ABSTRACT
Dufulin (DFL) is a potent, new-generation agrochemical that protects crops, such as tobacco, from viruses. Herein, for the first time, we developed a sensitive and specific polyclonal antibody-based indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) to detect DFL residues in samples. The assay showed high sensitivity and specificity to DFL, with a half maximal inhibitory concentration (IC50) of 48.5 ng ml−1 and limit of detection (IC20) of 2.5 ng ml−1. The average recovery of DFL in different spiked samples, such as tobacco, rice, tomato, cucumber, soil and water, was in the range of 70.7–138.8%. A statistically significant correlation was observed between our developed ELISA and previously established methodology of high-performance liquid chromatography with a diode array detector. These results indicate our assay is a potentially useful analytical tool for rapid detection of DFL residue in actual samples.
Introduction
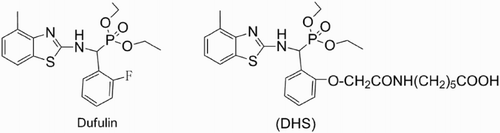
Dufulin (DFL, O,O′-diethyl-2-(4-methylbenzothiazol)-amino-(2-florinephenyl)phosphonate, ) is a novel α-amino phosphonate antiviral agent developed and synthesized by the Center for Research and Development of Fine Chemicals of Guizhou University. With subsequent registration of potentially bioactive DFL granted by the Ministry of Agriculture of China (Song et al., Citation2005; Song, Yang, Jin, & Bhadury, Citation2009; Song, Zhang, Yang, Hu, & Jin, Citation2006; Yang, Song, Hong, Jin, & Hu, Citation2005; Zhang et al., Citation2005). With growing popularity and application of DFL, standardized protocols monitoring residual DFL in agricultural products is of significant importance. So far, several analytical methods, such as high-performance liquid chromatography (HPLC)–ultraviolet (HPLC–UV) (Chen et al., Citation2008), ultraperformance liquid chromatography coupled with photodiode array detection (Zhang et al., Citation2014) and liquid chromatography with tandem mass spectrometry (Li et al., Citation2015) have been used to detect DFL in environmental and agricultural samples. Although these methods can qualitatively and quantitatively determine the DFL, these methods require expensive instruments and skilled analysts and are time consuming. For these reasons, the development of new and simple analytical methods to determine peptides in environmental and agricultural samples with adequate sensitivity and at lower cost is warranted (Hennion & Barcelo, Citation1998).
Toward this end, methods such as an enzyme-linked immunosorbent assay (ELISA) are a low cost, portable and sensitive means of screening a large amount of samples (Gui, Jin, Chen, Cheng, & Zhu, Citation2006; Pyo, Lee, & Choi, Citation2005; Watanabe et al., Citation2006; Zhu, Song, Liu, Kuang, & Xu, Citation2016). Therefore, an ELISA is a practical tool for routine monitoring of residual DFL in agricultural products. However, to the best of our knowledge, there has not been development of a polyclonal antibody (pAb)-based indirect competitive (ic)-ELISA method utilized to ascertain DFL levels. We describe, for the first time, the preparation of a pAb that specifically recognizes DFL, and we developed a simple and sensitive indirect ELISA (ic-ELISA) to allow for simultaneous detection of DFL in environmental and agricultural samples and confirmed the results of our developed method via HPLC.
Materials and methods
Reagents and materials
The DFL standard was obtained from our centre. Phoxim, parathion, fenthion, fenitrothion, chlorpyrifos, and 6-aminohexanoic acid were purchased from the National Standards Co. (Beijing, China). Bovine serum albumin (BSA, molecular weight [MW] = 67,000), ovalbumin (OVA, MW = 45,000), complete Freund’s adjuvant (CFA), incomplete Freund’s adjuvant (IFA), goat anti-rabbit IgG–horseradish peroxidase (HRP) conjugate, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride) (EDC), gelatine and skim milk were purchased from Sigma-Aldrich (St. Louis, MO, USA). O-phenylenediamine (OPD) polyethylene sorbitan monolaurate (Tween 20) was procured from Shanghai Chemical Reagents Co. (Shanghai, China). Polystyrene 96-well microtiter plates were obtained from Costar (Corning, MA, USA). All reagents were of analytical grade unless otherwise specified.
