ABSTRACT
Carbofuran is a highly toxic pesticide used in fruits and vegetables. In this study, we produced a specific and sensitive monoclonal antibody (mAb) which was prepared based on a hapten that was derivatized with benzofuranol against carbofuran. Following mice immunization and cell fusion, we obtained three monoclonal cell lines: 6D5, 3H2, and 3C6. The cell line 3H2 generated mAb with the highest affinity and sensitivity. The half maximal inhibitory concentration was 0.3 ng/mL, and the cross-reactivity was <1%. Based on this mAb, an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) and immunochromatographic test strip (ICS) assay were developed to detect carbofuran residues in cucumbers and apples. The working range of ic-ELISA was 0.1–1 ng/mL, and the cutoff value of ICS was 1 ng/mL. The analytical recovery of carbofuran in cucumber and apple samples ranged from 81% to 97%. Both methods represent rapid screening tools for carbofuran detection in fruits and vegetables.
Introduction
Carbofuran, an active acetylcholinesterase inhibitor, is widely used as an acaricide, insecticide, and nematicide in fruits and vegetables. Among the carbamate pesticides, carbofuran is highly toxic especially to birds, bees, aquatic animals (Gbadegesin, Owumi, Akinseye, & Odunola, Citation2014; Harabawy & Ibrahim, Citation2014; Saxena, Gupta, & Murthy, Citation2014), and mammals (oral median lethal dose is 5–50 mg/kg in rats). Carbofuran is currently classified as a highly hazardous category 1B pesticide by the World Health Organization (Gammon, Liu, & Becker, Citation2012). In addition to its neurotoxic effects, carbofuran causes muscle hypercontraction, infertility (Chauhan, Pant, Gupta, & Srivastava, Citation2000; Tonomura, Mori, Torii, & Uehara, Citation2009), acute pancreatitis, and myocardial injury (Brkic, Vitorovic, Gasic, & Neskovic, Citation2008; Rizos, Liberopoulos, Kosta, Efremidis, & Elisaf, Citation2004; Yen, Hsieh, Tsai, & Chen, Citation2015). Due to its poor degradability in acidic and neutral soils, carbofuran easily pollutes soils and underground water and poses a public health risk (Campbell, David, Woodward, & Li, Citation2004).
The current methods used in the detection of carbofuran residues include gas chromatography, electrochromatography, high-performance liquid chromatography with post-column derivatization, and photo-induced chemiluminescence detection (Chen et al., Citation2011; Chien Hua, Cho Chun, & Tai Chia, Citation2012; Lópezpaz, Cataláicardo, & Langasánchez, Citation2014; Xu & Rohrer, Citation2012). Although the sensitivity of these methods is very high, the limit of detection (LOD) of photo-induced chemiluminescence is as high as 100 ng/L, these methods require expensive equipment and complex sample preparation and pre-concentration steps. Therefore, a rapid and sensitive carbofuran detection method is required.
Immunological assays, which are inexpensive, simple, sensitive, and rapid, have been extensively used to detect the presence of carbofuran residues. Antonio Abad et al. produced a monoclonal antibody (mAb) specific to carbofuran with a half maximal inhibitory concentration (IC50) of 1.8 ng/mL (Abad, Moreno, & Montoya, Citation1997). Xia Sun et al., who developed an amperometric immunosensor for carbofuran, reported a detection limit of 0.33 ng/mL, while Ying Zhu et al. improved its sensitivity to 0.03 ng/mL (Sun, Du, Wang, Zhao, & Li, Citation2011; Zhu, Cao, Sun, & Wang, Citation2013). Jin-Yi Yang et al., who developed a fluorescence polarization immunoassay for carbofuran detection in food and environmental water samples, obtained an IC50value of 48.8 ng/mL (Yang et al., Citation2014). Pei Zhou et al. developed a nanocolloidal gold-based immunoassay for carbofuran with a detection limit of 120 ng/mL, and Yirong Guo et al. reported a detection limit of 32 ng/mL (Guo, Liu, Gui, & Zhu, Citation2009; Zhou et al., Citation2004). In this study, we designed a hapten containing a carbofuran fragment and produced a highly sensitive and specific mAb against carbofuran. The developed mAb was used in immunochromatographic strip (ICS) assay and indirect competitive enzyme-linked immunosorbent (ic-ELISA) for the detection of carbofuran residues in fruits and vegetables.
