of PBS (10 mL) containing 14 mg of EDEA. The solution was adjusted to pH 6.5. EDC (18.2 mg dissolved in water) was added drop-wise to the mixture, which was stirred at room temperature for 2 h, adjusted to pH 7.5, and allowed to react for 1 h. Uncoupled EDEA was removed via dialysis against PBS for 2 days using a membrane of molecular weight cutoff of 1400 Da. PBS was replaced three times daily. Cationized OVA was prepared by the same method.
Covalent coupling of LFLX to BSA-EDEA: The immunogen LFLX-EDEA-BSA was prepared by a modified carbodiimide active ester method as previously reported (Mukunzi et al., Citation2016; Wang, Zhang, Ni, Zhang, & Shen, Citation2014). As shown in (b), LFLX (10 mg), EDC (15 mg), and NHS (9.0 mg) dissolved in 5 mL of 0.05 M MES at pH 4.7 were incubated for 6 h at room temperature under constant stirring. This solution was slowly added to a solution of BSA-EDEA (0.3 μmol) in 10 mL of CBS (0.05 M, pH 9.6), and the combined solution was stirred for 3 h before being dialyzed for 3 days. Coating antigens were prepared by the same method using OVA and OVA-EDEA as carrier proteins. UV absorbance was used to confirm conjugation. Conjugates were stored at −20°C.
Immunization and antibody production
Freund’s complete and incomplete adjuvants were used to emulsify LFLX-EDEA-BSA. Emulsified LFLX-EDEA-BSA was subcutaneously administered to 15 BLB/c female mice (Tochi et al., Citation2016). Cell fusion and screening were performed as reported by Peng et al. (Citation2014), and antibody production and purification were achieved by the method reported by (Isanga et al., Citation2016; Kuang et al., Citation2013). Purified anti-LFLX mAb was stored at −20°C.
Ic-ELISA development and optimization
Ic-ELISA was performed as previously reported (Mukunzi et al., Citation2016). A checkerboard titration procedure was used to optimize coating antigen and anti-LFLX mAb interaction. Briefly, 100 µL of coating antigen (LFLX-EDEA-OVA) dissolved in carbonate buffer (0.05 M, CB, pH 9.6) were added to each well of a 96-well plate and incubated at 37°C for 2 h. The plate was washed three times (200 µL per well) with blocking buffer (2% gelatin in 0.05 M CB, pH 9.6) and incubated at 37°C for 2 h. After washing three times, the plate was loaded with 50 µL of LFLX standard and 50 µL of anti-LFLX mAb at different concentrations and incubated at 37°C for 30 min. HRP-labeled goat anti-mouse IgG (100 µL per well) was added, and the plate was incubated at 37°C for 30 min. The reaction was completed by washing the plate four times and adding 100 µL of 3, 3′, 5, 5′-tetramethylbenzidine substrate per well. Color development was allowed at 37°C for 15 min and stopped with an addition of 50 µL of 2 M sulfuric acid per well. Optical density (OD) was measured at 450 nm using a microplate reader.