Instruments
Ultraviolet–visible (UV–VIS) spectra were recorded on a spectrophotometer (TU-1901, Persee, China). Plate washing was carried out in an ELx405 microplate washer (BioTek Instruments; Winooski, VT, USA), and absorbance was measured with a 680-plate reader (Bio-Rad, Hercules, CA, USA). Regarding HPLC instrumentation, Agilent 1100 chromatographic equipment was fitted with a diode array detector (DAD) and a Kromasil C18 column (5 μm particle size, 250 × 4.6 mm, Agilent, USA). Ultrapure water (18.2 MΩ) obtained from a Millipore Milli-Q ultrapure water system (Millipore, Bedford, MA, USA)
Buffers and solutions
Multiple buffers and solutions were used for ELISA. The coating buffer was a 50 mM carbonate buffer (pH 9.6); phosphate-buffered saline (PBS, pH 7.4) was composed of 138 mM NaCl, 1.5 mM KH2PO4, 7 mM Na2HPO4 and 2.7 mM KCl, and the washing solution (PBST) consisted of PBS and 0.05% Tween 20 (v/v). The blocking buffer was 5% skim milk in PBS (w/v), and the dilution buffer was identical to that of the blocking buffer. The substrate diluent solution contained 24.3 ml of 100 mM citric acid and 25.7 ml of 200 mM Na2HPO4 per 100 ml of water, pH 5.0. The substrate solution was prepared by adding 0.4 g l−1 OPD and 10 μl of 30% H2O2 per 25 ml of above-mentioned substrate diluent solution; 2 M H2SO4 was used as a stop solution and used throughout our study. Analyte stock solutions (1 mg ml−1) were prepared in methanol.
Immunogens and coating antigen preparation
The haptens (6-[2-((methyllbenzothiazol-2-yl)-1-(2-ethoxy)-O,O-diethyl-α-aminophosphonate) acetamido)] hexanoic acid, DHS) used for immunogen preparation to obtain antibodies and the competitors in the immunoassay (coating antigens) are presented in . The DHS hapten was first synthesized as previously outlined (Lu, Yang, Hu, Ding, & Shi, Citation2013).
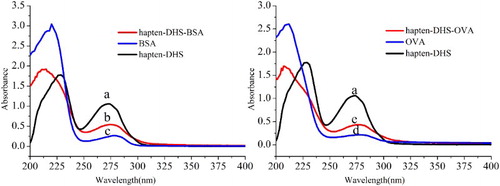
Hapten–DHS was conjugated to an immunogenic carrier protein, BSA or OVA, via the carbodiimide method (EDC) (Bai et al., Citation2013; Wu et al., Citation2011). The carboxylic acid on the hapten was activated by EDC to produce an activated intermediate that could react with the amine functionality of BSA or OVA to form an amide linkage. To execute this experiment, 10 mg of Hapten (DHS) was dissolved in 2.5 ml of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, pH 7.2), and 20 mg of EDC, freshly prepared in 0.5 ml water was then added dropwise. The reaction took place at 25°C with gentle stirring for 2 h. Then the mixture was very slowly added to the carrier protein solution (20 mg dissolved in 1.5 ml of PBS) and stirred slowly for 4 h at 4°C. The resulting conjugation (hapten–DHS–BSA) and coating antigen (hapten–DHS–OVA) were dialysed against three changes of PBS for 3 days to remove uncoupled, free hapten and EDC, and the conjugates were stored at −20°C until further use (Zhang et al., Citation2008). Scans of the hapten, BSA (OVA) and the conjugates were obtained on a UV–VIS spectrophotometer to identify conjugation.