Materials and methods
Chemicals
Benzofuranol, dichloromethane, triethylamine, bis(p-nitrophenyl) carbonate, ethyl acetate, petroleum ether, methyl 4-aminobutyrate hydrochloride, anhydrous sodium sulfate, sodium chloride, 1,4-dioxane, trifluoroacetic acid, sodium bicarbonate, ovalbumin(OVA), keyhole limpet hemocyanin (KLH; MW: 4,000,000 Da), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide, N,N-dimethyl carboxamide (DMF), QuickAntibody-Mouse5W adjuvant was obtained from Kang Biquan Biological Co., Ltd. (Beijing, China). Enzyme immunoassay-grade horseradish peroxidase-labelled goat anti-mouse immunoglobulin from Jackson Immuno Research, 3,3′,5,5′-tetramethylbenzidine, Tween-20, horseradish peroxidase, and gelatin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Reagents required for cell fusion and cell culture (e.g. polyethylene glycol solution, hypoxanthine-aminopterin-thymidine supplement, hypoxanthine-thymidine supplement, and 1640 cell culture) were obtained from Life Technologies Corporation (Shanghai, China). Material for ICS, for example, glass fiber membrane for the sample pad, nitrocellulose membrane (NC membrane) used for immobilizing the coating antigen and antibody, H5076 filter paper for the absorbent pad, Ahlstrom 8964 for the conjugated pad, and polyethylene adhesive card, were supplied by JieYi Biotech Co. Ltd. (Shanghai, China).
Solutions
We prepared phosphate-buffered saline (PBS, 0.01 M, pH 7.4), borate-buffered saline (BB, 0.01 M, pH 8.6), coating buffer (0.05 M carbonate bicarbonate, pH 9.6), blocking buffer (0.2% gelatin in coating buffer), washing buffer (PBST: 0.05% Tween-20 (v/v) in 0.01 M PBS), and termination solution (2 M sulfuric acid). A color-substrate solution was prepared with solutions A (citric acid, H2O2, and Na2HPO4) and B (0.06% v/v3,3,5,5-tetramethylbenzidinein glycol) in a 5:1 (v/v) ratio.
Hapten synthesis
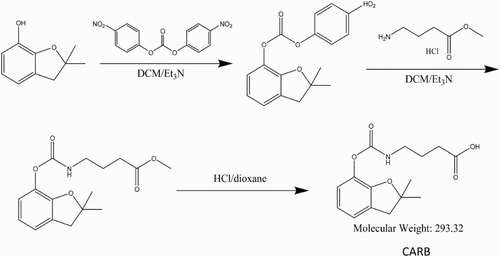
The structure of the hapten synthesized in this study is shown in . Benzofuranol (1.64 g, 10 mmol), previously dissolved in dichloromethane (50 mL), was mixed with triethylamine (1.11 g, 11 mmol) under ice cooling and stirred for 10 min. Subsequently, bis(p-nitrophenyl) carbonate (3.19 g, 10.5 mmol) was added, gradually warmed to room temperature (RT, 30°C), and stirred for 1 h. Thin layer chromatography was used to assess whether the reaction was complete, the ratio of the distance from the center of the spot to the origin and the distance from the leading edge of the solvent to the origin was 0.6 (petroleum ether: ethyl acetate = 10:1). Under ice cooling, methyl 4-aminobutyrate hydrochloride (1.697 g, 10 mmol), which was basified with triethylamine (2.22 g, 22 mmol) in methylene chloride solution (10 mL), was added to the reaction mixture, stirred overnight at RT, rinsed twice with water and saturated brine (each solution was 50 mL), and dried over anhydrous sodium sulfate. The drying agent was removed by filtration. The resulting filtrate was spin-dried under vacuum and purified by column chromatography (silica gel of 200–300 mesh, mobile phase consisted of petroleum ether: ethyl acetate = 15:1 to 10:1 elution) resulting in 1.8 g of product. The intermediate ester (1.8 g, 5.86 mmol) was dissolved in 1,4-dioxane (15 mL) and mixed with trifluoroacetic acid (18 mL) and water (15 mL). The reaction was heated to 60°C for 4 h, cooled to RT, extracted twice with ethyl acetate (30 mL), rinsed twice with water, saturated aqueous sodium bicarbonate, water, and saturated brine (each solution was 30 mL), and dried over anhydrous sodium sulfate. The drying agent was removed by filtration, and the filtrate was vacuum-dried and purified by column chromatography on silica gel (200–300 mesh; mobile phase consisted of petroleum ether: ethyl acetate = 5:1), resulting in 730 mg of hapten. We named this hapten as CARB.
