ABSTRACT
The aim of this study was to evaluate the immunoglobulin E (IgE) sensitivity to common pandora (Pagellus erythrinus) parvalbumin (CPP) in a Moroccan population from the Fez region, and then to study the effect of temperature and enzymatic digestion on the allergenicity of CPP. This work was conducted with a questionnaire completed by a sera-bank, obtained from 500 patients recruited from Fez hospitals. Their sera were analyzed for specific IgE against CPP. Evaluation of specific IgE showed that 11.8% of patients present higher values (>150 IU/ml). Further indirect ELISA and dot-blot results indicated that CPP showed a decrease in the binding of anti-IgE under heating with an average diminution of 41.9%, while pepsin hydrolysis reduced IgE recognition by 22.9%. These results demonstrate that this population was sensitive to CPP and the sensitivity could be reduced by heating and pepsin hydrolysis with an action higher with temperature than enzymatic digestion processing.
Introduction
Fish is a valuable source of proteins containing physiologically active substances such as eicosapentaenoic acid and docosahexaenoic acid, and minerals such as calcium. Thus, fish plays an important role in human nutrition and health. In Morocco, a large variety of fish are consumed. Common pandora, Pagellus erythrinus, is one of the most widely consumed fish species in Morocco. It is a demersal marine fish species of the Sparidae family. Common pandora is widely distributed in the Mediterranean, along the European and African coasts of the Atlantic Ocean and it is one of the main target species of commercial fisheries and a promising candidate for Mediterranean marine aquaculture (Klimogianni, Koumoundouros, Kaspiris, & Kentouri, Citation2004). However, fish allergy, mediated by immunoglobulin E (IgE), is becoming a serious problem worldwide, especially in coastal regions. In neighboring country like Spain, fish was the third most common food sensitivity (after egg and cow’s milk) (Pascual et al., Citation2008). In Morocco, previous works by our laboratory showed that 9.8% of 500 patients, recruited from Fez hospitals, reported adverse reactions to fish and shellfish, while 10.2% of patients had a high specific IgE to shrimp tropomyosin (>150 IU/ml) (Mejrhit, Azdad, Chda et al., Citation2017). In addition, other study by our laboratory concerning sardine parvalbumin (SP) found that 7.8% of 1008 patients present specific IgE to SP values higher than >150 IU/ml (Mejrhit, Azdad, El Kabbaoui et al., Citation2017). However, to our knowledge, there are neither previous publications nor thesis that have been conducted or focused on a common pandora allergy.
Among the various allergens characterized in fish, parvalbumin, a calcium-binding protein, has been recognized as the major allergen (Beale, Jeebhay, & Lopata, Citation2009; Bugajska-Schretter et al., Citation1998). It is a globular protein of about 12 kDa in size and is abundant in fish muscle (Girija & Rehbein, Citation1988). Research had demonstrated that more than 95% of fish-allergic patients have been found to have specific IgE to this protein and many of the IgE-binding epitopes on this allergen were present in various fish species (Bugajska-Schretter et al., Citation1998; de Martino et al., Citation1990).
In order to reduce the risk of fish allergy, various food processing techniques described in the literatures such as heating, enzyme hydrolysis and pH can influence the allergenic potential of food proteins. These modifications may increase or decrease the allergenicity of proteins, depending on the severity of the treatment (Bousfiha & Aarab, Citation2013; Cabanillas et al., Citation2012; Ouahidi, Aarab, & Dutau, Citation2010; Ouahidi, El Hamsas, & Aarab, Citation2011; Thomas et al., Citation2007; Wang et al., Citation2014; Xu, Shi, Yao, Jiang, & Luo, Citation2016).
The aim of this study was first to evaluate the IgE sensitivity to common pandora parvalbumin (CPP) in a Moroccan population from the Fez region, and then to study the effect of temperature and enzymatic digestion on the allergenicity of CPP.
Material and methods
Human sera
Human sera were collected from 500 volunteers at the University Hospital Centre of Fez and Fez laboratories, who had come for different medical tests. The sample was composed of 320 females (64%) and 180 males (36%). There were 82.2% patients, represented by adults, aged between 20 and 60 years, and 17.8%, represented by children, aged between 1 and 19 years. A questionnaire was provided to all patients concerning food allergy characteristics and fish allergy in particular. Patients were asked to provide information on the species they tried, the presence of allergic symptoms and types of fish allergy (sardine, mackerel, common pandora, whiting, shrimps, etc.). This work was conducted from May 2014 to June 2015 and was approved by the Ethic committee of the University Hospital Center of Fez. After formal consent of the patients, a blood sample of 3 ml was collected in a dry tube. After centrifugation at 3000 rpm during 5 min, sera were separated and stored at −20°C until use.
