ABSTRACT
3-monochloro-1,2-propanediol (3-MCPD) is a member of a group of chemicals known as chloropropanols that has been detected in a wide range of foods. In the present study, we found that its α-chlorohydrin structure (3-MCPD(+)) has toxic effects on activated T cells in mice. Specifically, the data showed that 1, 2, and 4 mM 3-MCPD(+) markedly inhibited ConA-induced T cell proliferation and Th1/Th2 cytokine production. Furthermore, Western blotting was used to study the mechanism of action of 3-MCPD(+). The data revealed that 3-MCPD(+) inhibited the activation of the Ca2+/CaM/I-κB-NF-κB and Ca2+/CaM/CaN/NFAT signaling pathways. In vivo, 3-MCPD(+) treatment significantly inhibited 2,4-dinitrofluorobenzene-induced delayed-type hypersensitivity reactions in mice. These observations indicated that 3-MCPD(+) is toxic to activated mouse T cells in vitro and in vivo.
1. Introduction
3-MCPD is a member of a group of contaminants known as chloropropanols (Hamlet, Sadd, Crews, Velisek, & Baxter, Citation2006). It is produced during food processing and is thought to be a representative chloropropanol due to its significant contribution to pollution and its strong toxicity. More recently, 3-MCPD has attracted renewed attention because large amounts of 3-MCPD fatty acid esters have been found in dietary products, especially in refined vegetable oils (Zelinkova, Svejkovska, Velisek, & Doležal, Citation2006). 3-MCPD fatty acid esters can release free 3-MCPD via hydrolysis in the human body. Thus, 3-MCPD may be significantly more dangerous than previously estimated. Previous studies showed that 3-MCPD exerts a variety of toxic effects, such as genotoxicity (Silhankova, Smid, Cerna, Davidek, & Velisek, Citation1982; Zeiger, Anderson, Haworth, Lawlor, & Mortelmans, Citation1988), immunotoxicity (Lee et al., Citation2004), carcinogenicity (Lynch, Bryant, Hook, Nestmann, & Munro, Citation1998) and infertility (Kwack et al., Citation2004). However, the effect of 3-MCPD on mouse T cells has not yet been studied.
T cells are known to play an important role in the immune system by extensively interacting with chemical poisons in the circulation. Thus, T cell suppression may be an ideal marker to detect toxicity before organ damage. T cell activation, which triggers the T cell receptor–CD3 complex in collaboration with costimulatory and adhesion receptors, is a complex process. Many studies have demonstrated that Ca2+ influx is crucial for T cell activation upon antigen stimulation (Pearce, Citation2010), and changes in the intracellular calcium concentration ([Ca2+]i) control diverse cellular processes, including the translocation of transcription factors from the cytosol to the nucleus, such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)and nuclear factor of activated T cells (NFAT) (Aifantis, Gounari, Scorrano, Borowski, & von Boehmer, Citation2001; Pfeifhofer et al., Citation2003). NF-κB and NFAT are the most relevant transcription factors that regulate the expression of activation-associated and pro-inflammatory genes. Specifically, they are activated and translocated to the nucleus by an increase in the cytosolic Ca2+ level, which induces the expression of gene products that mediate innate and adaptive immunity, thereby contributing to the control of cell proliferation, cytokine secretion, and apoptosis (Ma et al., Citation2007).
2. Methods
2.1. Reagents
3-MCPD(+) (As No. 57090-45-6, purity 98%) was provided by Bio Tech Inc., Shanghai. Dimethyl sulfoxide (DMSO), concanavalin (ConA), cyclophosphamide (CTX), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and Griefs reagent were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 2,4-Trinitrotoluene (DNFB) was purchased from BD Bio-Sciences (CA, USA). interleukin-2 (IL-2), interferon gamma (IFN-γ), interleukin-4 (IL-4), and interleukin-6 (IL-6) ELISA kits were purchased from Bio legend. RPMI 1640 medium and fetal bovine serum (FBS) were obtained from Estrogen-Gibbon (Grand Island, NY). Phosphate-specific antibodies for IκB as well as antibodies against p65 nuclear factor κB (NF-κB), IκB, β-actin, and nuclear factor of activated T cells 2 (NFAT2) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Fluo-3/AM was purchased from Bedtime Institute (Changchun, Jilin, China). Primary antibodies used for Western blotting against calmodulin (CaM) and calcineurin (CaN) were purchased from cell signaling (Beverly, MA). Horseradish-peroxidase-conjugated goat anti-mouse IgG (H+L) and Horseradish-peroxidase-conjugated goat anti-rabbit IgG (H+L) were purchased from PTG (Chicago, IL, USA).