Antigen preparation
To prepare the immunogen CARB–EDC–KLH, CARB was covalently attached to KLH with EDC (Gu, Liu, Song, Kuang, & Xu, Citation2015). Briefly, CARB (4.5 mg), EDC (10 mg), and NHS (6 mg) were dissolved in 300 μL of anhydrous DMF (referred to as solution A) and stirred for 8 h at RT. KLH (3 mL; 5 mg/mL) was mixed with an equal volume of BB, resulting in solution B. Solution A was mixed with solution B and allowed to react overnight at RT. The resulting product was the immunogen CARB–EDC–KLH. A similar method was used to prepare the coating antigen CARB–EDC–OVA.
Carbofuran mAb preparation
CARB–EDC–KLH was mixed with an equal volume of QuickAntibody-Mouse5W adjuvant and injected into the thigh muscle of female BALB/c mice (6–8 weeks of age). The first immunization dose was 100 μg/mouse; booster immunizations dose was 50 μg/mouse. Each immunization was performed every 21 days. Eight days following each immunization, serum titer values and IC50 were determined by ic-ELISA. The mouse with the highest serum titer value and lowest IC50 was selected for the last intraperitoneal injection immunization (25 μg/mouse without adjuvant). Cell fusion was performed three days later.
Mouse spleen cells were fused with Sp 2/0 myeloma cells using polyethylene glycol 1500 for hybridoma cell production. Hybridoma cells were selected by ELISA and sub-cloned three times by the limiting dilution method (Guo et al., Citation2015; Huang et al., Citation2009). Selected hybridoma cells were cultured and injected into the peritoneal cavity of mice for the production of ascites. MAbs were purified by caprylic acid and ammonium sulfate precipitation, dialyzed against PBS for three days (PBS was replaced every 8 h), and stored at ‒20°C.
Development of ic-ELISA for carbofuran detection
In this experiment, ic-ELISA was developed as previously reported (Guo et al., Citation2015). Checkerboard assays were performed to determine the most appropriate coating antigen and antibody concentration for ic-ELISA. CARB-EDC-OVA (coating antigen) was diluted with coating buffer to 0.03, 0.1, 0.3, and 1 µg/mL, and mAb was diluted with antibody diluents to 0.03, 0.1, 0.3, and 1 µg/mL. To improve the stability and sensitivity of the assay, different parameters were evaluated including pH (5.0, 6.0, 7.4, 8.6, and 9.6), NaCl concentration (0%, 0.4%, 0.8%, 1.6%, and 3.2%), and methanol concentration (0%, 5%, 10%, 15%, and 20%, v/v). Standard mAb curves were generated by plotting OD450 values on the y-axis against the concentration of carbofuran (log10) on the x-axis. Antibody sensitivity was determined by IC50.
Cross-reactivity (CR)
Carbosulfan, 3-hydroxycarbofuran, benzofuranol, isoprocarb, m-tolyl methylcarbamate, and methomyl were used to assess CR with carbofuran by ic-ELISA. CR was calculated by the following equation, CR (%) = [IC50 (carbofuran)/IC50 (analogs)] × 100%.
Development of an ICS assay for carbofuran detection
Preparation of gold nanoparticles (GNPs)
GNPs were prepared by reducing gold chloride with sodium citrate as previously reported (Li et al., Citation2016; Wu et al., Citation2013; Xu et al., Citation2016; Yan et al., Citation2012). First, 100 mL of AuCl3·HCl·4H2O solution (0.01%, w/v) was boiled under constant stirring. Subsequently, 1.5 mL of freshly prepared trisodium citrate (1%, w/v) was added under constant stirring. After the color of the solution changed to dark red (1 min), the solution was boiled for 15 min and its volume was adjusted to 100 mL with Millipore-Q water. The solution was allowed to cool to RT and stored at 4°C. GNPs were visualized under ultraviolet–visible spectrophotometer and transmission electron microscope. The maximum absorbance was 520 nm, and the average diameter of the GNPs was 18 ± 2 nm.