PAb preparation
The pAb was generated as previously described (Sun & Zhuang, Citation2015; Wang et al., Citation2011; Wang, Xu, Zhang, Wang, & Dong, Citation2011). Two female New Zealand white rabbits were immunized by subcutaneous injection at multiple sites on their backs at 2-week intervals over a period of 10 weeks. Initial immunization was performed by injecting 1 mg of DHS–BSA dissolved in 1 ml of 0.9% NaCl and emulsified with an equal volume of CFA. The subsequent four immunizations were administered using IFA. Seven days after each boost, a blood sample was collected from the ear vein. Antibody titre was determined by indirect ELISA. When no further titre enhancement was observed, rabbits were euthanized after being anaesthetized using aether. Whole blood samples were collected and left to coagulate at 25°C for 1 h and then at 4°C overnight. This was followed by centrifugation at 10,000 rpm for 10 min. The clear supernatant phase was carefully collected. pAb was purified from antiserum via ammonium sulphate precipitation and divided into aliquots and stored at −20°C until further use. Blood was drawn one week before immunization to obtain control serum.
Establishment of the ic-ELISA method
Polystyrene 96-well microplates were coated with 100 μl per well of hapten–DHS–OVA conjugates in coating buffer at optimized concentrations overnight at 4°C. After washing three times with PBST, the plates were treated with blocking buffer and sealed and stored for 2 h at 37°C. The sensitized plates were washed with PBST and patted dry onto paper towels; then, 50 μl per well of the optimal antibody dilution (1/320,000 in dilution buffer) and 50 μl per well of a series of DFL standard concentrations (0, 0.1, 1, 10, 50, 100, 500 and 1000 ng ml−1) or sample extracts were added to the well (in triplicate) and incubated for 1.5 h at 37°C. The plates were washed again, and horseradish peroxidise-labelled goat anti-rabbit IgG (1/10,000 in DFL buffer, 100 μl per well) was added for an additional 30 min at 37°C. The plates were washed again, 100 μl per well of OPD substrate solution was added. Colorimetric development was monitored at 37°C and stopped after 15 min with 2 M H2SO4, and the plate was read on an ELISA plate reader at 490 nm with a reference wavelength at 650 nm to obtain the absorbance of each well (Francesc, Antonio, & Josep, Citation2011). Sigmoidal curves were mathematically fitted to a four-parameter logistic equation and were developed by plotting the concentration (Log C) versus B/B0 (mean absorbance for the DFL standard divided by the mean absorbance for the zero standards), from which a half maximal inhibitory concentration (IC50) values (concentration at which binding of the antibody to the coating antigen is inhibited by 50%) were determined (Wang et al., Citation2010).
ic-ELISA optimization
In this assay, the chequerboard method, in which several serum dilutions are titrated against various amounts of coating antigens (hapten–DHS conjugated to OVA), was used to select the most suitable antiserum and to approximate appropriate antigen coating and antibody concentrations for our competition assays. When the OD490 value was approximately 1.0, the corresponding dilution was considered suitable for the chequerboard assays (Josep & Antonio, Citation2009). This procedure was the same as for the competition assays except that the addition of the pesticide standard or sample was omitted at the competition step. Moreover, a set of experimental parameters, including ionic strength, pH, organic solvents and blocking agents, were sequentially studied to improve immunoassay sensitivity (Liang, Jin, Gui, & Zhu, Citation2007; Kim, Kim, Lee, & Lee, Citation2007). The optimized buffer condition was used in the subsequent ELISA assay. Regarding ionic strength, the influence of Na+ concentration, in skim milk-PBS, at 0.05, 0.15, 0.5 and 1.5 mol l−1 was tested. The effect of pH was evaluated using different skim milk-PBS solutions ranging from pH 5.2 to 9.0. The tolerance of our ELISA assay to methanol concentrations (0%, 5%, 10%, 20% and 30% in skim milk-PBS) was also studied. Moreover, the effect of blocking reagents (0.05% PBST, 0.1% BSA, 0.1% OVA, 1% gelatine and 5% skim milk powder) was investigated.