2.2. Animals
BALB/c female mice weighing 18–22 g were purchased from Jilin University Experimental Animal Center and acclimatized for 1 week before use. Rodent laboratory chow and tap water were provided ad libitum, and the animals maintained under controlled conditions at a temperature of 24 ± 1°C, humidity of 40–80%, and a 12-h light/12-h dark cycle. All procedures were in strict accordance with the guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and the Policy of Animal Care and Use Committee of Jilin University.
2.3. Cell preparation
BALB/c female mice were sacrificed, and their spleens were aseptically removed and homogenized by passing them through a sterile plastic strainer under aseptic conditions. After centrifuging the cells twice at 100g for 5 min, erythrocytes were lysed in Eris–NH4Cl buffer, and the cell pellets were washed twice with RPMI-1640 medium. The cells were then re-suspended in complete RPMI-1640 medium. The cells were counted using a hemocytometer, and cell viability was assessed using the Trypan-blue dye exclusion technique. In all cases, viability exceeded 95%. The cultures were maintained in a humidified atmosphere of 5% CO2 at 37°C.
2.4. T cells purifications
T cells were purified from whole splenocyte preparations using the Pan T Cell Isolation Kit according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, splenocytes were collected, and a single-cell suspension was generated in MACS buffer (PBS, 0.5% BSA and 2 mM EDTA). The cells were then incubated with an antibody cocktail and magnetic beads that allow for the negative selection of T cells in the presence of a magnetic column. The purity of mouse CD3 T cells consistently exceeded 95%.
2.5. T cell cytotoxicity assay
Purified T cells were plated at a density of 2 × 106 cells/mL in 96-well plates (Costar USA) containing 100 μL of RPMI 1640 complete medium (tetraplicate wells). The cells were treated with multiple concentrations of 3-MCPD(+) (0–32 mM). After 44 h, 20 μL of MTT (5 mg/mL) was added to each well, and the cells were further incubated for 4 h at 37°C in 5% CO2. Cell-free supernatants were then removed and replaced with 150 μL/well DMSO. The optical density was measured at 570 nm on a microplate reader.
2.6. T cell proliferation assay
Purified T cells (2 × 106 cells/mL) collected as described above were treated with 3-MCPD(+) (final concentration: 1, 2, or 4 mM) plus ConA (final concentration: 5 μg/mL) or ConA alone for 48 h. After 44 h, 20 μL of MTT (5 mg/mL) was added to each well, and the cells were further incubated for 4 h at 37°C with 5% CO2. The plate was centrifuged at 1800 rpm for 10 min, and supernatants were then removed and replaced with 150 μL/well DMSO. The optical density was measured at 570 nm on a microplate reader.
2.7. Determination of cytokines
Purified T cells (2 × 106 cells/mL) were incubated with 3-MCPD(+) (final concentration: 1, 2 or 4 mM) plus ConA (final concentration: 5 μg/mL) or ConA alone for 48 h. The concentrations of the cytokines IFN-r, IL-2, IL-4, and IL-6 in the supernatants were measured by ELISA according to the manufacturer’s instructions (Biolegend, Inc, Camino Santa Fe, Suite E San Diego, CA, USA).
2.8. RT-PCR analysis
Purified T cells were prepared as described above. Total RNA was extracted from the ConA-activated T cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and then pooled for the RT-PCR analysis. The total RNA was reverse-transcribed using the BioRT cDNA first strand synthesis kit (Bioer Technology) according to the manufacturer’s recommendations. The following primers were used in this study: β-actin (forward primer 5′-ATCATGTTTGAGACCTTCAACA-3′ and reverse primer 5′-CATCTCTTGCTCGAAGTCCA-3′), IFN-r (forward primer 5′-TCTGAGACAATGAACGCTAC-3′ and reverse primer 5′-TTCCACATCTATGCCACT-3′), IL-2 (forward primer 5′-CTACAGCGGAAGCACAGC-3′ and reverse primer 5′-TCCTCAGAAAGTCCACCA-3′), IL-4 (forward primer 5′-TCGGCATTTTGAACGAGGTC-3′ and reverse primer 5′-GAAAAGCCCGAAAGAGTCTC-3′), and IL-6 (forward primer 5′-TCCAGTTGCCTTCTTGGGAC-3′ and reverse primer 5′-GTGTAATTAAGCCTCCGACTTG-3′). The cDNA served as the template in a 20-µL reaction mixture and was processed using an initial step at 94°C for 5 min followed by 30–35 amplification cycles (94°C for 30 s; 55–60°C for 30 s; and 72°C for 40 s) and a final extension step at 72°C for 10 min. The final PCR products were separated on 2% agarose gels, and the transcription amounts were normalized to the levels of β-actin transcript.