Preparation of gold-labelled anti-carbofuran mAb
The GNP solution (20 mL) previously adjusted to pH 8.2 with 0.1 M K2CO3 was mixed with anti-carbofuran mAb (2 g/L,70 μL) under constant stirring for 2 h at RT. Blocking solution (10% BSA, 100 mL) was added and stirred for 2 h. Subsequently, the solution was centrifuged three times at 6200 × g for 15 min to remove excess antibody and blocking reagent. The gold-labelled anti-carbofuran mAb was resuspended in borate buffer (0.002 M BB, 1% sucrose, 0.01% Tween-20, pH 7.2, w/v) and stored at 4°C (Guo et al., Citation2014; Sun et al., Citation2012).
ICS preparation
The test strip consists of four parts: a conjugate pad, an NC membrane, a sample pad, and an absorbent pad. First, the gold-labelled anti-carbofuran mAb was loaded onto the fiber membrane to form the pad and dried at 37°C for 2 h. Second, the coating antigen and antibody were sprayed onto the NC membrane using a Dispensing Platform (BioDot Inc., Irvine, CA, USA) resulting in a test band (T band) and control band (C band). The NC membrane, conjugate pad, and sample pad were loaded on the back of the plate. The absorbent pad was attached to the upper section of the plate. Finally, the assembled plate was cut into 3-mm wide strips and dried at 37°C for 4 h (Chen, Liu, Kuang, Song, & Xu, Citation2013).
ICS assay
A stock solution of 5 mg/L carbofuran was prepared in DMF. Carbofuran standards (0, 0.05, 0.1, 0.25, 0.5, 1, 2 ng/mL) were prepared by diluting the sock solution with PBS (0.01 M, pH 7.4). In this experiment, 80 µL of each carbofuran standard was added to the sample pad, which was placed horizontally. After 7 min, the color intensity of the T and C bands was quantified using a BioDot TSR3000 Membrane Strip Reader (BioDot Inc.). In carbofuran-positive samples, free carbofuran competes with the immobilized carbofuran for the gold-labeled mAb, thereby contributing to no color on the T band.
Sample pre-treatment and spiked sample analyses
Chopped cucumber and apple samples (10 g) were weighed, placed in 50 mL centrifuge tubes, and spiked with different amounts of carbofuran (0, 10, 20, 50, 100, and 200 ng), added 20 mL acetonitrile. The spiked samples were subjected to ultrasonic extraction for 15 min and centrifugation at 2400 × g for 10 min. An aliquot of the resulting supernatant (1 mL) was dried with nitrogen, adjusted to 5 mL with PBS, and analyzed by ic-ELISA and ICS.
Results and discussion
Synthesis of hapten and artificial antigen
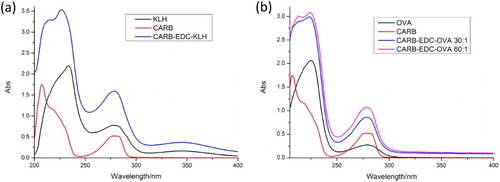
Carbofuran contains no carboxy or amino groups for protein conjugation. Therefore, it is necessary to use another compound that reacts with carbofuran and contains carboxy or amino groups (Abad, Moreno, & Montoya, Citation1999). In this study, benzofuranol was used. Following a series of reactions, we obtained hapten CARB, which contained a carboxyl group with four carbon chains. Long carbon chains contribute to fully exposed carbofuran functional group derivatives. To produce immunogen and coating antigen, we used EDC to couple CARB to KLH and OVA, respectively. The synthesis of immunogen CARB–EDC–KLH and of coating antigen CARB–EDC–OVA was confirmed by ultraviolet–visible spectroscopy (). CARB and KLH have absorption peaks at 276–282 nm and 280–350 nm, respectively; CARB–EDC–KLH had an absorption peak at 270–284 nm. The conjugated antigen had an absorption peak at 350 nm. Similar results were obtained for CARB–EDC–OVA. Therefore, the results revealed that the immunogen and coating antigen were successfully synthetized.
