ABSTRACT
Aloe vera (Aloe barbadensis Miller) has been used as topical and oral therapeutic. This research highlights the phenolic constituents’ profile and antioxidant activity of 70% ethanol extracts of Aloe vera flower for the first time. The ethanol-based extracts showed the inhibition for linoleic acid oxidation and free radical-induced DNA damage. Among about 11 phenolic constituents of the extract, identified by using high performance liquid chromatography (HPLC), the content of vanillic acid was highest, corresponding to strong antioxidant activities of the extract. The extracts elevated superoxide dismutase, catalase, and glutathione peroxidase enzymes activities in the liver tissue of hydrogen peroxide-treated BALB/c mice. The radical-scavenging activities of the extracts were well-correlated to the total phenolic content. Therefore, Aloe bardadensis flower might be an effective source of natural antioxidant.
GRAPHICAL ABSTRACT
Introduction
In living organisms, oxidation is a crucial biological process for energy production, which is responsible for an excessive production of reactive oxygen species (ROS) (Arunachalam, Parimelazhagan, & Saravanan, Citation2011). The formation of the ROS causes aging and a number of diseases including inflammation, cancer, and other degenerative diseases in the living body (Samad et al., Citation2013). A wide range of phytochemicals including polyphenols and flavonoids are plenty in natural products, such as flower, fruits, and vegetables. Recently, many researchers have reported that the phytochemicals may afford protection against some chronic diseases, caused by oxidative stress (Debnath, Kim, & Lim, Citation2013; Samad et al., Citation2013). These phytochemicals can scavenge the free radicals by inhibiting their initiation and chain propagation. In this regard, natural antioxidants are capturing the interest of the research community.
Aloe plants include over 300 species, of which Aloe barbadensis is most widely known in the world. It is a tropical plant and belongs to the Asphodelaceae (Liliaceae) family. It grows in the dry regions of Asia, Africa, and Europe (Surjushe, Vasani, & Saple, Citation2008). It is a stem-less or very short-stemmed succulent plant. Its flowers appear in summer. Each flower looks pendulous, having a 2–3 cm long yellow tubular corolla (Gong, Wang, & Chen, Citation2002). The aloe has been reported to have significant therapeutic effects, such as inhibition of cancer cells’ activation and proliferation as well as anti-inflammatory, anti-viral, and anti-oxidation properties (Yoo et al., Citation2008). The alcoholic extract of Aloe vera showed higher antibacterial and anti-fungal activities than that of aqueous extract, reported elsewhere (Choi et al., Citation2001). The detailed phytochemical analysis of skin and gel of Aloe vera leaf showed the presence of medicinal value-added chemical constituents such as tannin, phlobatannins, saponin, flavonoids, steroids, terpenoids, and cardiac glycosides anthroquinones (Sathyaprabha, Kumaravel, Ruffina, & Praveenkumar, Citation2010). However, the phytochemicals analysis and antioxidant activity of the Aloe vera flower extracts have not been widely investigated. In this regard, we investigated the phytochemical profiles and antioxidant activity of ethanol extracts of the Aloe barbadensis flower in hydrogen peroxide-treated BALB/c mice.
Materials and methods
Materials and chemicals
All the chemicals including Folin-Ciocalteu (FC) reagent, gallic acid (GA), 2,2-diphenyl-1-picrylhydrazyl (DPPH), trichloroacetic acid (TCA), 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), linoleic acid, ascorbic acid (AA), and 5,5-dimethylpyrroline 1-oxide (DMPO) were purchased from Sigma Chemical Co. (St. Louis, MO), USA. Dulbecco’s modified Eagle’s medium (DMEM), FeCl3, and NaOH were purchased from Wako Pure Chemical Industries Ltd. (Tokyo, Japan).
Preparation of extracts
Dried Aloe barbadensis flowers (800 g) was extracted with 70% ethanol at room temperature (RT) for 3 days. The extracts were then filtered through filter paper followed by evaporation on a rotary vacuum evaporator at 40°C under reduced pressure. The concentrated mass was dried and kept in a freezer at –20°C. The percentage of yields (w/w) was determined using the equation, yield (%) = (total extracted sample mass/total dry sample mass) × 100. The stock solution was prepared by dissolving it into distilled water (DW) for different analyses.