2.9. Fluorescence measurement
Photochemical measurements were obtained under an inverted fluorescence microscope (Motic AE31, Motic Medical Diagnostic Systems Co., Ltd, Xiamen, China) to measure changes in the intracellular calcium levels. Purified mouse T cells were treated with 3-MCPD(+) (final concentration: 1, 2, or 4 mM) plus ConA (final concentration: 5 μg/mL) and ConA for 30 min. According to the number of cells in each sample, Fluo-3/AM was added to the suspension to estimate [Ca2+]i in the cytoplasm. The cells were incubated with Fluo-3/AM for 30 min prior to measurement, which was conducted at 480–500 nm.
2.10. Western blot analysis
Purified T cells (2 × 106 cells/mL) were pre-incubated with different concentrations of 3-MCPD(+) (1, 2, or 4 mM) in 6-well plates at 37°C for 60 min, followed by a 30-min incubation with ConA (final concentration 5 μg/mL). The cells were lysed using Cytoplasmic Extraction Reagent (Beyotime, Jiangsu, China) according to the manufacturer’s instructions to obtain cytoplasmic protein, and corresponding Nuclear Protein Extraction Reagent (Beyotime, Jiangsu, China) was used to obtain nuclear protein. The protein concentration was determined using a Bradford assay (Bio-Rad, Munich, Germany) prior to storage at −80°C. For NFAT2, 60–80 µg of protein extract was separated using 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane. Western blotting was performed with the appropriate antibodies, and the proteins were visualized using enhanced chemiluminescence (ECL).
2.11. DNFB-induced delayed-type hypersensitivity (DTH) response
Six-week-old female BALB/c mice were divided into 6 groups, each consisting of 10 mice. On days 1 and 2, the BALB/c mice were initially sensitized with 20 mL of 5% DNFB dissolved in acetone–olive oil (4:1), which was applied to the shaved abdominal skin. Beginning on the day of immunization, the immunized mice were gavage-fed water, 3-MCPD(+) in water at dose of 10, 20, 40 mg/kg/day, or CTX (20 mg/kg in 2% DMSO) daily for 6 consecutive days. The control groups received the same volume of saline. The DTH reaction was evaluated based on the increase in the ear patch weight (8-mm punches) between the left and right ear 36 h after the second challenge.
2.12. Statistical analysis
All data are presented as the mean + standard deviation (SD). Differences between groups were analyzed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA), and significance was examined using a one-way analysis of variance (ANOVA). A P value of <.05 was considered to be significant.
3. Results
3.1. Effect of 3-MCPD(+) on ConA-induced T cell proliferation
The T cell cytotoxicity assay showed that 3-MCPD(+) did not significantly affect the viability of T cells at concentrations ranging from 0 to 4 mM (data not shown). Thus, we selected 1, 2, and 4 mM as experimental doses.
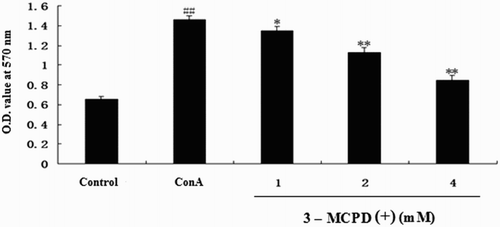
Proliferation was measured with an MTT assay. As shown in , 3-MCPD(+)dose-dependently suppressed ConA-induced T lymphocyte proliferation at the concentrations of 1–4 mM. These results indicated that 3-MCPD suppressed ConA-induced T cell proliferation.
Figure 1. Effect of 3-MCPD (+) on ConA-induced T cell proliferation. T cells collected from mice were treated with 3-MCPD(+) (final concentration: 1, 2, or 4 mM) plus ConA (final concentration: 5 μg/mL) or ConA (5 μg/mL) for 48 h. T cell proliferation was assessed with an MTT assay. The results were obtained from three independent experiments and are presented as the mean ± standard deviation. Significant differences from the control group are indicated by ##P < .01 versus control group; *P < .05 or **P < .01 versus ConA group.