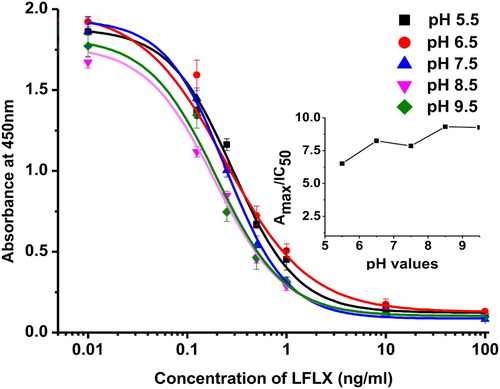
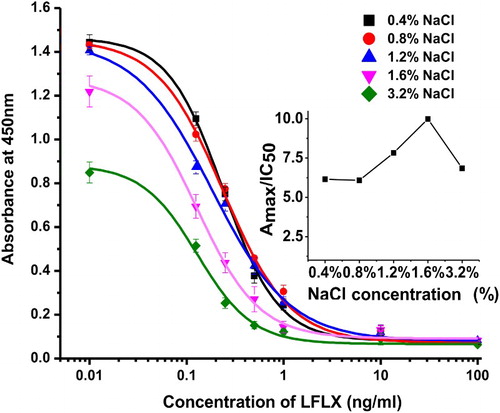
The effect of ionic strength on the sensitivity of ic-ELISA was evaluated by considering the following parameters; pH value (5.5, 6.5, 7.5, 8.5, and 9.5) and salt concentration (0.4 to 3.2%). Maximum absorbance (Amax), half-inhibition concentration (IC50), and maximal Amax/IC50 ratio were used to assess the optimum condition. A standard curve was generated using a four-parameter sigmoidal logistic dose response (Orginpro 8.5 software, Corporation, Northampton, MA, USA).
The specificity of anti-LFLX mAb was evaluated by studying cross-reactivity (CR) with FQ analogs. CR was calculated using the following equation,
Recovery studies by ic-ELISA
The performance of ic-ELISA was evaluated by spiking different LFLX-negative bovine milk samples with LFLX standards (0.0, 20, 40, and 80 ng/mL). Each sample was thoroughly vortexed and centrifuged at 8445 × g for 20 min at 4°C. An aliquot of the supernatant was diluted 100-fold with PBS and analyzed by ic-ELISA. The recovered amount of LFLX was calculated from the standard curved generated under the optimum ic-ELISA condition. Intra-assay coefficient of variation (CV) was determined from four repeat samples analyzed on a single day, and inter-assay CV was determined from samples analyzed on six consecutive days.
Synthesis of gold nano particle-labeled mAb
Colloidal gold nano particles (GNPs) were prepared using the trisodium citrate reduction method (Kong et al., Citation2015; Xu et al., Citation2016). The diameter of the GNPs was characterized by UV-VIS spectroscopy and transmission electron microscopy. GNP-labeled anti-LFLX mAb was prepared by adding 50 µL of anti-LFLX mAb (4.8 mg/mL) in borate buffer (0.1 M, pH 8.5) to a GNP solution (15 mL) previously adjusted to pH 8.2 with 0.1 M K2CO3. Following a 1-h incubation period, 2 mL of 5% BSA was slowly added to stabilize the GNPs and block any residual surface on the GNPs. After 30 min, the mixture was centrifuged at 6000 rpm for 25 min, and the precipitates were collected and washed with gold-labeled re-suspension buffer (0.02 M PBS, containing 1% BSA, 5% sucrose, and 0.5% PEG 6000, pH 7.4). The conjugates were dissolved in 5 mL PBS containing 0.2% NaN3 and stored at 4°C.
Immunochromatographic strip preparation
The immunochromatographic strip was prepared as previously reported (Liu, Xing, Yan, Kuang, & Xu, Citation2014). The immunochromatographic strip consists of an absorbent pad, a NC membrane, a conjugate pad, and a sample pad assembled in layers. The NC membrane was pre-coated with LFLX-EDEA-BSA (1 mg/mL) on the test (T) line and with goat anti-mouse IgG (0.5 mg/mL) on the control (C) line. The anti-LFLX mAb-colloid gold conjugates move along the membrane chromatographically to the T line, generating a visible line when the coating antigen and GNP-labeled anti-LFLX mAb complex forms.
Application of the strip device
Lateral-flow immunochromatrographic detection of LFLX was based on the competitive interaction between LFLX-GNP-labeled LFLX mAb and coated LFLX-OVA antigen located on the T line. The efficiency of the developed immunostrip was evaluated by testing different concentrations of LFLX in PBS buffer. Additionally, bovine milk samples were spiked with different concentrations of LFLX and detected without any further pretreatment steps. The color of the T line was allowed to develop for 5 min and visually recorded.