Determination of total phenolic and flavonoid contents
The phenolic content of the extract was measured according to the method of Debnath et al. (Citation2011) and Im et al. (Citation2016). Briefly, 10 mg of the dried extract was dissolved into 1 mL DW. GA was used as the standard and its different solutions (0–500 µg/mL) were prepared in ethanol. A 40 μL aliquot of each sample or the standard was mixed with 20 μL of 1 N FC reagent followed by incubation at RT for 3–5 min. Then, 20% NaCO3 solution was added in the mixture, followed by incubation at RT for 30 min. Finally, the absorbance was measured at 700 nm wavelength by using a UV–Vis spectrophotometer. The result was expressed as percentage of gallic acid equivalents (GAEs) per 100 g dry mass. Furthermore, the total flavonoid content was measured by the reported colorimetric assay (Debnath et al., Citation2011; Lee, Choi, Park, & Kim, Citation2014).
DPPH and ABTS radicals’ scavenging activities
The activities of the DPPH radical of the extract were measured according to the report of Samad, Debnath, Ye, Hasnat, and Lim (Citation2014). The butylated hydroxytoluene (BHT) with different contents (0.12–4.00 mg/mL) was used as standard. Then, 80 μL solution of sample or standard was added to 80 μL DPPH. The mixture was kept at ambient condition for 30 min. Then, the activity was determined using the equation, activity (%) = {(a − b) − (x − y)/(a − b)} × 100, where x = absorbance of DPPH + sample/standard at 517 nm; y = absorbance of sample/standard + methanol at 517 nm; a = absorbance of DPPH + DW (or ethanol)/methanol at 517 nm; and b = absorbance of methanol + DW (or ethanol) at 517 nm.
7 mM aqueous ABTS solution was mixed with a potassium persulfate solution to make a solution having the concentration of 2.45 mM. It was then left undisturbed at RT for 12–16 h under dark before use (Jeong et al., Citation2009). The freshly prepared ABTS solution was used for all experiments. The absorbance of ABTS solution was adjusted to 0.70 ± 0.02 at 734 nm wavelength by dilution with 0.01 M phosphate buffer saline (PBS, pH 7.4). Then, 0.9 mL of ABTS solution was mixed with 0.1 mL of each sample (0.12–4.00 mg/mL) and BHT, followed by the incubation at RT for 5 min. The ABTS scavenging activity was determined by using a UV–Vis spectrometer at 734 nm.
Reducing power activity
The activity for reducing power of the extract was estimated according to the method of Hu, Shen, and Wang (Citation2009) after some modification. Briefly, 1 mL of extract with different concentrations (0.12–4.00 mg/mL) or BHT (0.12–4.00 mg/mL) was added to the mixture of 2.5 mL of 0.2M sodium phosphate buffer (pH 6.6) and 2.5 mL of potassium ferricyanide (10 mg/mL), followed by incubation at 50°C in water bath for 30 min. A 2.5 mL of TCA (100 mg/mL) was then added into the mixture, followed by the centrifuge at 3000 rpm for 10 min. After that, 2.5 mL of each DW and 1 mL of FeCl3 solution (1 mg/mL) were added to the above mixture successively. The effective concentration of extract (EC50) at 0.5 value of absorbance was measured at 700 nm.
Nitrite scavenging activity
The scavenging activity for nitrite was measured following the method of Lee et al. (Citation2009) with some modifications. Briefly, 1 mL of sample or standard (BHT) with different concentrations (0.125−2.0 mg/mL) was added to 1 ml of NaNO2 (1 mM). The pH of the solution was decreased up to 2.0 by adding 0.1 N HCl acid slowly. Then, 10 mL DW was added to the solution. It was maintained at 37°C in a water bath for 1 h. Then, 500 μL each of DW and 100 μL of Griess reagent was added to the solution successively. It was again incubated at RT for 15 min. The scavenging activity of each sample/standard was estimated by measuring the absorbance at 540 nm wavelength.