3.2. Effect of 3-MCPD(+) exposure on Th1 and Th2 cytokine secretion and mRNA expression in vitro
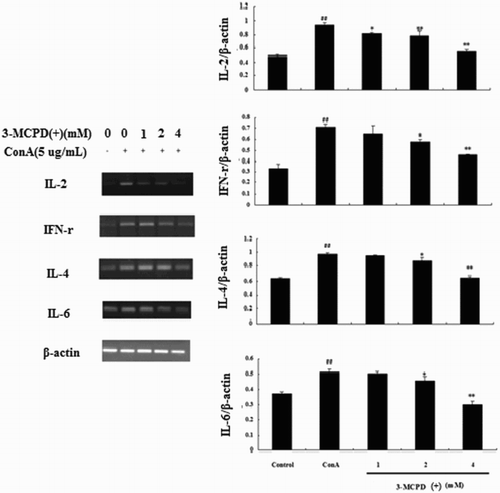
In this study, we used ConA as a T cell mitogen and selected IL-2, IFN-r as Th1 cytokines and IL-4, IL-6 as Th2 cytokines to test the effect of 3-MCPD(+) on cytokine production in vitro. As shown in , treating mouse splenocytes with ConA alone significantly increased cytokine production compared to control cells. Moreover, treatment with 1, 2, or 4 mM of 3-MCPD(+) significantly and dose-dependently decreased the levels of the four cytokines compared the ConA-treated group. Total RNA was also extracted from T cells treated with 3-MCPD(+) using TRIzol to measure the IL-2, IL-4, IL-6, and IFN-r mRNA levels by PCR, and the results of this assay corroborated the results observed at the protein level ().
Figure 2. Effect of 3-MCPD (+) exposure on ConA-induced cytokine production in the cell culture supernatant. The levels of the cytokines IL-2, IFN-γ, IL-4, and IL-6 were analyzed by ELISA. The values are the mean ± standard deviation of three independent experiments. ##P < .01 vs. Control group, *P < .05 or **P < .01 vs. Con A group.
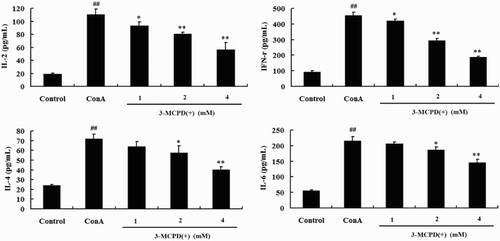
Figure 3. Effect of 3-MCPD (+) exposure on Th1 and Th2 cytokine mRNA expression. IL-2, IFN-γ, IL-4, and IL-6 mRNA expression levels were measured by RT-PCR. The lower panel shows data from three independent experiments. A representative Western blot is shown in here. ##P < .01 versus control group; *P < .05 or **P < .01 versus ConA group.
3.3. Effects of 3-MCPD(+) on [Ca2+]i in T cells
We used Fluo-3 AM to examine the effects of 3-MCPD(+) on intracellular Ca2+ mobilizations in mouse T cells. As shown in , the level of intracellular Ca2+ significantly increased in the ConA group, whereas 1, 2, or 4 mM 3-MCPD(+) significantly decreased intracellular free Ca2+ fluorescence.
Figure 4. Effect of 3-MCPD (+) on the [Ca2+]i in ConA-induced T cells. T cells were pretreated with 20 μM Fluo-3/AM and examined under a confocal laser microscope. The fluorescence intensity in the ConA group was increased compared with the control group, and 3-MCPD (+) decreased this fluorescence intensity. The results were obtained from three independent experiments and are presented as the mean ± standard deviation of three independent experiments. ##P < .01 versus control group; *P < .05 or **P < .01 versus ConA group.