Specificity determination
Cross-reactivity (CR) was assessed to determine antibody specificity. Five structurally related compounds (phoxim, parathion, fenthion, fenitrothion and chlorpyrifos; ) were selected and evaluated. Standard solutions of these compounds were analysed via our ELISA procedure described earlier. CR was calculated via the following equation (Wortberg, Kreissig, Jones, Rocke, & Hammock, Citation1995):
Samples pretreatment
To evaluate the accuracy and reproducibility of our developed ELISA, tobacco, tomato, cucumber, rice, soil and water samples were selected, spiked with different concentrations of DFL and analysed in a blinded fashion. For the spiked-and-recovery test, four solutions (0, 0.12, 1.2 and 12 μg ml−1) of DFL in methanol were prepared. The tomato and cucumber samples were cut into pieces and homogenized prior to test, the tomato samples were chopped analysis, while the tobacco and rice samples were crushed into a powder. Sample pretreatment procedures were performed as follows: 12 g of prepared sample was spiked with 1 ml of standard solution, and then 29 ml of benzene/ethyl acetate (1:1, v/v) was added as the extracting solvent. The resulting mixture was oscillated for 1 min with a vortex mixer, and then 1 g of NaCl and 4 g MgSO4 were added. The oscillation was continued for another 1 min. After centrifugation (10 min, 6000 rpm), 3 ml of supernatant was transferred and evaporated at 35°C under a nitrogen stream. The dry residue was dissolved into 1 ml of skim milk-PBS (containing 5% methanol) and analysed via our optimized ELISA protocol.
HPLC analysis
To validate the ELISA method, samples were further analysed by HPLC. The sample preparation procedure involved homogenization (tomato and cucumber samples) and sonic oscillation (soil, rice and tobacco samples) with acetone, then filtration and filtrate extraction using benzene/ethyl acetate (1:1, v/v). For the rice and tobacco samples, the organic phase was purified by column chromatography. The spiked water samples were extracted with methylene chloride. All mixtures were concentrated to near dryness in a vacuum rotary evaporator at 40°C. The residues obtained were dissolved in 1 ml of methanol and passed through a syringe philtre (0.22 µm, 13 mm) for HPLC analysis.
The HPLC operation conditions were as follows: analyses were performed on an Agilent 1100 chromatography fitted with a DAD and a Kromasil C18 column, (5 µm particle size, 250 × 4.6 mm). The injection volume was 20 µl, the temperature of column oven was 25°C. The chromatograph was run with a mobile phase of methanol-water (80:20, v/v) at a flow rate of 1.0 ml min−1. DFL was determined at 270 nm.
Results and discussion
Hapten synthesis and conjugation
Hapten synthesis is a key step in the rapid development of immunoassays for ascertaining pesticide residue levels. Generally, a suitable hapten for immunization should preserve the structure of the target compound as much as possible. A spacer length of four to six C atoms is considered the most suitable for this purpose (Jung, Meyer, & Hamm, Citation1989; Li & Helm, Citation1995). DFL is a rare member of the pesticides containing P. It has a typical phosphonate (RO)2P(=O)-group (R=ethyl), a benzo heterocyclic moiety and an o-fluorophenyl group attached to the α-carbon (). Based on the standard rules for hapten synthesis, in the present investigation, we designed the DHS hapten bearing a reactive 6-C atom carboxylic acid group (–OCH2CONH(CH2)5COOH) by replacing the fluorine atom attached to the benzene ring of DFL. This subtle change in the structure led to high specificity in the DFL ELISA (). Finally, the covalently coupled hapten with the amino group in the carrier protein by the EDC forms an immunogen and coating antigen conjugate, respectively.
Antibody production and antisera screening
The immunogen was inoculated into two New Zealand rabbits, and after each boost, the antisera were screened against the DHS–OVA coating antigen by indirect ELISA. After the fifth injection, the sensitivities of two antisera samples (named pAb 1 and pAb 2) were also studied (). The final titre values of pAb 1 and pAb 2 were 1/160,000 and 1/240,000, respectively, while the IC50 values were recorded as 133 and 128 ng ml−1, respectively. It is apparent from that although the titres were different, the sensitivity of the two antisera was almost the same. In subsequent experiments, the pAb 2 antiserum was used for ic-ELISA development to detect DFL residues.
Table 1. Summary of titresa of antisera and competitive inhibition of DFL.
ic-ELISA optimization
To establish a highly sensitive and specific ELISA method, optimal assay conditions were determined via adjusting several parameters, such as the coating antigen concentration and dilutions of antiserum and goat anti-rabbit IgG-HRP (Watanable et al., Citation2002). According to the chequerboard titration assay results, the optimized concentration of coating antigen, hapten–DHS–OVA, was 0.625 μg ml−1. The pAb 2 dilution was 1:320,000, and the suitable dilution of goat anti-rabbit IgG-HRP was 1:12,000.