Hydroxyl and superoxide radicals’ scavenging activities
The activities for hydroxyl and superoxide radicals were determined by using electron spin resonance (ESR) spectra as reported by Debnath, Kim, Nath, and Lee (Citation2017). Briefly, 20 μL each of 0.3 M 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and 10 mM H2O2 was mixed with PBS of pH 7.4. Then, 20 μL each of 10 mM iron sulfate and sample or standard was added to the above mixture, followed by ESR measurement after 2.5 min. The reaction mixture was prepared by adding 20 μL of various concentrations of sample or standard to 20 μL of 0.8 M DMPO, 1.6 mM EDTA, and 0.8 mM riboflavin. Before the ESR measurement, the mixture was illuminated under a UV lamp (λ = 365 nm) for 1 min.
Inhibition of linoleic acid oxidation
The inhibition effect of sample for lipid peroxidation was measured using the method of Lee et al. (Citation2009) with some modifications. 2.5 mL of sample or standard was mixed with 1 mL of 0.1 M PBS (pH 7.0), followed by the addition of 1 mL of 50 mM ethanoic linoleic acid. It was diluted with 0.5 mL of DW followed by incubation at 40°C in a water bath under dark condition. Then, 2.5 mL of 75% ethanol and 50 μL of 30% ammonium thiocyanate were added to 50 μL of the above mixture successively. After 3 min, 50 μL of 20 mM FeCl2 solution was added to the mixture. It was incubated again for 3 min. The absorbance was measured at 500 nm wavelength of light and continued up to 7 days. Both AA and BHT were used as positive controls. The scavenging activity of linoleic acid was calculated by using the equation, activity (%) = 100 − (A/B) × 100, where A is the absorbance of sample reaction indext = maxh. and B is the absorbance of control reaction indext = maxh.
High-performance liquid chromatography
High-performance liquid chromatography (HPLC) was performed on an analytical column of Supelco C18 (25 cm×46 mm, 5 μm). The mobile phase composed of DW and acetonitrile (ACN) using the following gradient program: 0–12 min, 10% ACN; 12–18 min, 10–20% ACN; 18–24 min, 20–50% ACN; 24–30 min, 50% ACN; 30–35 min, 50–10% ACN. The flow rate of mobile phase was 0.8 mL/min. The sample injection volume was 20 µL. The detection was performed with a UV–Vis spectrophotometer at a 290 nm wavelength of light.
Preparation of liver tissue samples
Male BALB/c mice (average body weighing 18.2 ± 1.6 g) at 4 weeks of age were purchased from Orient Bio (Seongnam, Korea). The mice were kept in cages in a closed room with the cycle of 12 h light and dark. The mice were sacrificed on the 7th day of adaptation. The liver sample was separated in six tubes, each containing 5 mL of 0.1 M Tris-HCl buffer (pH 7.4), which were denoted by control sample (A), damage sample (B), and treatment sample (from C to F). The liver tissue was homogenized. Then, 100 μg/mL of Aloe flower extracts and 10 μg/mL of AA were added to C and D tubes, respectively. They were incubated at 37°C for 30 min. Then, H2O2 was added to all tubes except A. It was again incubated at 37°C for 30 min, followed by the centrifuge at 2500 rpm and 4°C. The supernatants were used for antioxidant assays.
Superoxide dismutase-like activity
The superoxide dismutase (SOD)-like activity of the extract was measured according to the method described by Debnath et al. (Citation2011). Briefly, 200 μL of 7.2 mM pyrogallol aqueous solution and 3 mL of 50 mM Tris-HCl buffer (pH 8.5) were added to 200 μL of samples successively. The SOD-like activity was estimated by measuring the absorbance at 420 nm wavelength of light.
Glutathione peroxidase and catalase activity assay
The enzymatic activity of the extract was investigated with Cayman’s catalase assay kit, performed according to the manufacturer’s instructions.
Statistical analysis
The statistical analyses were assessed with GraphPad Prism (v5.0, GraphPad Software Inc, La Jolla, CA, USA). The differences of P < .05 were marked as significant.