Determination of antibody sensitivity in ic-ELISA
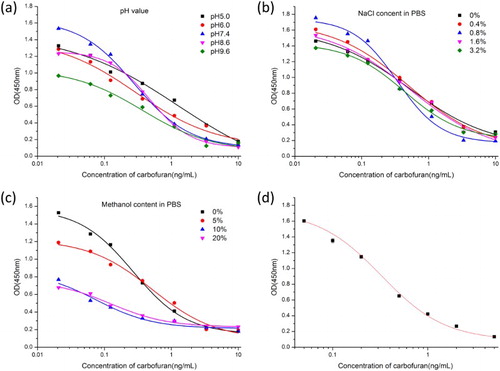
MAb was obtained using 6D5, 3H2, and 3C6 cell lines. Carbofuran standards (0–5 ng/mL) were prepared in PBS. Among the cell lines, 3H2 had the lowest IC50 value (0.3 ng/mL); therefore, the optimization experiments were performed with the mAb 3H2. The coating antigen and antibody concentrations were 0.1 and 0.3 µg/mL, respectively. Based on the pH results ((a)), the lowest IC50 value and highest absorbance value (Absmax) were obtained at pH 7.4. While NaCl (0–3.2%) had little effect on absorption values, 0.8% NaCl resulted in the lowest IC50 value ((b)). With increasing methanol concentration, Absmax decreased and IC50 increased ((c)). IC50 was lower at 0% methanol than at other methanol concentrations. Therefore, the optimal conditions for ic-ELISA were pH 7.4, 0.8% NaCl in PBS buffer, and 0% methanol. Under these conditions, IC50 was 0.3 ng/mL ((d)).
Antibody specificity
Antibody specificity was determined by measuring CR. The IC50 values and CR of the analogs used in this study are shown in . The IC50 values of carbosulfan and 3-hydroxycarbofuran were 30 and 50 ng/mL, respectively, while the IC50 values of the other analogs were >1000 ng/mL. The CR values of carbosulfan and 3-hydroxycarbofuran were 1% and 0.6%, respectively, while the CR value of the other analogs was <0.03%. Therefore, the antibody was specific to carbofuran.
Table 1. Cross-reactivity of mAb to carbofuran and analogs.
Carbofuran recovery tests
Due to the highly volatile nature of acetonitrile, we dried 1 mL of extracting solution with nitrogen and subsequently adjusted its volume to 5 mL with PBS. In this manner, we transformed the acetonitrile extraction solution into a PBS system, and the extracting solution was diluted five times resulting in different concentrations of carbofuran (0–2 ng/mL). Additionally, the dilution minimized matrix interferences. Carbofuran recovery from cucumber and apple samples was calculated (). Carbofuran recovery was 81–85% from cucumber samples and 91–97% from apple samples. The standard deviation was 0.004–0.064, and the coefficient of variation was 0.62–6.50%. Therefore, the sample pre-treatment was suitable. The water content of the samples affected the recovery rates. Different cutoff values of cucumber and apple samples were associated with different carbofuran recovery rates.
Table 2. Accuracy evaluation of immunoassay methods for samples.
Carbofuran detection by ICS
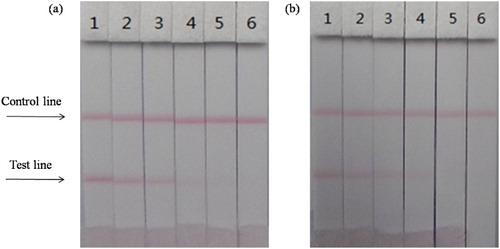
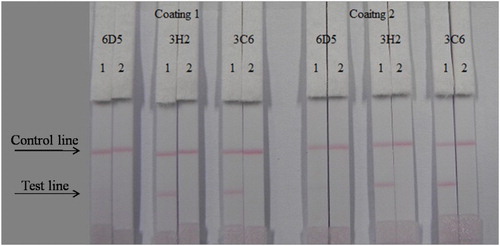
The ICS assay was performed by the wet method. We optimized different kinds of antibodies (6D5, 3H2, and 3C6) and coating antigens (CARB–EDC–OVA–30 and CARB–EDC–OVA–60), and optimized 1 mL of gold nanoparticle with 4L K2CO3 and 8 g/mL antibody. The result can be seen in . Firstly, mixed 7 L of gold nanoparticle with 43 L resuspends, then mixed with 150 L PBS (for 0 ppb). The concentration of the coating antigen was 0.5 mg/mL. Concentrations of carbofuran standards were 0 and 5 ng/mL. From the results, antibody 3H2 and coating antigen CARB–EDC–OVA-60 were the most suitable.