![Figure 4. Effect of 3-MCPD (+) on the [Ca2+]i in ConA-induced T cells. T cells were pretreated with 20 μM Fluo-3/AM and examined under a confocal laser microscope. The fluorescence intensity in the ConA group was increased compared with the control group, and 3-MCPD (+) decreased this fluorescence intensity. The results were obtained from three independent experiments and are presented as the mean ± standard deviation of three independent experiments. ##P < .01 versus control group; *P < .05 or **P < .01 versus ConA group.](/cms/asset/d900ee0d-11b4-4c52-b7d4-60adacc972ef/cfai_a_1309357_f0004_c.jpg)
3.4. Effects of 3-MCPD(+) on the NFAT pathway
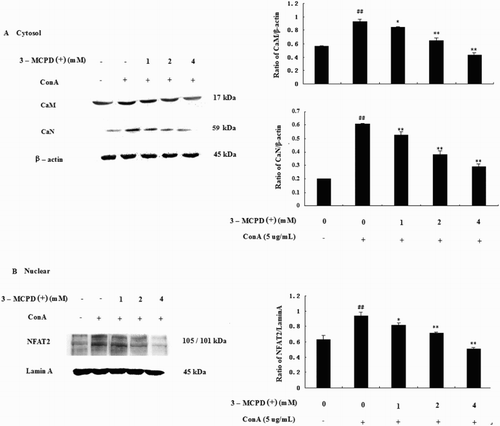
We examined the effect of 3-MCPD (+) on Con A-induced NFAT2 activity by Western blotting. As shown in , the expression of CaM, CaN, and NFAT2 increased when T cells were stimulated with ConA compared with the control group, and 3-MCPD (1, 2, or 4 mM) dose-dependently suppressed the expression of CaM, CaN, and NFAT2.
Figure 5. Effect of 3-MCPD (+) on the NFAT pathway. Protein was extracted from mouse T cells according to the manufacturer's instructions. (A) CaM, CaN; (B) nucleic NFAT2 protein expression was measured by Western blotting. The lower panels show the means ± SD. These results were obtained from three independent experiments. A representative Western blot is shown in the upper panel. Significant differences from the control group are designated by ##P < .01 versus control group; *P < .05 or **P < .01 versus ConA group.
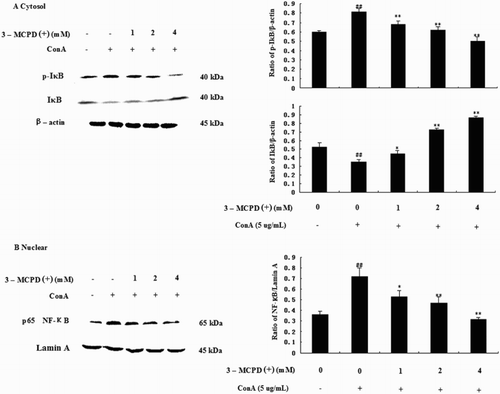
3.5. Effects of 3-MCPD(+) on the NF-κB pathway
We also examined the effect of 3-MCPD(+) on the ConA-induced NF-κB, p-IκB, and IκB activities by Western blotting. The results showed that NF-κB and IκB phosphorylation increased in purified mouse T cells after ConA administration but was significantly inhibited by 3-MCPD treatment. Moreover, IκB decreased after ConA treatment but was significantly increased by 3-MCPD in a dose-independent manner ().
Figure 6. Effect of 3-MCPD (+) on the NF-κB pathway. Protein was extracted from mouse T cells according to the manufacturer's instructions. (A) The IκB, P-IκB, and (B) nuclear NF-κB protein expression levels were measured by Western blotting. The lower panel shows the means ± SD. These results were obtained from three independent experiments. A representative Western blot is shown in the upper panel. Significant differences from control group were designated by ##P < .01 versus control group; *P < .05 or **P < .01 versus ConA group.
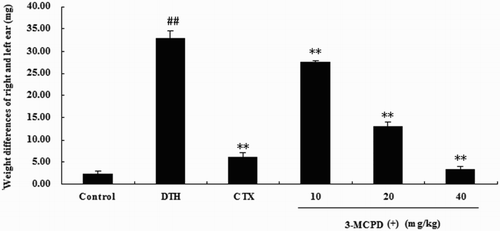
3.6. Effect of 3-MCPD(+) on the DNFB-induced DTH response in mice
To test the immunotoxicity of 3-MCPD(+) to the T cell-mediated immune response in vivo, we analyzed the effects of 3-MCPD(+) on the DNFB-induced DTH response in BALB/c mice. As shown in , ear swelling was significantly increased in the DTH group compared with the control group. This result indicated that the DNFB-induced DTH model was successful. 3-MCPD(+) at doses of 10, 20, or 40 mg/kg also significantly reduced ear swelling in DNFB-induced mice compared with the DTH group. Moreover, high-dose 3-MCPD(+) (40 mg/kg) was more immunotoxic than the control drug, CTX.