Results and discussion
The yield for the ethanol-based aloe flower extract was 5.9% (w/w). The phenolics are considered as the main component of the plant, which can suppress free radicals (Debnath et al., Citation2011). The total phenolic content was 17.52 mg GAE per 100 g of dry mass for the ethanol extracts (). The total flavonoid content was found to be 13.20 mg catechin equivalent (CE)/100 g dry mass, expressed in CEs (). Flavonoids are found naturally in fruit, vegetables, and nuts as well as in tea, as a polyphenolic compound with various chemical structures and characteristics (Mcclure, Citation1975). It shows the metal chelating activity and lipid peroxidation inhibition (Cook & Samman, Citation1996). It exhibits antioxidant and anti-inflammatory activities (Middleton, Kandaswami, & Theoharides, Citation2000).
Table 1. The total phenolic and flavonoid contents of ethanol extract of Aloe barbadensis flower.
Then, the ethanol extracts exhibited the suppression of DPPH free radicals in a concentration-dependent manner. The activity varied from 20.4% to 85.0% with the concentrations, ranging from 0.125 to 1.0 mg/mL. The IC50, concentration at which 50% of the DPPH radical was scavenged, was 0.25 mg/mL (). However, the value of the extracts was lower than that of BHT (94.7%) at the concentration of 1.0 mg/mL, described in (P < .05). The scavenging activity for DPPH was well consistent with the phenolic and flavonoid contents with the values of regression factor, r2 of 0.9875 and 0.9781, respectively. The scavenging activity for the ABTS radical varied from 27.8% to 87.4% for the extracts with the variation of concentrations from 0.125 to 1.0 mg/mL. Its IC50 was 0.30 mg/mL. The radical-scavenging activity of the extract was nearly equal to that of BHT (88.7%) at the concentration of 1.0 mg/mL, described in (P > .05). The higher scavenging activity for ABTS could be attributable to high phenolic and flavonoid contents with r2 of 0.99 and 0.9856, respectively (Sayd, Hanan, Taie, & Taha, Citation2010).
Table 2. 1,1-diphenyl-2-picryl-hydrazyl (DPPH) [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt] (ABTS), nitrite, hydroxyl and super oxide scavenging activities (IC50 values), and reducing power activity (EC50) of the extracts of Aloe barbadensis and AA as well as BHT as positive control.
Reducing power (Fe3+ to Fe2+) activity is one of most important role of phenolic compounds (Dorman, Peltoketo, Hiltunen, & Tikkanen, Citation2003). It is an indicator of the antioxidant capacity of natural products. The extracts exhibited Fe3+ reducing power in a dose-dependent (0.12–2.5 mg/mL) manner, showing higher absorbance. The activity of the extract increased from 0.13 to 0.55. However, the observed EC50 of the extract (2.1 mg/mL) was significantly lower (P > .05) than those of the BHT and AA ().
The protein-containing foods and both the leafy and root vegetables contain nitrite and amine compound that can produce nitrosamine. Nitrosamines are responsible for cell damage and cancer (Lee et al., Citation2009). The scavenging activity of the extracts for nitrite was increased with the concentration, ranging from 0.125 to 1.0 mg/mL. The extract showed 38.03% to 55.48% scavenging activities while it was 35.97% to 58.77% for AA. The IC50 values were 0.92 and 0.89 mg/mL for the extracts and AA, respectively, indicating comparable scavenging activity of extracts with AA ().
The hydroxyl and superoxide radicals play a key role in causing diseases including arthritis, ischemia, gastric problems, cancer, and acquired immune deficiency syndrome (Kumpulainen & Salonen, Citation1999). The radicals can react rapidly with foods containing fatty acids, proteins, and sugars (Halliwell, Citation1994). Therefore, the scavenging activities of the extract for these radicals are very important. In an ESR spectrum, the highest peak represents the relative amounts of the DMPO–OH adduct. The flower extracts reduced both the hydroxyl and superoxide radicals from 10% to 60%, and from 20% to 65%, respectively, at the concentration range 0.12–1 mg/ml with the IC50 values 0.92 and 0.85 mg/mL, respectively (). However, the activities of AA were more pronounced for both hydroxyl and superoxide radicals.