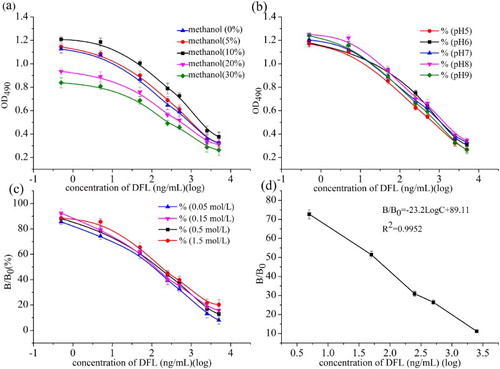
Sensitivity was expressed as the IC50 value. A lower IC50 value is indicative of higher assay sensitivity. To obtain a low IC50 value, the effects of different chemical conditions, including ionic strength, pH, organic solvent content and blocking agents, were investigated. In the pAb 2/ DHS–OVA system, a lower salt concentration (≤0.15 M) resulted in a lower IC50 value, whereas a higher salt concentration (≥0.5 M) in a lower R2 value of its linear equation due to lower OD values. As for the effect of blocking reagents, PBST, BSA, OVA, gelatine and skim milk were tested to eliminate nonspecific binding. The IC50 values of these blocking solutions were as follows: PBST (365 ng ml−1), 0.1% BSA (119 ng ml−1), 0.1% OVA (176 ng ml−1), 1% gelatine (97 ng ml−1) and 5% skim milk (90 ng ml−1). We selected 5% skim milk, which was the most cost effective and had the lowest IC50 value. Gleaning from data presented in (a), with the increase in methanol concentration, assay sensitivity decreased, confirming that organic solvents affect antigen-antibody interaction and inhibits DFL from binding to the antibody. Consequently, the lowest IC50 was observed at 5% methanol concentration. When analytes were dissolved in buffer pH values ranging from 5.2 to 9.0, no significant effect on the IC50 value was detected, but, interestingly, both a strong acid and alkali matrix resulted in lower sensitivity, indicating that the enzymatic reaction and the appetency reaction of the antigen and antibody were optimal at neutral conditions. Thus, a pH value of 7.4 was selected for the assay (as shown in (b)). Based on the data depicted in (c), a salt concentration of 0.05 M was selected in our ic-ELISA system because the corresponding R2 value was > 0.99, and the IC50 value was the lowest while the Amax was close to 1.0. (d) shows the standard curve of DFL under optimized conditions.
Establishment of ic-ELISA for DFL residue determination
With the optimized parameters in hand, the calibration curve for DFL using ic-ELISA was then established ((d)). Seven concentrations of DFL (10,000, 5000, 1000, 500, 100, 10, 1, 0.1 and 0 ng ml−1) were used, and the linear regression equation was B/B0 = –23.2 LogC + 89.11, with a correlation (R2) value of 0.9952. The IC50 value of was 48.5 ng ml−1, the limit of detection (IC20) was 2.5 ng ml−1, and the workable range (IC20–IC80) was 2.5–952.6 ng ml−1 (Hennion & Barcelo, Citation1998).
Specificity
To determine the specificity of the optimized ic-ELISA, several compounds of similar structure and raw hapten material were tested for CR. displays the CR results. The interference observed from the competitors was almost negligible. The highest interference was recorded for 6-aminohexanoic acid, which showed a CR of 1.01%. The low cross-reactivity of the antibody for the spacer arm raw material indicated that the 6-C spacer arm kept most of the characteristic structural features and preserved the antigenic determinants of DFL. Therefore, our immunoassay for DFL was highly specific, with minimal chances of false positives.
Table 2. The antibody specificity and CR.