Results and discussion
Antigen synthesis
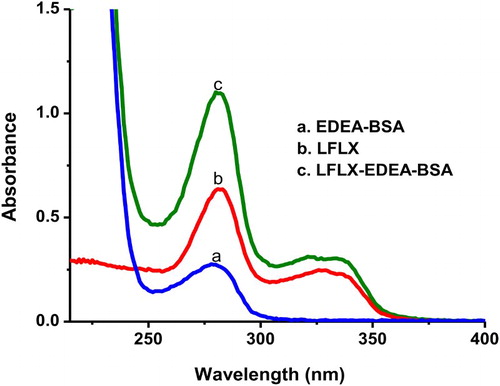
Unless attached to a large molecule, small molecules cannot induce an immune response by themselves (Wang et al., Citation2014). LFLX is a small molecule that contains a carboxyl group (–COOH), which is ideal for amino-acid conjugation. However, it is important to consider the distance between the hapten and the carrier protein to improve immunogenicity of the hapten. In this study, EDEA was used to cationize BSA protein prior to conjugation to LFLX (). The generated immunogen (LFLX-EDEA-BSA) was spectrophotometrically analyzed, and its UV absorbance was compared to those of BSA and LFLX UV. As depicted in , LFLX-EDEA-BSA and LFLX had similar UV characteristics at 326 and 335 nm. This result confirmed that the conjugation was successful because the carrier protein BSA-EDEA absorbs at 280 nm. Two coating antigens were prepared using the same EDC method. One coating antigen (LFLX-EDEA-OVA) involved EDEA as the linker between LFLX and OVA and t second coating antigen was produced with a zero length linker (LFLX-OVA). Both coating antigens had similar UV characteristics to those of the immunogen.
Optimization and specificity of ic-ELISA
The coating antigens (LFLX-EDEA-OVA and LFLX-OVA) and mAb were tested at different concentrations (0.001–1.0 µg/mL). The LFLX standard was kept constant at 0.3 ng/mL. At 0.05 µg/mL LFLX-EDEA-OVA and 0.25 µg/mL mAb, we observed a 62% inhibition, while at 0.03 µg/mL LFLX-OVA and 0.25 µg/mL mAb, we obtained a 68% inhibition. Therefore, 0.03 µg/mL LFLX-OVA was chosen for subsequent experiments. Other parameters were evaluated, including pH values and NaCl concentrations. We observed that pH had a little effect on Amax values. However, IC50 was affected by pH to the extent where the Amax/IC50 ratio exhibited two maximum points: a relative maximum at pH 6.5 and an absolute maximum at pH 8.5 (), suggesting that LFLX was better detected in alkaline than in acidic buffer. Therefore, pH 8.5 was chosen for subsequent ELISA experiments. The addition of 0.4–1.2% NaCl had no significant effects on Amax. However, Amax/IC50 increased with 1.2% NaCl, and Amax decreased from 1.45 to 0.884 as the salt concentration reached 3.2% (). Therefore, 1.2% NaCl was selected for subsequent experiments.
CR study and establishment of standard curves
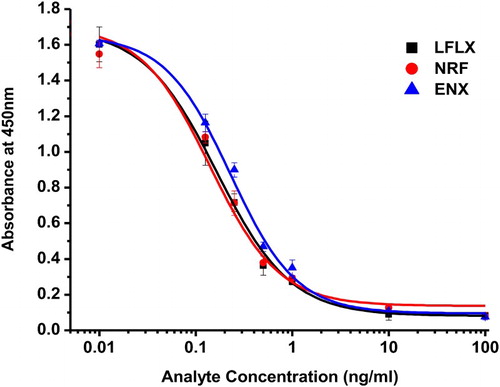
In general, an antibody is specific only to its corresponding hapten. However, the hapten may recognize other drugs from the same family. shows that anti-LFLX mAb had high sensitivity towards LFLX, NRFLX, and ENX compounds and moderate sensitivity towards ciprofloxacin, ofloxacin, fleroxacin, and pefloxacin. The CR of anti-LFLX mAb with other FQs was <0.1%. Based on these results, LFLX, NRFLX, and ENX were selected for subsequent experiments. depicts the calibration curves for these drugs.