Figure 7. Effect of 3-MCPD on the DNFB-induced DTH reaction in BALB/c mice. (A) The ear swelling (Δmears) was assessed by calculating the difference between the weights of untreated and DNFB-treated ear punches 36 h after challenge. The values are presented as the means ± SD (n = 10). Significant differences from the control group are designated by ##P < .01 versus control group; *P < .05 or **P < .01 versus DTH group.
4. Discussion
Activated T cells are well known to play an important role in immunity, and the T cell-mediated immune response is clearly suppressed in many diseases, including cancer (Sandoval, Hocher, & Verdeil, Citation2003). In the present study, we focused on the toxic effects of 3-MCPD on activated T cells both in vitro and in vivo to elucidate the mechanisms underlying 3-MCPD immunotoxicity.
First, we tested lymphocyte proliferation with an MTT assay, which is the most widely used method to assess immunological function, and ConA, a plant mitogen that only stimulates T cell proliferation (Ohsawa, Citation2009). Our results show that 3-MCPD(+) dose-dependently suppressed ConA-induced splenocyte proliferation at concentrations of 1–4 mM (). These results indicated that 3-MCPD(+) suppressed ConA-induced T cell proliferation.
Cytokines play a critical role as messenger molecules in the immune system, and they are also the potential targets for immunomodulation. Th1 cells produce IL-2, IFN-r, and IL-12 and drive cell-mediated immune responses, whereas Th2 cells produce IL-4, IL-6 and IL-10 and promote the humoral immune response. The two types of cytokines collaborate to modulate immune function. In this study, we found that the levels of four cytokines in the cell supernatant significantly and dose-dependently decreased upon treatment with 1, 2, or 4 mM 3-MCPD (+) compared to the ConA group. Moreover, we extracted total RNA from T cells treated with 3-MCPD (+) using TRIzol and measured the IL-2, IL-4, IL-6, and IFN-r mRNA levels by PCR. The resultant data corroborated the results obtained at the protein level.
Intracellular Ca2+ is a quintessential intracellular messenger, and many cell functions, such as maturation, differentiation, and signal transduction, are regulated by changes in [Ca2+]i (Honda et al., Citation2001; Poenie, Alderton, Tsien, & Steinhardt, Citation1985). We used Fluo-3/AM to examine the effects of 3-MCPD (+) on intracellular Ca2+ mobilization in mouse T cells, which indicated that 3-MCPD (+) strongly inhibited the intracellular free Ca2+ levels in T cells.
Moreover, the NFAT family plays a crucial role in T cell activation, and many studies have revealed that NFAT activation depends on the Ca2+/calmodulin/calcineurin pathway. Calmodulin (CaM) is a key regulator of numerous cellular processes and the predominant intracellular receptor for Ca2+ signals (Wang, Wang, Yao, Guo, & Liu, Citation2012). Specifically, the Calcium/CaM complex regulates several downstream targets including protein kinases and phosphatases. Calcineurin (CaN) is a serine/threonine phosphatase enzyme that is expressed in many types of cells, such as immune cells, muscle cells, and neurons, and regulates NFAT phosphorylation. Once activated by stimuli, NFAT translocates from the cytosol to the nucleus to regulate the transcription of genes, including those that encode cytokines (IL-2, IFN-γ, and TNF-a) and regulatory enzymes, such as COX-2 (Strauss, Osen, & Debatin, Citation2002). We examined the effect of 3-MCPD (+) on ConA-induced NFAT2 expression by Western blotting, which showed 3-MCPD (+) dose-dependently suppressed the expression of CaM, CaN and NFAT2.
NF-κB is also Ca2+ dependent transcription factor that is responsible for the activation of the immune response, especially in T cells. The calcium/calmodulin-dependent protein kinase II (CaMKII), which is activated by the calcium-binding protein CaM, is also an important downstream target of the Ca2+ signaling pathway. Thus, NF-κB activation depends on the Ca2+/calmodulin/CamKII pathway (Ishiguro et al., Citation2006). NF-κB mostly participates in the regulation of genes in inflammation, immunity, cell proliferation, cell apoptosis, and other physiological and pathological processes. Normally, NF-κB is sequestered in the cytoplasm by interacting with inhibitory IκB molecules. Once stimulated, NF-κB-IκB is activated by the phosphorylation of conserved serine residues in IκB. IκB is then ubiquitinated and degraded, allowing NF-κB to migrate to the nucleus and activate transcription. Thus, the phosphorylation of IκB plays an important role in the activity of NF-κB (Lee, Jeong, Kim, Kim, & Lee, Citation2009; Rahighi et al., Citation2009). We examined the effect of 3-MCPD (+) on the ConA-induced production of NF-κB, p-IκB, and IκB by Western blotting, which showed that NF-κB and the phosphorylation of IκB increased in purified mouse T cells after ConA administration but were significantly inhibited by 3-MCPD (+) treatment. Moreover, the level of IκB was decreased after ConA treatment but significantly increased by 3-MCPD (+) in a dose-independent manner (). These data indicated that 3-MCPD (+) may inhibit mouse T cells by activating the NF-κB pathway.