Analysis of spiked samples
The spiked recoveries were used to represent the accuracy of our ic-ELISA. Particularly, DFL residues in rice, tobacco, soil, tomato, cucumber and water samples were detected using our optimized assay and pretreatment scheme as outlined above. As indicated in , the mean recoveries were 98.2% (89.5–105.1%), 92.1% (87.7–99.0%), 114.5% (98.8–138.8%), 84.1% (70.7–95.1%), 99.7% (93.7–103.3%) and 85.0% (79.0–93.4%) for the rice, soil, tobacco, tomato, cucumber and water samples, respectively. These data could ideally meet the requirement of an analytical method for residue determination. However, overestimated recovery data (>120%) were also found, which reduced the reliability of the developed ELISA.
Table 3. Recoveries of spiked real samples determined by ELISA and HPLC (n = 3)a.
Comparison of ic-ELISA with HPLC
To confirm the accuracy of ic-ELISA, the spiked samples were also analysed by HPLC, which was developed in our laboratory. Results are provided in . The average recoveries of DFL by HPLC from all samples ranged from 68.5% to 112.0%, with a relative standard deviation range of 3.0–13.2%. With regard to accuracy and precision, the recovery values determined by ic-ELISA were better compared with those obtained from HPLC. Nevertheless, selecting either ic-ELISA or HPLC would depend on the prioritized needs and requirements for rapidness and accuracy.
Conclusions
To our knowledge, this is the first report of a highly sensitive and effective ic-ELISA that rapidly detects DFL in agricultural and environmental samples on the basis of a specific pAb for DFL. The sample preparation was simple, and CR with analogous compounds and structures was negligible, indicating exceptional specificity. Our experimental ic-ELISA data were in good agreement with data obtained via confirmatory HPLC. Therefore, our developed method is a novel, safe, efficient and sensitive analytical tool for routine monitoring of DFL residues in environmental and agricultural samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Yuling Chen is now a master student in the Center for Research and Development of Fine Chemicals of Guizhou University. Her research is focused on organic pesticide residue analysis and fast detection technology.
Jing Li is now a master student at Guizhou University, Guiyang, China. Her research interests are toxicology of dufulin and pesticide residue analysis.
Ping Lu obtained her Ph.D. in pesticide science from Guizhou University in 2012 and then began to work as a faculty in the Center for Research and Development of Fine Chemicals of Guizhou University. She is an associate professor, her research interests are fast detection technology, pesticide residue analysis and food safety.
Deyu Hu is a full professor of pesticide chemistry of Guizhou University. Her research is focused on green pesticide innovation and pesticide residue analysis.
Wei Xue got his Ph.D. in pesticide science in 2011 from Guizhou University, Guiyang, China, and then became a faculty in the Center for Research and Development of Fine Chemicals of Guizhou University. His research interests includes green pesticide innovation and natural products chemistry.
Xiaoyan Ding is now a master student in the Center for Research and Development of Fine Chemicals of Guizhou University. Her research is focused on organic pesticide residue analysis.
Additional information
Funding
References
- Bai, Y. H., Liu, Z. H., Wang, H. J., Bi, Y. F., Huang, Y. L., Sun, L., … Xu, S. X. (2013). Development of a rapid and sensitive enzyme-linked immunosorbent assay to detect clonidine residues in swine urine samples. Food and Agricultural Immunology, 26(1), 1–12. doi: 10.1080/09540105.2013.858663
- Chen, Z., zhang, Y. L., Zeng, S., Song, B. A., Lu, P., Hu, D. Y., … Xue, W. (2008). Residue analysis and dissipation of 30% dufulin wettable powder in soil and tobacco leaves. Guizhou University, Master Dissertation. Retrieved from http://www.paper.edu.cn/releasepaper/content/200801-101.