Table 1. Cross-reactivity percentage of anti-LFLX mAb with related FQ compounds.
Recovery results of spiked bovine milk samples by ic-ELISA
Matrix effects affect the evaluation and validation of immunoassay kits. To overcome matrix effects, non-spiked bovine milk samples were diluted in the assay buffer at different ratios (1:0, 1:50, 1:100, 1:200, and 1:400 v/v). As a result of the 100-fold dilution, there was consistency in the OD values between the samples and the assay buffer. This technique of reducing matrix effects in milk samples has been applied by Peng, Liu, Kuang, Cui, and Xu (Citation2017) and Tochi et al. (Citation2016). In this study, bovine milk samples were spiked with different concentrations (0.0, 20, 40, and 80 ng/mL) of LFLX, NRFLX, and ENX to evaluate the performance of the developed ic-ELISA. As shown in , the intra- and inter-assay recoveries were 98.02–107.40% and 100.65–107.82% for LFLX, 92.17–105.55% and 96.50–102.30% for NRFX, 87.46–99.12% and 92.47–105.52% for ENX, respectively. Moreover, intra- and inter-assay CVs showed no significant changes, which suggest that ic-ELISA is suitable for the detection of LFLX, NRFX, and ENX in bovine milk samples ().
Table 2. Recovery results of LFLX, NRF, and ENX from spiked bovine milk samples.
Lateral-flow immunochromatographic assay
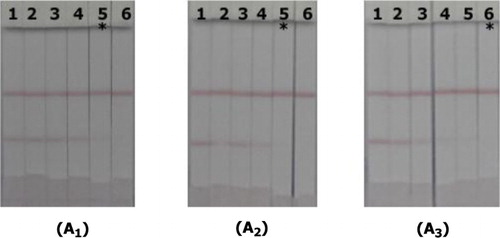
The immunochromatographic strip detection of LFLX, NRFLX, and ENX was performed by testing different concentrations of each compound in 0.01 M PBS and in milk. Theoretically, a positive test would result in a red C line, whereas a negative test would result in red C and T lines (Kim & Kim, Citation2009; Mukunzi et al., Citation2016). The color development on the T line was inversely proportional with the drug concentration ((A1–3)). The visual cutoff values for LFLX, NRFLX, and ENX were 1.0, 1.0, and 2.5 ng/mL, respectively, in PBS and 5.0, 2.5, and 10.0 ng/mL, respectively, in milk (). Due to matrix effects, the performance of the strip in bovine milk samples showed a slight shift in the visual cutoff values compared to the performance of the strip in PBS. However, the outcome was considerable compared to the device required to detect FQs below the established MRLs. On December 31st, 2016, the Ministry of Agriculture of China banned the use of LFLX, NRFLX, pefloacin, and ofloxacin (China Ministry of Agriculture, Citation2015). Therefore, this developed strip method would be useful in the detection of FQs in food products.
Conclusion
We produced a mAb against LFLX with a highly sensitive immunogen (LFLX-EDEA-BSA) and used it in the development of ic-ELISA and a lateral-flow immunochromatographic assay. Under optimized conditions, ic-ELISA had IC50 values of 0.19 ng/mL, 0.17 ng/mL, and 0.23 ng/mL for LFLX, NRFLX, and ENX, respectively, with moderate CR with ciprofloxacin, fleroxacin, ofloxacin, and pefloxacin and no CR with other tested FQs. The intra- and inter-assay recoveries were 98.02–107.40% and 100.65–107.82% for LFLX, 92.17–105.55% and 96.50–102.30% for NRFX, 87.46–99.12% and 92.47–105.52% for ENX, respectively. The immunochromatographic strip results had visual cutoff values of 2.5 ng/mL, 0.5 ng/mL, and 10.0 ng/mL for LFLX, NRFLX, and ENX, respectively, in bovine milk samples. Based on recent safety regulations on the use of antibiotics, the developed ic-ELISA and strip method may assist in the detection of FQs in foods.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Daniel Mukunzi was born in Rwanda and began to study as a Ph.D. student in the School of Food Science and Technology of Jiangnan University in 2012. His research interests are immunoassay’s development for agonists.