Extraction of CPP
CPP was extracted as previously described, with modifications (Hamada, Nagashima, et al., Citation2003; Hamada, Tanaka, et al., Citation2003). Briefly, the raw common pandora muscle was defatted with chloroform to remove lipids. Once this preparation was filtered, the powder was dried overnight at room temperature. The samples were subsequently extracted with 10% of 0.15 mol/l NaCl in 0.01 mol/l phosphate buffer (pH 7.0), and boiled for 10 min. The heated extract was filtered and centrifuged at 3000 rpm for 15 min. Then, the resulting supernatants were dialyzed against distilled water overnight at 4°C. The dialyzed proteins were stored at –20°C until use for subsequent experiments. Extract quality was confirmed by means of sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS-PAGE).
Treatment of extracted CPP with heating and enzymatic digestion
Extracted CPP (0.5 mg/ml) was exposed to different temperatures (70°C, 80°C and 90°C) for various times (30, 60 and 120 min). For enzymatic treatment, CPP was digested with pepsin (30 μg/ml) in an acid environment (pH 2) for 30, 60 and 120 min at 37°C. Thereafter, CPP treated was deposited into a 96-well microplate and human IgE binding was evaluated by ELISA and dot-blot assay.
Estimation of specific IgE
Specific IgE binding to CPP was assayed by indirect ELISA as previously described (Bousfiha & Aarab, Citation2013; Ouahidi et al., Citation2010, Citation2011). Briefly, 100 µl of CPP (0.5 mg/ml) was deposited per well in 96-well microplates and incubated for 60 min at 37°C. Then, 200 µl of 0.5% bovine serum albumin (BSA) was added to every well for an hour at 37°C. After removal of BSA, human sera were added (100 µl/well) before incubation with goat anti-human IgE peroxidase conjugate for 60 min at 37°C. Binding of anti-IgE was revealed by adding 100 µl of 0.05% orthophenylenediamine. The reaction was stopped by adding 3 M HCl. Then the developed color was measured by absorbance at 490 nm.
SDS-PAGE analysis
SDS-PAGE was performed according to the method described by Laemmli (Citation1970). Protein samples (100 μl per well) were mixed with loading buffer (10% SDS, 10% glycerol, 10% β-mercaptoethanol and 2.5% bromphenol blue), heated at 100°C (5 min), electrophoresed in 15% analytical SDS-polyacrylamide gels, and resolved by SDS-PAGE. Proteins were stained using Coomassie Brilliant Blue R-250.
The dot-blot assay
Dot-blot assay was performed as previously described (Cai et al., Citation2010; Zheng, Lin, Pawar, Li, & Li, Citation2011) with some modifications. In brief, purified CPP was spotted onto nitrocellulose membranes (5 µl for each dot) and left to dry at room temperature 37°C. The spotted membranes were incubated for 1 h at 37°C in blocking buffer BBST (2.5% Tween in borate-buffered saline, pH 7.4) to block the non-specific binding sites. After washing with BBST/0.1% for 5 min, the membranes were then incubated with human sera overnight (diluted 1:2 with BBS). After incubation for 1 h with anti-IgE peroxidase conjugate (diluted 1:1000 with BBS), the reaction was revealed visually by the incubation of membranes in a solution containing 0.05% of diaminobenzidine in BBS buffer. The intensity of spot indicates the reactivity of specific IgE to CPP.
Statistical analysis
Descriptive statistics were presented as numbers with percentages or as average values. Statistical analysis was based on the Student’s t-test taking p < .05 as the limit of the significant value. The results are expressed as mean ± SEM (standard errors of mean), and the SEM was calculated by the following relation: SEM = SD (standard deviation)/√ (sample size). All statistical analyses were performed using Epi Info™ version 7.2.0.1.