- Francesc, A. E., Antonio, A. F., & Josep, V. M. (2011). Determination of fenhexamid residues in grape must, kiwifruit, and strawberry samples by enzyme-linked immunosorbent assay. Food Chemistry, 124(4), 1727–1733. doi: 10.1016/j.foodchem.2010.07.112
- Gui, W. J., Jin, R. Y., Chen, Z. L., Cheng, J. L., & Zhu, G. N. (2006). Hapten synthesis for enzyme-linked immunoassay of the insecticide triazophos. Analytical Biochemistry, 357(1), 9–14. doi: 10.1016/j.ab.2006.07.023
- Hennion, M. C., & Barcelo, D. (1998). Strengths and limitations of immunoassays for effective and efficient use for pesticide analysis in water samples: A review. Analytica Chimica Acta, 362(1), 3–34. doi: 10.1016/S0003-2670(97)00608-9
- Josep, V. M., & Antonio, A. F. (2009). Monoclonal antibody generation and direct competitive enzyme-linked immunosorbent assays evaluation for the analysis of the fungicide fenhexamid in must and wine. Journal of Agricultural and Food Chemistry, 57, 5129–5135. doi: 10.1021/jf900867u
- Jung, F., Meyer, H. D., & Hamm, R. T. (1989). Development of a sensitive enzyme-linked immunosorbent assay for the fungicide fenpropimorph. Journal of Agricultural and Food Chemistry, 37(4), 1183–1187. doi: 10.1021/jf00088a080
- Kim, Y. J., Kim, Y. A., Lee, Y. T., & Lee, H. S. (2007). Enzyme-linked immunosorbent assays for the insecticide fenitrothion: Influence of hapten conformation and sample matrix on assay performance. Analytica Chimica Acta, 591(2), 183–190. doi: 10.1016/j.aca.2007.03.072
- Liang, C. Z., Jin, R. Y., Gui, W. J., & Zhu, G. N. (2007). Enzyme-linked immunosorbent assays based on a monoclonal antibody for the detection of the insecticide triazophos; assay optimization and application to environmental samples. Environmental Science & Technology, 41(19), 6783–6788. doi: 10.1021/es070828m
- Li, K. C., & Helm, R. F. (1995). Synthesis and rearrangement reactions of ester-linked lignin-carbohydrate model compounds. Journal of Agricultural and Food Chemistry, 43(8), 2098–2103. doi: 10.1021/jf00056a026
- Li, Y. J., Lu, P., Hu, D. Y., Bhadury, P. S., zhang, Y. P., & zhang, K. K. (2015). Determination of dufulin residue in vegetables, rice, and tobacco using liquid chromatography with tandem mass spectrometry. Journal of AOAC International, 98(6), 1739–1744. doi: 10.5740/jaoacint.15-134
- Lu, P., Yang, S., Hu, D. Y., Ding, X. Y., & Shi, M. M. (2013). Synthesis of hapten and development of immunoassay based on monoclonal antibody for the detection of dufulin in agricultural samples. Journal of Agricultural and Food Chemistry, 61(43), 10302–10309. doi: 10.1021/jf4025954
- Pyo, D., Lee, J., & Choi, E. (2005). Trace analysis of microcystins in water using enzyme-linked immunosorbent assay. Microchemical Journal, 80(2), 165–169. doi: 10.1016/j.microc.2004.07.015
- Song, B. A., Yang, S., Hong, Y. P., Zhang, G. P., Jin, L. H., & Hu, D. Y. (2005). Synthesis and bioactivity of fluorine compounds containing isoxazolylamino and phosphonate groups. Journal of Fluorine Chemistry, 126, 1419–1424. doi: 10.1016/j.jfluchem.2005.08.005
- Song, B. A., Yang, S., Jin, L. H., & Bhadury, P. S. (2009). Envionment-Friendly antiviral agents for plant. Beijing: Chemical Industry Press.