Joel Isanga was born in Uganda and began to study as a Ph.D. student in the School of Food Science and Technology of Jiangnan University in 2013. His research interests are monoclonal antibody development and applications.
Steven Suryoprabowo was born in Indonesia and obtained his bachelor’s degree from Pelita Harapan University (Indonesia); then he obtained his master’s degree in Food Science (2014) from Jiangnan University, Wuxi, China. His research interests are monoclonal antibody development and immunochromatographic strip test and application.
Liqiang Liu earned his Ph.D. in Food Science in 2014 from Jiangnan University, Wuxi, China, and then became a faculty in College of Food Science and Technology of Jiangnan University. His research interests include immunochromatographic strip design and application.
Hua Kuang obtained her Ph.D. from China Agricultural University in 2009 and then began to work as a faculty in College of Food Science and Technology of Jiangnan University. She is currently a full professor in food safety. Her research interests are biosensor development.
Additional information
Funding
References
- Adachi, T., Satou, Y., Satou, H., Shibata, H., Miwa, S., Iwase, Y., … Masutomi, N. (2015). Assessment of 8-methosypsoralen, lomefloxacin, sparfloxacin, and pirfenidone phototoxicity in long-evans rats. International Journal of Toxicology, 34(1), 16–23. doi:10.1177/1091581814559397 doi: 10.1177/1091581814559397
- Asif, M. (2013). Antimicrobial and anti-tubercular activity of quinolone analogues. Science International, 1(10), 336–349. doi:10.17311/sciintl.2013.336.349 doi: 10.17311/sciintl.2013.336.349
- Cao, Z., Lu, S., Liu, J., Zhan, J., Meng, M., & Xi, R. (2011). Preparation of anti-lomefloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for detection of lomefloxacin residue in milk. Analytical Letters, 44(6), 1100–1113. doi:10.1080/00032719.2010.511733 doi: 10.1080/00032719.2010.511733
- Chang, C.-S., Wang, W.-H., & Tsai, C.-E. (2010). Simultaneous determination of 18 quinolone residues in marine and livestock products by liquid chromatography/tandem mass spectrometry. Journal of Food and Drug Analysis, 18(2), 87–97.
- China Ministry of Agriculture. (2015). Gazette of the Ministry of Agriculture of the People’s Republic of China, no. 2292. Retrieved from http://www.moa.gov.cn
- El-Shanawany, A. A., El-Adl, S. M., Abdel-Aziz, L. M., Hashem, H. A., & Sebaiy, M. M. (2011). Rapid RP-HPLC method for simultaneous estimation of levofloxacin hydrochloride, lomefloxacin hydrochloride, gatifloxacin and sparfloxacin. Asian Journal of Research in Chemistry, 4(11), 1688–1694.