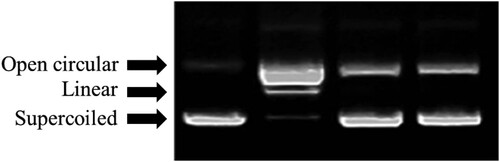
We further determined the protective effect of the extracts against DNA damage induced by H2O2. DNA breaks into supercoiled (SC), open circular, and linear forms (LFs), when it is exposed to hydroxyl radical derived from Fenton reaction. An SC circular DNA comprises 4361 base pairs. This DNA system model has the advantage to damage. It might be easily assessed owing to a simple gel electrophoretic protocol (Bates & Maxwell, Citation2005). shows the protected effect of extracts against free radical-induced DNA damage. Herein, the SC from the DNA was completely converted to the linear form under the treatment of the hydroxyl radical generated from the Fenton reaction (Lane 2), compared with the control (Lane 1). The protection of hydroxyl-induced DNA damage by the extracts was dose dependent manner (Lanes 3–4) for the ethanol extract.
Figure 1. Agarose gel electrophoretic patterns of plasmid DNA breaks by •OH (hydroxyl radical) generated from a Fenton reaction in the presence of ethanol extract from Aloe flower. An amount of 1 μg of pBR 322 DNA was incubated at 37°C for 1 h in 0.04 mM FeSO4 and 30% H2O2 with the following additive combinations: Lane 1, no addition (plasmid DNA control); Lane 2, FeSO4 and H2O2 (DNA damage control); and Lanes 3–4, FeSO4 and H2O2 in the presence of the ethanol extracts Aloe flower with concentrations of 0.5 and 1 mg/mL, respectively.
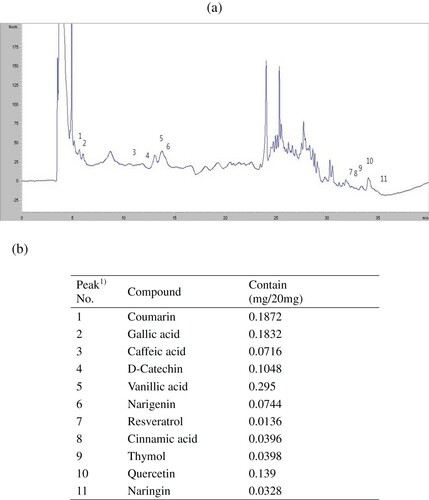
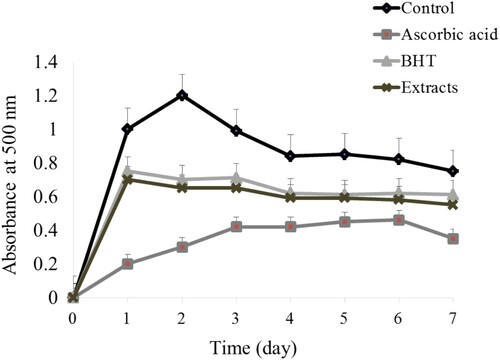
Linoleic acid is an unsaturated fatty acid. It produces peroxide by autooxidation. Here, H2O2 converts Fe2+ to Fe3+. Then, the red-colored ferric thiocyanate is formed. Then, we examined the inhibition of peroxide formation by the extracts up to 7 days. The monitoring absorbance at 500 nm of light, described in , indicates that the auto-oxidation of linoleic acid in control was robust (P < .05) from the 1st day and reached a maximum point on the 3rd day. The activity started to drop from the 4th day. The activity of the extract was comparable to the activity of BHT (P > .05) (). However, the activity of AA was more pronounced than extracts. It is well-known that the polyphenols are able to inhibit the lipid peroxidation (Debnath et al., Citation2011). Therefore, this result suggests that the extract of aloe flowers is capable of dismissing the lipid peroxidation chain reaction. The high inhibition capacity of flower extracts might be attributable to its phenolic contents. Therefore, we further analyse the phenolic constituents of aloe flower extract (). Eleven components were determined by HPLC analysis of the tested extract. The eleven components were identified as coumarin, GA, caffeic acid, D-catechin, vanillic acid, nariqenin, resveratrol, cinnamic acid, thymol, quercetin, and naringin. Among the phenolic components investigated here, content of vanillic acid was highest, which might be associated with its strong antioxidant activities.