To confirm the immunotoxicity of 3-MCPD(+) to T cell in vivo, we analyzed the effects of 3-MCPD(+) on the DNFB-induced DTH response in BALB/c mice. The DTH reaction is a classic T cell-mediated pathologic response associated with T cell activation and the production of Th1-type cytokines (Usuda, Fujii, & Nonogaki, Citation2016). In this assay, CTX, an immunosuppressant, served as a positive control for the DTH model. The results of the in vivo study demonstrated that 3-MCPD(+) significantly suppressed the DNFB-induced DTH immune response.
5. Conclusions
This work characterized the toxicity of 3-MCPD(+) to ConA-induced mouse T cell activation. Specifically, 3-MCPD(+) dose-dependently suppressed ConA-induced splenocyte proliferation and Th1/Th2 cytokine levels in vitro as well as the DNFB-induced DTH response in BALB/c mice. This effect may be mediated by the suppression of Ca2+/CaM/NF-κB and Ca2+/CaM/CaN/NFAT signaling. During the experiment, no obvious signs of toxicity were observed in the 3-MCPD(+)-treated mice based on body weight changes and an examination of individual organs (data not shown), which indicated that low-dose 3-MCPD(+) negatively impacted the immune system of mice prior to detectable organ damage.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Shuang Guan is a Ph.D in Animal medicine. His research interests are food toxicology and immune regulation for contaminants. Presently, he is working in the department of food science and engineering, Jinlin university, Changchun, China.
Xin Yu, student.
Baochen Fang, student.
Yixuan Huang, student.
Linli Xu, student.
Jing Lu is a Ph.D in Animal medicine. His research interests are food toxicology and immune regulation for contaminants. Presently, he is working in the department of food science and engineering, Jinlin university, Changchun, China.
Additional information
Funding
References
- Aifantis, I., Gounari, F., Scorrano, L., Borowski, C., & von Boehmer, H. (2001). Constitutive pre-TCR signaling promotes differentiation through Ca2+ mobilization and activation of NF-kappaB and NFAT. Nature Immunology, 2, 403–409.
- Hamlet, C. G., Sadd, P. A., Crews, C., Velisek, J., & Baxter, D. E. (2006). Occurrence of 3-chloro-propane-1,2-diol (3-MCPD) and related compounds in foods: a review. Food Additives and Contaminants, 19, 619–631. doi: 10.1080/02652030210132391
- Honda, J., Fumimori, T., Yosida, N., Yonemitsu, J., Kitajima, H., & Oizumi, K. (2001). Extracts from the Mackerel (BM-1) Inhibit the production of IL-2 and IFNγ from Lymphocytes via calcium mobilization. Campbell, A. K.(Ed). Food and Agricultural Immunology, 13(4), 265–273. doi: 10.1080/09540100120094528
- Ishiguro, K., Green, T., Rapley, J., Wachtel, H., Giallourakis, C., Landry, A., … Xavier, R. J. (2006). Ca2+/calmodulin-dependent protein kinase II is a modulator of CARMA1-mediated NF-kappaB activation. Molecular and Cellular Biology, 26, 5497–5508. doi: 10.1128/MCB.02469-05
- Kwack, S. J., Kim, S. S., Choi, Y. W., Rhee, G. S., Da Lee, R., Seok, J. H., … Lee, B. M. (2004). Mechanism of antifertility in male rats treated with 3-monochloro-1,2-propanediol (3-MCPD). Journal of Toxicology and Environmental Health, Part A, 67, 2001–2011. doi: 10.1080/15287390490514651
- Lee, J. K., Byun, J. A., Park, S. H., Kim, H. S., Park, J. H., Eom, J. H., & Oh, H. Y. (2004). Evaluation of the potential immunotoxicity of 3-monochloro-1,2-propanediol in Balb/c mice I. 2160;. Effect on antibody forming cell, mitogen-stimulated lymphocyte proliferation, splenic subset, and natural killer cell activity. Toxicology, 204, 1–11. doi: 10.1016/j.tox.2004.04.005