- Feng, M., Yong, Q., Wang, W., Kuang, H., Wang, L., & Xu, C. (2013). Development of a monoclonal antibody-based ELISA to detect Escherichia coli O157:H7. Food and Agricultural Immunology, 24(4), 481–487. doi:10.1080/09540105.2012.716026 doi: 10.1080/09540105.2012.716026
- Gery, v. V., Janosi, A., Bordin, G., Toussaint, B., Maghuin-Rogister, b. a. G., & Edwin De Pauw, A. R. R. (2002). Multiresidue determination of (fluoro)quinolone antibiotics in swine kidney using liquid chromatography–tandem mass spectrometry. Journal of Chromatography A, 952, 121–129. doi: 10.1016/S0021-9673(02)00092-4
- Helm, F., & Fricker, G. (2015). Liposomal conjugates for drug delivery to the central nervous system. Pharmaceutics, 7(2), 27–42. doi:10.3390/pharmaceutics7020027 doi: 10.3390/pharmaceutics7020027
- Hu, R., Liu, T., Zhang, X., Yang, Y., Chen, T., Wu, C., … Tan, W. (2015). DLISA: A DNAzyme-based ELISA for protein enzyme-free immunoassay of multiple analytes. Analytical Chemistry, 87(15), 7746–7753. doi:10.1021/acs.analchem.5b01323 doi: 10.1021/acs.analchem.5b01323
- Hubicka, U., Zmudzki, P., Zuromska-Witek, B., Zajdel, P., Pawlowski, M., & Krzek, J. (2013). Separation and characterization of ciprofloxacin, difloxacin, lomefloxacin, norfloxacin, and ofloxacin oxidation products under potassium permanganate treatment in acidic medium by UPLC-MS/MS. Talanta, 109, 91–100. doi:10.1016/j.talanta.2013.01.055 doi: 10.1016/j.talanta.2013.01.055
- Isanga, J., Tochi, B. N., Mukunzi, D., Chen, Y., Liu, L., Kuang, H., & Xu, C. (2016). Development of a specific monoclonal antibody assay and a rapid testing strip for the detection of apramycin residues in food samples. Food and Agricultural Immunology, 1–18. doi:10.1080/09540105.2016.1202211
- Jain, R. (2012). Eco friendly spectrophotometric method for quantitative estimation of lomefloxacin using hydrotropic approach. Journal of Applied Pharmaceutical Science, 111–114. doi:10.7324/japs.2012.2425 doi: 10.7324/JAPS.2012.2425
- Kim, Y.-K., & Kim, H. (2009). Immuno-strip biosensor system to detect enrofloxacin residues. Journal of Industrial and Engineering Chemistry, 15(2), 229–232. doi:10.1016/j.jiec.2008.10.007 doi: 10.1016/j.jiec.2008.10.007
- King, D. E., Malone, R., & Lilley, S. H. (2000). New classification and update on the quinolone antibiotics. American Family Physician, 61(9), 2741–2748.
- Kong, N., Song, S., Peng, J., Liu, L., Kuang, H., & Xu, C. (2015). Sensitive, fast, and specific immunoassays for methyl testosterone detection. Sensors (Basel), 15(5), 10059–10073. doi:10.3390/s150510059 doi: 10.3390/s150510059
- Kuang, H., Xing, C., Hao, C., Liu, L., Wang, L., & Xu, C. (2013). Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors (Basel), 13(4), 4214–4224. doi:10.3390/s130404214 doi: 10.3390/s130404214
- Liu, L., Luo, L., Suryoprabowo, S., Peng, J., Kuang, H., & Xu, C. (2014). Development of an immunochromatographic strip test for rapid detection of ciprofloxacin in milk samples. Sensors (Basel), 14(9), 16785–16798. doi:10.3390/s140916785 doi: 10.3390/s140917621
- Liu, L., Xing, C., Yan, H., Kuang, H., & Xu, C. (2014). Development of an ELISA and immunochromatographic strip for highly sensitive detection of microcystin-LR. Sensors (Basel), 14(8), 14672–14685. doi:10.3390/s140814672 doi: 10.3390/s140814672