Figure 2. Inhibition of linoleic acid oxidation by the ethanol extracts of Aloe flower at various concentrations. Negative control was made instead of test samples. AA and BHT were used as positive control. Statistical comparisons were made between the extracts and positive control versus the negative control (P < .05) and the extracts versus the standard (not significant).
The antioxidant capacity of plants corresponds to its medicinal values. Therefore, the antioxidant activities of the plant extracts are broadly used as important parameters to evaluate its bioavailability as medicinal foodstuffs. However, the one-dimensional analysis is not sufficient to evaluate these properties (Frankel & Meyer, Citation2000). Therefore, to ensure the effectiveness of plant extracts, their antioxidant properties should be verified in different ways. The antioxidant activities were further evaluated by using different methods along with the measurement of its phenolic constituents tested.
The antioxidant enzymes such as SOD, glutathione peroxidase (GPx), and catalase (CAT) are essential to maintain a stable and normal cell function and control its internal environment. The highly reactive superoxide radical can be converted to H2O2 by SOD. Especially, CAT is rich in the liver. It originates in peroxisomes of liver and helps to metabolize H2O2 to H2O and O2. On the other hand, GPx catalyzes the reduction of hydroperoxides such as ROOH and H2O2 (Fridovich, Citation1989; Kono & Fridovich, Citation1982; Kathirvel, Chen, Morgan, French, & Timothy, Citation2010). In the current report, the activities of SOD, CAT, and GPx for sample B (damage) were decreased more compared with that of sample A. Then, these activities were increased for the treatment samples “C” (). It suggested that H2O2 caused oxidative damage in sample B. It is consistent with the report by Wang et al. (Citation2010), in which the oxidative stress induced by H2O2 (0.5 mM) in a normal human liver cell was decreased by the antioxidant enzymes.
Table 3. SOD, GPx, and CAT activities of Aloe barbadensis in mice liver damaged by H2O2.
In conclusion, the aloe ethanol extracts show excellent scavenging activities for DPPH, ABTS, hydroxyl, and superoxide radicals, and have high reducing activity. They inhibited lipid peroxidation and prevented DNA damage. The extracts increased SOD, CAT, and GPx enzymes activities in the liver tissue of hydrogen peroxide-treated BALB/c mice. The extracts had high levels of vanillic acid, which might be responsible for their strong antioxidant activities. These data suggest that the extract is a good source of natural antioxidant.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Trishna Debnath
Trishna Debnath obtained her B.Sc. and M.Sc. degree in Botany from Bangladesh. She obtained her Ph.D. in Advanced Applied Life Science from Konkuk University, Republic of Korea in 2014. Currently she is working as an assistant professor in the Department of Food Science and Biotechnology, Dongguk University, Republic of Korea. Her research interests include functional foods chemistry and immunology.
Mithun Ghosh
Mithun Ghosh has been graduated from University of Gour Banga, West Bengal, India. Currently, he is doing M.Sc. at the Department of Life Science in Konkuk University (Glocal Campus), South Korea. His research interests include functional foods chemistry and drug materials.
Young Min Lee
Young Min Lee has completed his graduation and post-graduation at the Department of Life Science in Konkuk University, Glocal campus. Currently he is doing Ph.D. course in the same University, South Korea. His Interests include functional foods and Medical research.
Narayan Chandra Deb Nath
Narayan Chandra Deb Nath received B.Sc. and M.Sc. degree in chemistry in 2000 and 2002, respectively, from University of Dhaka, Bangladesh. He received Ph.D. in 2013 from Konkuk University, Korea. His current research concentrates on fundamental issues on the development of high efficiency solar cells and its application to artificial photosynthesis, biosensor, and functional foods chemistry.
Kwang-Geun Lee
Kwang-Geun Lee received the B.S. and M.S. degrees in food science and technology from Seoul National University, Korea, in 1991 and 1994, respectively, and the Ph.D. degree in food science from the University of California, Davis, in 2000. His research interests include risk assessments and analysis of food toxins.
Beong Ou Lim
Beoung Ou Lim is an assistant professor at Department of Life Science in Konkuk University, Glocal Campus, South Korea. He earned his master of science from Kyushu University in 1994 and achieved his Ph.D. in Food Chemical Engineering from the same university in the year of 1997. During his Ph.D. he did some noticeable work. Currently he has over 50 publications as a corresponding author. His current research interest includes the food medical bio-compound.