Figure 4. Optimization of different antibodies and coating antigens. Antibody 3H2 and coating 2 were better. 1 = 0 ng/mL, 2 = 5 ng/mL.
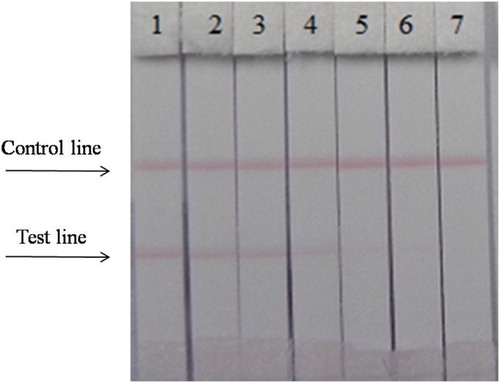
The concentration of coating antigen was 0.5 mg/mL (CARB–EDC–OVA-60). Different carbofuran standard concentrations (0–2 ng/mL) in PBS were used. shows that the strip cut-off value was 1 ng/mL.
Figure 5. The sensitivity of the immunochromatographic assay in PBS for carbofuran. 1 = 0 ng/mL, 2 = 0.05 ng/mL, 3 = 0.1 ng/mL b, 4 = 0.25 ng/mL, 5 = 0.5 ng/mL, 6 = 1 ng/mL, 7 = 2.5 ng/mL. Cut off at 1 ng/mL.
The extracting solution of cucumber and apple samples used in ICS was similar to that used in ic-ELISA. As shown in and , the strip performed well. The cutoff values of cucumber and apple samples were 2 and 1 ng/mL, respectively, in agreement with the ic-ELISA results.
Conclusions
In this study, we produced a highly sensitive and specific mAb against carbofuran with an IC50 value of 0.3 ng/mL. Additionally, we developed ic-ELISA and ICS for the rapid detection of carbofuran in cucumbers and apples. The working range of ic-ELISA was 0.1–1 ng/mL. The cutoff values of cucumber and apple samples were different (2 and 1 ng/mL carbofuran, respectively). The two immunoassays generated similar results. The ICS assay, which is quick, convenient, simple, and sensitive, could be used to detect carbofuran residues in fruits and vegetables.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Leijun Yao obtained her bachelor’s degree from Guangxi University, Nanning, China, in 2014. Subsequently, she started studies as a graduate student in Food Science at Jiangnan University (Wuxi, China). Her research interests include immunoassay applications in food.
Liqiang Liu obtained his Ph.D. in Food Science in 2014 from Jiangnan University, Wuxi, China. Later, he became a faculty at the College of Food Science and Technology of Jiangnan University. His research interests include immunochromatographic strip design and application.
Shanshan Song obtained her master’s degree in Food Science in 2012 from Jiangnan University, Wuxi, China. Later, she became a research assistant at the College of Food Science and Technology of Jiangnan University. Her research interests include monoclonal antibody development.
Hua Kuang obtained her Ph.D. from China Agricultural University in 2009 and then began to work as a faculty at the College of Food Science and Technology of Jiangnan University. She is currently a full professor in food safety. Her research interests include biosensor development.
Chuanlai Xu is a full professor of Food Science and Technology of Jiangnan University. He obtained his Ph.D. in Food Science in 2002. His research interests include fast detection technology and food safety evaluation.
Additional information
Funding
References
- Abad, A., Moreno, M. J., & Montoya, A. (1997). A monoclonal immunoassay for carbofuran and its application to the analysis of fruit juices. Analytica Chimica Acta, 347(1–2), 103–110. doi: 10.1016/S0003-2670(97)00077-9
- Abad, A., Moreno, M. J., & Montoya, A. (1999). Development of monoclonal antibody-based immunoassays to the N-methylcarbamate pesticide carbofuran. Journal of Agricultural & Food Chemistry, 47(6), 2475–2485. doi: 10.1021/jf981184s
- Brkic, D. V., Vitorovic, S., Gasic, S. M., & Neskovic, N. K. (2008). Carbofuran in water: Subchronic toxicity to rats. Environmental Toxicology and Pharmacology, 25(3), 334–341. doi: 10.1016/j.etap.2007.11.002
- Campbell, S., David, M. D., Woodward, L. A., & Li, Q. X. (2004). Persistence of carbofuran in marine sand and water. Chemosphere, 54(8), 1155–1161. doi: 10.1016/j.chemosphere.2003.09.018
- Chauhan, L. K. S., Pant, N., Gupta, S. K., & Srivastava, S. P. (2000). Induction of chromosome aberrations, micronucleus formation and sperm abnormalities in mouse following carbofuran exposure. Mutation Research/Fundamental & Molecular Mechanisms of Mutagenesis, 465(1–2), 123–129.