- Song, B. A., Zhang, G. P., Yang, S., Hu, D. Y., & Jin, L. H. (2006). Synthesis of N-(4-bromo-2-trifluoromethylphenyl)-1-(2-fluorophenyl)-O,O-dialkyl-α-aminophosphonates under ultrasonic irradiation. Ultrasonics Sonochemistry, 13(2), 139–142. doi: 10.1016/j.ultsonch.2005.03.003
- Sun, R. Y., & Zhuang, H. S. (2015). Development of a highly sensitive biotin–streptavidin enzyme-linked immunosorbent assay for detecting diethyl phthalate based on a specific polyclonal antibody. Food and Agricultural Immunology, 26(5), 1–15. doi: 10.1080/09540105.2015.1027666
- Wang, C. M., Li, X. B., Liu, Y. H., Guo, Y. R., Xie, R., Gui, W. J., & Zhu, G. N. (2010). Development of a mab-based heterologous immunoassay for the broad-selective determination of organophosphorus pesticides. Journal of Agricultural and Food Chemistry, 58(9), 5658–5663. doi: 10.1021/jf904575k
- Wang, Y., Xu, Z. L., Xie, Y. Y., Tian, Y. X., Shen, Y. D., Yong, G. M., … Sun, Y. M. (2011b). Development of polyclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for sodium saccharin residue in food samples. Food Chemistry, 126(2), 815–820. doi: 10.1016/j.foodchem.2010.11.076
- Wang, Y., Xu, Y., Zhang, X., Wang, E. L., & Dong, Y. (2011a). Preparation of an immunoaffinity column and its application in sample cleanup for methandrostenolone residues detection. Journal of Chromatography B, 879(22), 2149–2154. doi: 10.1016/j.jchromb.2011.05.053
- Watanabe, E., Miyake, S., Ito, S., Baba, K., Eun, H., Ishizaka, M., & Endo, S. (2006). Reliable enzyme immunoassay detection for chlorothalonil: Fundamental evaluation for residue analysis and validation with gas chromatography. Journal of Chromatography A, 1129(2), 273–282. doi: 10.1016/j.chroma.2006.06.095
- Watanable, E., Kanzaki, Y., Tokumoto, H., Hoshino, R., Kubo, H., & Nakazawa, H. (2002). Enzyme-linked immunosorbent assay based on a polyclonal antibody for the detection of the insecticide fenitrothion. Evaluation of antiserum and application to the analysis of water samples . Journal of Agricultural and Food Chemistry, 50(1), 53–58. doi: 10.1021/jf0108359
- Wortberg, M., Kreissig, S. B., Jones, G., Rocke, D. M., & Hammock, B. D. (1995). An immunoarray for the simultaneous determination of multiple triazine herbicides. Analytica Chimica Acta, 304, 339–352. doi: 10.1016/0003-2670(94)00651-2
- Wu, G. Q., Wang, S. L., Wang, X. Y., Li, X. F., Deng, X. P., Shen, Z. L., & Xi, T. (2011). Determination of a new antibacterial peptide S-thanatin in rat plasma by an indirected-ELISA. Peptides, 32(7), 1484–1487. doi:10.1016/j.peptides.2011.05.009
- Yang, S., Song, B. A., Hong, Y. P., Jin, L. H., & Hu, D. Y. (2005). Synthesis and crystal structure of N-(6methoxylbenzothiazol-2-yl)-1-(4-fluorophenyl)O,O-dipropyl-α-aminophosphonate. Journal of Chemical Crystallography, 35(11), 891–895. doi:10.1007/s10870-005-5134-8
- Zhang, K. K., Meng, X. G., Jiang, D., Hu, D. Y., Zhang, Y. P., Lu, P., … Song, B. A. (2014). Dissipation rates of dufulin residues in paddy, soil, and water determined by ultra-performance liquid chromatography coupled with photo-diode array detection. International Journal of Environmental Analytical Chemistry, 94(4), 370–380. doi: 10.1080/03067319.2013.853760
- Zhang, G. P., Song, B. A., Yang, S., Jin, L. H., Hu, D. Y., & He, W. (2005). Crystal structure of N-(4-methylbenzothiazole-2-yl)-1-(4-trifluoromethylphenyl)-O,O-dipropyl-α-aminophosphonate. Analytical Sciences, 21, 105–106. doi:10.2116/analscix.21.x105.
- Zhang, Q., Sun, Q., Hu, B. S., Shen, Q., Yang, G., Liang, X., … Liu, F. Q. (2008). Development of a sensitive ELISA for the analysis of the organophosphorous insecticide fenthion in fruit samples. Food Chemistry, 106(3), 1278–1284. doi: 10.1016/j.foodchem.2007.07.049
- Zhu, Y. T., Song, S. S., Liu, L. Q., Kuang, H., & Xu, C. L. (2016). An indirect competitive enzyme-linked immunosorbent assay for acrylamide detection based on a monoclonal antibody. Food and Agricultural Immunology, 27(6), 1–10. doi: 10.1080/09540105.2016.1160369