References
- Arunachalam, K., Parimelazhagan, T., & Saravanan, S. (2011). Phenolic content and antioxidant potential of Sarcostigma kleinii Wight. & Arn. Food and Agricultural Immunology, 22, 161–170. doi:https://doi.org/10.1080/09540105.2010.549211
- Bates, A. D., & Maxwell, A. (2005). DNA topology (pp. 26–43). Oxford: Oxford University Press.
- Choi, S. W., Son, B. W., Son, Y. S., Park, Y. I., Lee, S. K., & Chung, M. H. (2001). The wound-healing effect of a glycoprotein fraction isolated from Aloe vera. British Journal of Dermatology, 145, 535–545. doi:https://doi.org/10.1046/j.1365-2133.2001.04410.x
- Cook, N. C., & Samman, S. (1996). Flavonoids-chemistry, metabolism, cardioprotective effects, and dietary sources. The Journal of Nutritional Biochemistry, 7, 66–76. doi:https://doi.org/10.1016/0955-2863(95)00168-9
- Debnath, T., Kim, D. H., & Lim, B. O. (2013). Natural products as a source of anti-inflammatory agents associated with inflammatory bowel disease. Molecules, 18(6), 7253–7270. doi: https://doi.org/10.3390/molecules18067253
- Debnath, T., Kim, E.-K., Nath, N. C. D., & Lee, K.-W. (2017). Therapeutic effects of Ligularia stenocephala against inflammatory bowel disease by regulating antioxidant and inflammatory mediators. Food and Agricultural Immunology. doi:https://doi.org/10.1080/09540105.2017.1332008
- Debnath, T., Park, P. J., Debnath, N. C., Samad, N. B., Park, H. W., & Lim, B. O. (2011). Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chemistry, 128, 697–703. doi:https://doi.org/10.1016/j.foodchem.2011.03.090
- Dorman, H. J. D., Peltoketo, A., Hiltunen, R., & Tikkanen, M. J. (2003). Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chemistry, 83, 255–262. doi:https://doi.org/10.1016/S0308-8146(03)00088-8
- Frankel, E. N., & Meyer, A. S. (2000). The problems of using one dimensional methods to evaluate multifunctional foods and biological antioxidants. Journal of the Science of Food and Agriculture, 80, 1925–1941. doi: https://doi.org/10.1002/1097-0010(200010)80:13<1925::AID-JSFA714>3.0.CO;2-4
- Fridovich, I. (1989). Superoxide dismutases. An adaptation to a paramagnetic gas. The Journal of Biological Chemistry, 264, 7761–7764.
- Gong, M., Wang, F., & Chen, Y. (2002). Study on application of arbuscular-mycorrhizas in growing seedlings of Aloe vera (in Chinese). Journal of Chinese Medicine, 25, 1–3.
- Halliwell, B. (1992). Reactive oxygen species and the central nervous system. Journal of Neurochemistry, 59, 1609–1623. doi:https://doi.org/10.1111/j.1471-4159.1992.tb10990.x
- Halliwell, B. (1994). Free radicals and antioxidants: A personal view. Nutrition Research, 52, 253–265.
- Hu, W., Shen, W., & Wang, M.-H. (2009). Free radical scavenging activity and protective ability of methanolic extract from Duchenea indica against protein oxidation and DNA damage. Journal of Food Science and Nutrition, l4, 277–282.
- Im, K., Lee, J. Y., Byeon, H., Hwang, K. W., Kang, W., Whang, W. K., & Min, H. (2016). In vitro antioxidative and anti-inflammatory activities of the ethanol extract of eggplant (Solanum melongena) stalks in macrophage RAW 264.7 cells. Food and Agricultural Immunology, 27, 758–771. doi:https://doi.org/10.1080/09540105.2016.1150427
- Jeong, C.-H., Choi, G. N., Kim, J. H., Kwak, J. H., Heo, H. J., Shim, K.-H., … Choi, J.-H. (2009). In vitro antioxidant activities and phenolic composition of hot water extract from different parts of Cudrania tricuspidata.Journal of Food Science and Nutrition,14, 283–289.