Results
Reported adverse food reactions
The self-reported food adverse reaction, evaluated in this population, was 14.8%. It was mostly associated with fish and shellfish (11.8%), followed by eggs (4.4%) and milk (2.8%). Regarding fish and shellfish (), we observed sardine (11%) was the most common species causing adverse reactions in patients, followed by horse mackerel (7.2%), mackerel (6.4%), whiting (2.2%) and common pandora (2%). From the 11.8% (n = 59) who reported fish/shellfish sensitivity, 64% had multiple fish sensitivities. As regard age and sex, self-reported fish sensitivity was higher in adults (12.4%) than in children (9%) and higher in female (13%) than in male (9.4%). Regarding the results of self-reported common pandora sensitivity, we noted that the prevalence was most marked in adults (90%) than in children (10%) as well as in female (80%) than in male (20%). The most frequent clinical signs were cutaneous reactions (98%) and respiratory symptoms (30%).
Table 1. Demographic characteristics of the study population.
Specific IgE measurement
Sera of 500 patients have been tested for specific IgE binding to CPP. Dosage of specific IgE to CPP indicated that the average of positive values (>2 IU/ml) was 97.3 IU/ml (n = 261) vary between 2.75 and 302.75 IU/ml. From them, 21.2% of patients present values higher than 100 IU/ml and 11.8% concerning the values higher than 150 IU/ml (). According to age and sex, the results showed a higher prevalence in female (13.7%) than in male (8.3%) and in children (13.5%) than in adults (11.4%) with a rate higher than 150 IU/ml.
Table 2. Distribution of IgE Levels among the study population.
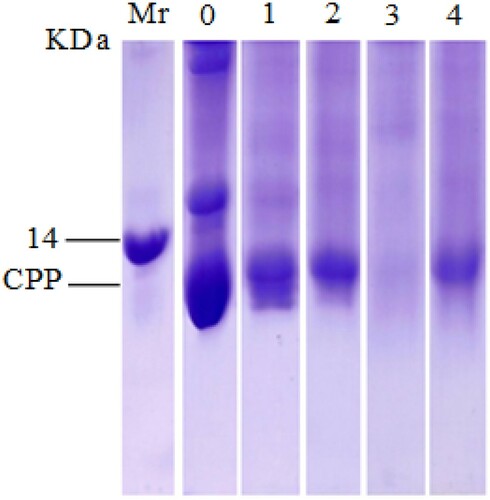
SDS-PAGE analysis of CPP
Protein profiles of untreated CPP and CPP treated by heating at 90°C and pepsin hydrolysis during 1 h were analyzed by SDS-PAGE. As shown in , parvalbumin proteins appeared as a band corresponding to a molecular mass of 12 kDa, which is considered to be the major allergen in fish. After heating at 90°C for 1 h, the SDS-PAGE profile showed the presence of 12 kDa CPP band in lane 2, but with a slight decrease in their intensity compared with the original band extract (lane 1). Concerning the effect of pepsin hydrolysis, no clear protein band appeared in lane 3, and the intensity of the CPP band almost disappeared. By contrast, when CPP was exposed to both treatments (heating followed by pepsin hydrolysis), we noted a CPP band in lane 4, but with a weaker intensity than that with heating effect.
Figure 1. SDS-PAGE analysis of untreated CPP and CPP treated by heating and pepsin.
Notes: Mr, markers of the molecular weights; 0, total proteins of common pandora extracted in phosphate buffer solution (10%, pH 7.4); 1, extracted native CPP; 2, CPP treated by temperature; 3, CPP treated by pepsin and 4, CPP treated by the combination of two treatments.
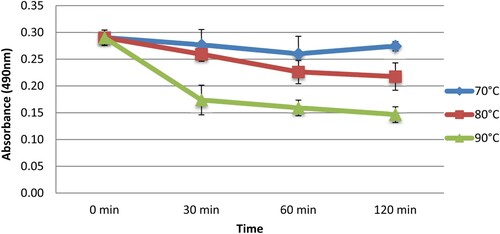
Effect of heating and enzymatic digestion on the detection of CPP by rabbit IgG using ELISA and dot-blot assays
The variation in immunoreactivity of CPP after heating and enzymatic digestion was firstly assessed by using rabbit IgG anti-CPP. The aim was to determine the parameters of reduction of the CPP binding to specific antibodies by using ELISA and dot-blot assays.