- Lee, S. O., Jeong, Y. J., Kim, M., Kim, G. H., & Lee, I. S. (2009). Suppression of PMA-induced tumor cell invasion by capillarisin via the inhibition of NF-kappaB-dependent MMP-9 expression. Biochemical and Biophysical Research Communications, 366, 1019–1024. doi: 10.1016/j.bbrc.2007.12.068
- Lynch, B. S., Bryant, D. W., Hook, G. J., Nestmann, E. R., & Munro, I. C. (1998). Carcinogenecity of monochloro-1,2-propanediol (alpha-chlorohydrin, 3-MCPD). International Journal of Toxicology, 17, 47–76. doi: 10.1080/109158198226756
- Ma, W., Mishra, S., Gee, K., Mishra, J. P., Nandan, D., Reiner, N. E., … Kumar, A. (2007). Cyclosporin A and FK506 inhibit IL-12p40 production through the calmodulin/calmodulin-dependent protein kinase-activated phosphoinositide 3-kinase in lipopolysaccharide-stimulated human monocytic cells. Journal of Biological Chemistry, 282, 13351–13362. doi: 10.1074/jbc.M611522200
- Ohsawa, M. (2009). Heavy metal-induced immunotoxicity and its mechanisms. Yakugaku Zasshi, 129(3), 305–19. doi: 10.1248/yakushi.129.305
- Pearce, E. L. (2010). Metabolism in T cell activation and differentiation. Current Opinion in Immunology, 22, 314–320. doi: 10.1016/j.coi.2010.01.018
- Pfeifhofer, C., Kofler, K., Gruber, T., Tabrizi, N. G., Lutz, C., Maly, K., … Baier, G. (2003). Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. The Journal of Experimental Medicine, 197, 1525–1535. doi: 10.1084/jem.20020234
- Poenie, M., Alderton, J., Tsien, R. Y., & Steinhardt, R. A. (1985). Changes of free calcium levels with stages of the cell division cycle. Nature, 315, 147–149. doi: 10.1038/315147a0
- Rahighi, S., Ikeda, F., Kawasaki, M., Akutsu, M., Suzuki, N., Kato, R., … Dikic, I. (2009). Specific recognitiong of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell, 136, 1098–1109. doi: 10.1016/j.cell.2009.03.007
- Sandoval, A., Hocher, V., & Verdeil, J. L. (2003). Flow cytometric analysis of the cell cycle in different coconut palm (Cocos nucifera L.) tissues cultured in vitro. Plant Cell Reports, 22(1), 25–31. doi: 10.1007/s00299-003-0651-4
- Silhankova, L., Smid, F., Cerna, M., Davidek, J., & Velisek, J. (1982). Mutagenecity of glycerol chlorohydrins and of their esters with higher fatty acids present in protein hydrolysates. Mutation Research Letters, 103, 77–81. doi: 10.1016/0165-7992(82)90090-2
- Strauss, G., Osen, W., & Debatin, K. M. (2002). Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clinical & Experimental Immunology, 128, 255–266. doi: 10.1046/j.1365-2249.2002.01777.x
- Usuda, H., Fujii, H., & Nonogaki, T. (2016). Sasa veitchii extracts suppress 2,4-dinitrofluorobenzene-induced contact hypersensitivity in mice. Food and Agricultural Immunology, 27(4), 523–534. doi: 10.1080/09540105.2015.1129597
- Wang, G. J., Wang, H. X., Yao, Y. S., Guo, L. Y., & Liu, P. (2012). The role of Ca2+/calmodulin-dependent protein kinase II and calcineurin in TNF-alpha-induced myocardial hypertrophy. Brazilian Journal of Medical and Biological Research, 45, 1045–1051. doi: 10.1590/S0100-879X2012007500121
- Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., & Mortelmans, K. (1988). Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environmental and Molecular Mutagenesis, 11, 1–18. doi: 10.1002/em.2850110602
- Zelinkova, Z., Svejkovska, B., Velisek, J., & Doležal, M. (2006). Fatty acid esters of 3 – chloropropane – 1,2 – diol in edible oils. Food Additives and Contaminants, 23, 1290–1298. doi: 10.1080/02652030600887628