- Kathirvel, E., Chen, P., Morgan, K., French, S. W., & Timothy, R. (2010). Oxidative stress and regulation of anti-oxidant enzymes in cytochrome P4502E1 transgenic mouse model of non-alcoholic fatty liver. Journal of Gastroenterology and Hepatology, 25, 1136–1143. doi:https://doi.org/10.1111/j.1440-1746.2009.06196.x
- Kono, Y., & Fridovich, I. (1982). Superoxide radicals inhibit catalase. Journal of Biological Chemistry, 257, 5751–5754.
- Kumpulainen, J. T., & Salonen, J. T. (1999). Natural antioxidants and anticarcinogens in nutrition, health and disease (pp. 178–187). London: The Royal Society of Chemistry.
- Lee, J.-H., Choi, E. J., Park, H.-S., & Kim, G. H. (2015). Evaluation of Compositae sp. plants for antioxidant activity, antiinflammatory, anticancer and antiadipogenic activity in vitro. Food and Agricultural Immunology, 25, 104–118. doi: https://doi.org/10.1080/09540105.2012.749394
- Lee, J.-H., Whang, J.-B., Youn, N.-R., Lee, S.-Y., Lee, H.-J., Kim, Y.-J., & Koh, K.-H. (2009). Antioxidant and oxygen radical scavenging capacities of the extracts of pear cactus, mulberry and Korean black raspberry fruits. Journal of Food Science and Nutrition, 14, 188–194.
- Mcclure, J. W. (1975). Physiology and functions of flavonoids. The Flavonoids, 970–1055. doi:https://doi.org/10.1007/978-1-4899-2909-9_18
- Middleton, E. J. R., Kandaswami, C., & Theoharidesl, T. (2000). The Effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer. Pharmacological Reviews, 52, 673–751.
- Samad, N. B., Debnath, T., Jin, H. L., Lee, B. R., Park, P. J., & Lim, B. O. (2013). Antioxidant activity of Benincasa hispida seeds. Journal of Food Biochemistry, 37, 388–395. doi:https://doi.org/10.1111/j.1745-4514.2011.00643.x
- Samad, N. B., Debnath, T., Ye, M., Hasnat, M. A., & Lim, B. O. (2014). In vitro antioxidant and anti-inflammatory activities of Korean blueberry (Vaccinium corymbosum L.) extracts. Asian Pacific Journal of Tropical Biomedicine, 4, 807–815. doi:https://doi.org/10.12980/APJTB.4.2014C1008
- Sathyaprabha, G., Kumaravel, S., Ruffina, D., & Praveenkumar, P. A. (2010). Comparative study on antioxidant, proximate analysis, antimicrobial activity and phytochemical analysis of Aloe vera and Cissus quadrangularis by GC-MS. Journal of Pharmacy Research, 3, 2970–2973.
- Sayd, S. S., Hanan, A. A., Taie, H. A. A., & Taha, L. S. (2010). Micropropagation, antioxidant activity, total phenolics and flavonoids content of Gardenia jasminoides ELLIS as affected by growth regulators. International Journal of Academic Research, 2, 184–191.
- Surjushe, A., Vasani, R., & Saple, D. G. (2008). Aloe vera: A short review. Indian Journal of Dermatology, 53, 163–166. doi:https://doi.org/10.4103/0019-5154.44785
- Wang, M., Ma, H. L., Liu, B., Wang, H. B., Xie, H., Li, R. D., & Wang, J. F. (2010). Pinus massoniana bark extract protects against oxidative damage in L-02 hepatic cells and mice. The American Journal of Chinese Medicine, 38, 909–919. doi:https://doi.org/10.1142/S0192415X10008342
- Yoo, E. A., Kim, S. D., Lee, W. M., Park, H. J., Kim, S. K., Cho, J. Y., … Rhee, M. H. (2008). Evaluation of antioxidant, antinociceptive, and anti-inflammatory activities of ethanol extracts from Aloe saponaria Haw. Phytotherapy Research, 22, 1389–1395. doi:https://doi.org/10.1002/ptr.2514