- Chen, L., Jia, C., Zhao, E., He, M., Yu, P., & Zhu, X. (2011). Gas chromatography–mass spectroscopy analysis of carbofuran and its metabolite 3-hydroxy-carbofuran in maize and soil in field. Communications in Soil Science & Plant Analysis, 42(11), 1316–1323. doi: 10.1080/00103624.2011.571736
- Chen, X., Liu, L., Kuang, H., Song, S., & Xu, C. (2013). A strip-based immunoassay for rapid determination of fenpropathrin. Analytical Methods, 5(21), 6234–6239. doi: 10.1039/c3ay41030g
- Chien Hua, H., Cho Chun, H., & Tai Chia, C. (2012). Analysis of carbofuran, carbosulfan, isoprocarb, 3-hydroxycarbofuran, and 3-ketocarbofuran by micellar electrokinetic chromatography. Journal of Separation Science, 35(10–11), 1359–1364. doi: 10.1002/jssc.201101108
- Gammon, D. W., Liu, Z., & Becker, J. M. (2012). Carbofuran occupational dermal toxicity, exposure and risk assessment. Pest Management Science, 68(3), 362–370. doi: 10.1002/ps.2270
- Gbadegesin, M. A., Owumi, S. E., Akinseye, V., & Odunola, O. A. (2014). Evaluation of hepatotoxicity and clastogenicity of carbofuran in Male wistar rats. Food and Chemical Toxicology, 65, 115–119. doi: 10.1016/j.fct.2013.12.034
- Gu, H., Liu, L., Song, S., Kuang, H., & Xu, C. (2015). Development of an immunochromatographic strip assay for ractopamine detection using an ultrasensitive monoclonal antibody. Food & Agricultural Immunology, 27(4), 471–483. doi: 10.1080/09540105.2015.1126808
- Guo, Y. R., Liu, S. Y., Gui, W. J., & Zhu, G. N. (2009). Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Analytical Biochemistry, 389(1), 32–39. doi: 10.1016/j.ab.2009.03.020
- Guo, J., Liu, L., Xue, F., Xing, C., Song, S., Kuang, H., & Xu, C. (2014). Development of a monoclonal antibody-based immunochromatographic strip for cephalexin. Food and Agricultural Immunology, 26(2), 282–292. doi: 10.1080/09540105.2014.907242
- Guo, L., Song, S., Liu, L., Peng, J., Hua, K., & Xu, C. (2015). Comparison of an immunochromatographic strip with ELISA for simultaneous detection of thiamphenicol, florfenicol and chloramphenicol in food samples. Biomedical Chromatography, 29(9), 1432–1439. doi: 10.1002/bmc.3442
- Harabawy, A. S., & Ibrahim, A. T. (2014). Sublethal toxicity of carbofuran pesticide on the African catfish Clarias gariepinus (Burchell, 1822): Hematological, biochemical and cytogenetic response. Ecotoxicology and Environmental Safety, 103, 61–67. doi: 10.1016/j.ecoenv.2013.09.022
- Huang, Y., Xu, H., Hui, L., Yang, H., Chen, Y., & Shi, X. (2009). Development of an enzyme-linked immunosorbent assay for residue analysis of the insecticide emamectin benzoate in agricultural products. Journal of Agricultural & Food Chemistry, 57(2), 359–364. doi: 10.1021/jf802788n
- Li, S., Xu, L., Ma, W., Wu, X., Sun, M., Kuang, H., … Xu, C. (2016). Dual-Mode ultrasensitive quantification of MicroRNA in living cells by chiroplasmonic nanopyramids self-assembled from gold and upconversion nanoparticles. Journal of the American Chemical Society, 138(1), 306–312. doi: 10.1021/jacs.5b10309
- Lópezpaz, J. L., Cataláicardo, M., & Langasánchez, A. (2014). Determination of methylcarbamate pesticides using flow injection with photoinduced chemiluminescence detection. International Journal of Environmental Analytical Chemistry, 94(6), 606–617. doi: 10.1080/03067319.2013.879295
- Rizos, E., Liberopoulos, E., Kosta, P., Efremidis, S., & Elisaf, M. (2004). Carbofuran-induced acute pancreatitis. Journal of the Pancreas, 5(1), 44–47.