- Lu, Y., Chen, B., Yu, M., Han, J., Wang, Y., Tan, Z., & Yan, Y. (2016). Simultaneous separation/enrichment and detection of trace ciprofloxacin and lomefloxacin in food samples using thermosensitive smart polymers aqueous two-phase flotation system combined with HPLC. Food Chemistry, 210, 1–8. doi:10.1016/j.foodchem.2016.04.074 doi: 10.1016/j.foodchem.2016.04.074
- Mukunzi, D., Tochi, B. N., Isanga, J., Liu, L., Kuang, H., & Xu, C. (2016). Development of an immunochromatographic assay for hexestrol and diethylstilbestrol residues in milk. Food and Agricultural Immunology, 27(6), 855–869. doi:10.1080/09540105.2016.1183601 doi: 10.1080/09540105.2016.1183601
- Park, H.-J., Kim, G.-D., Han, K.-H., & Lee, C.-H. (2013). The application of Parallux TM System for multi-detection of (fluoro)quinolone class antibiotics residues in Raw bovine milk. Korean Journal for Food Science of Animal Resources, 33(2), 198–204. doi:10.5851/kosfa.2013.33.2.198 doi: 10.5851/kosfa.2013.33.2.198
- Peng, J., Liu, L., Kuang, H., Cui, G., & Xu, C. (2017). Development of an icELISA and immunochromatographic strip for detection of norfloxacin and its analogs in milk. Food and Agricultural Immunology, 28(2), 288–298. doi:10.1080/09540105.2016.1263987 doi: 10.1080/09540105.2016.1263987
- Peng, J., Meng, X., Deng, X. F., Zhu, J. P., Kuang, H., & Xu, C. L. (2014). Development of a monoclonal antibody based sandwich ELISA for the detection of ovalbumin in foods. Food and Agricultural Immunology, 25, 1–8.
- Rubinstein, E. (2001). History of quinolones and their side effects. Chemotherapy, 47(3), 3–8. doi: 10.1159/000057838
- Suryoprabowo, S., Liu, L., Peng, J., Kuang, H., & Xu, C. (2014). Development of a broad specific monoclonal antibody for fluoroquinolone analysis. Food Analytical Methods, 7(10), 2163–2168. doi:10.1007/s12161-014-9863-1 doi: 10.1007/s12161-014-9863-1
- Tochi, B. N., Peng, J., Song, S., Liu, L., Kuang, H., & Xu, C. (2016). Determination of sarafloxacin and its analogues in milk using an enzyme-linked immunosorbent assay based on a monoclonal antibody. Analytical Methods, 8(7), 1626–1636. doi:10.1039/c5ay02702k doi: 10.1039/C5AY02702K
- Wang, Z., Zhang, H., Ni, H., Zhang, S., & Shen, J. (2014). Development of a highly sensitive and specific immunoassay for enrofloxacin based on heterologous coating haptens. Analytica Chimica Acta, 820, 152–158. doi:10.1016/j.aca.2014.02.043 doi: 10.1016/j.aca.2014.02.043
- Watabe, S., Morikawa, M., Kaneda, M., Nakaishi, K., Nakatsuma, A., Ninomiya, M., … Ito, E. (2016). Ultrasensitive detection of proteins and sugars at single-cell level. Communicative & Integrative Biology, 9(1), e1124201. doi:10.1080/19420889.2015.1124201 doi: 10.1080/19420889.2015.1124201
- Wu, X. L., Xiang, L., Yan, Q. Y., Jiang, Y. N., Li, Y. W., Huang, X. P., … Mo, C. H. (2014). Distribution and risk assessment of quinolone antibiotics in the soils from organic vegetable farms of a subtropical city, southern China. Science of the Total Environment, 487, 399–406. doi:10.1016/j.scitotenv.2014.04.015 doi: 10.1016/j.scitotenv.2014.04.015
- Xu, L., Peng, S., Liu, L., Song, S., Kuang, H., & Xu, C. (2016). Development of sensitive and fast immunoassays for amantadine detection. Food and Agricultural Immunology, 27(5), 678–688. doi:10.1080/09540105.2016.1148667 doi: 10.1080/09540105.2016.1148667
- Xu, Y., Liu, S., Guo, F., & Cui, F. (2015). Oxidation of enrofloxacin with permanganate: Kinetics, multivariate effects, identification of oxidation products, and determination of residual antibacterial activity. Journal of Chemistry, 2015, 1–8. doi:10.1155/2015/521395