- Saxena, P. N., Gupta, S. K., & Murthy, R. C. (2014). Comparative toxicity of carbaryl, carbofuran, cypermethrin and fenvalerate in metaphire posthuma and Eisenia fetida – a possible mechanism. Ecotoxicology and Environmental Safety, 100(1), 218–225. doi: 10.1016/j.ecoenv.2013.11.006
- Sun, X., Du, S., Wang, X., Zhao, W., & Li, Q. (2011). A label-free electrochemical immunosensor for carbofuran detection based on a sol-gel entrapped antibody. Sensors (Basel), 11(10), 9520–9531. doi: 10.3390/s111009520
- Sun, F., Liu, L., Ma, W., Xu, C., Wang, L., & Kuang, H. (2012). Rapid on-site determination of melamine in raw milk by an immunochromatographic strip. International Journal of Food Science & Technology, 47(7), 1505–1510. doi: 10.1111/j.1365-2621.2012.02998.x
- Tonomura, Y., Mori, Y., Torii, M., & Uehara, T. (2009). Evaluation of the usefulness of biomarkers for cardiac and skeletal myotoxicity in rats. Toxicology, 266(1–3), 48–54. doi: 10.1016/j.tox.2009.10.014
- Wu, X., Xu, L., Liu, L., Ma, W., Yin, H., Kuang, H., & Kotov, N. A. (2013). Unexpected chirality of nanoparticle dimers and ultrasensitive chiroplasmonic bioanalysis. Journal of the American Chemical Society, 135(49), 18629–18636. doi: 10.1021/ja4095445
- Xu, Q., & Rohrer, J. S. (2012). Rapid on-line-SPE HPLC determination of carbofuran and carbaryl in Tap and environmental waters. LC-GC North America, 30(9), 28–28.
- Xu, L., Zhao, S., Ma, W., Wu, X., Li, S., Kuang, H., … Xu, C. (2016). Multigaps embedded nanoassemblies enhance in situ Raman spectroscopy for intracellular telomerase activity sensing. Advanced Functional Materials, 26(10), 1602–1608. doi: 10.1002/adfm.201504587
- Yang, J.-Y., Zhang, Y., Wang, H., Xu, Z.-L., Eremin, S. A., Shen, Y.-D., & Sun, Y.-M. (2014). Development of fluorescence polarisation immunoassay for carbofuran in food and environmental water samples. Food and Agricultural Immunology, 26(3), 340–355. doi: 10.1080/09540105.2014.914890
- Yan, W., Xu, L., Xu, C., Ma, W., Kuang, H., Wang, L., & Kotov, N. A. (2012). Self-assembly of chiral nanoparticle pyramids with strong R/S optical activity. Journal of the American Chemical Society, 134(36), 15114–15121. doi: 10.1021/ja3066336
- Yen, C. C., Hsieh, M. C., Tsai, M. J., & Chen, H. C. (2015). Human carbofuran intoxication with myocardial injury mimicking acute myocardial infarction. The Kaohsiung Journal of Medical Sciences, 31(2), 112–113. doi: 10.1016/j.kjms.2014.11.006
- Zhou, P., Lu, Y., Zhu, J., Hong, J., Li, B., Zhou, J., & Montoya, A. (2004). Nanocolloidal gold-based immunoassay for the detection of the N-methylcarbamate pesticide carbofuran. Journal of Agricultural & Food Chemistry, 52(14), 4355–4359. doi: 10.1021/jf0499121
- Zhu, Y., Cao, Y., Sun, X., & Wang, X. (2013). Amperometric immunosensor for carbofuran detection based on MWCNTs/GS-PEI-Au and AuNPs-antibody conjugate. Sensors (Basel), 13(4), 5286–5301. doi: 10.3390/s130405286