Results showed that the IgG binding to CPP was decreased with the increase in heating temperature and time (). The maximum reduction observed was 45% when CPP was heated at 90°C. Concerning the effect of enzymatic treatment (), we noticed that CPP pepsin hydrolysis altered the binding of IgG to CPP with a maximum reduction of 69%. Furthermore, pepsin hydrolysis combined with thermal treatment at 90°C completely blocked the IgG binding to CPP. The maximum reduction observed was 69.2%. This decrease in reactivity was confirmed by dot-blot analysis (). The dot-blot assay achieved by the anti-CPP rabbit IgG showed the presence of a spot corresponding to parvalbumin, indicating the reactivity of rabbit IgG toward CPP. However, the intensity of the blotting spot was reduced when CPP was heated at 90°C, while pepsin hydrolysis (alone or in combination with heating) has removed this reactivity.
Effect of heating and enzymatic treatments on human IgE binding to CPP using ELISA and dot-blot assays
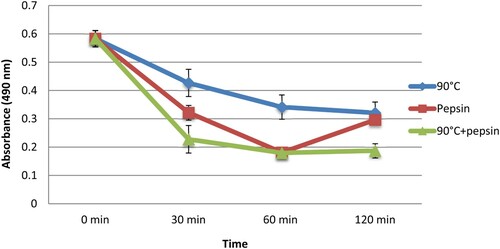
Human sera were analyzed for their IgE sensitivity to CPP. Human sera of 20 patients with higher IgE levels (>100 IU/ml) were selected and used to estimate the binding variation of IgE to CPP under temperature at 90°C and enzymatic treatment (during 1 h) using ELISA () and dot-blot assays ().
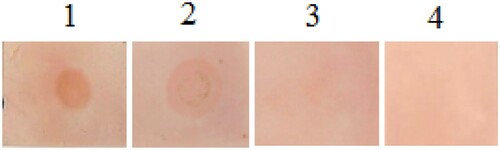
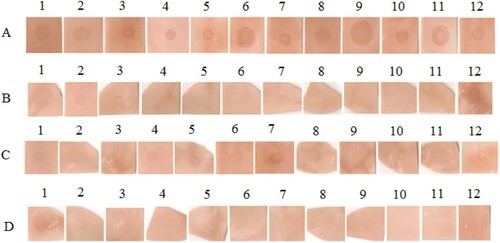
Figure 6. Results of dot-blot assay with sera of 12 patients.
Notes: (A) dot-blot with native CPP; (B) dot-blot with CPP treated by temperature; (C) dot-blot with CPP treated by pepsin and (D) dot-blot with CPP treated by the combination of two treatments.
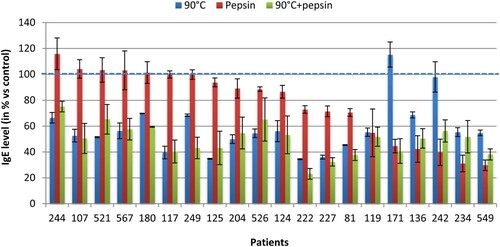
Under heat treatment (), we observed that 19 of 20 patients showed a decrease in the IgE binding to protein CPP from 2.1% to 65.6%, with an average diminution of 41.9%. In contrast, we noticed a small increase in recognition under this treatment in one patient. Where CPP was treated by pepsin, we observed for 15 patients a reduction in their IgE recognition varying from 0.2% to 70.3% with an average of 22.9%, while we showed in 5 patients a slight increase in recognition by this treatment. When the two treatments were combined (heating 90°C followed by pepsin), we observed for all patients an average diminution in the IgE binding to CPP of 50.5%. This reduction varied from 24.9% to 76.9%.
In the blotting assays, the sera from 12 patients with high IgE levels were used to recognize CPP. As shown in , results noted that all the sera tested detected the spots, indicating their reactivity toward native CPP. After heating at 90°C, the intensity of the blotting spots was decreased in all patients tested. Under digestion with pepsin, the intensity of the spots was also reduced in 11 patients, but it seems that IgE binding capacity of heated CPP was more decreased than that of CPP pepsin hydrolysis. When the two treatments were combined, the IgE reactivity of CPP was eliminated almost completely, as no spot was detected in all patients tested.
Discussion
The objective of this work was to evaluate the IgE sensitivity to CPP in a Moroccan population from the Fez region, and then to study the effect of temperature and enzymatic digestion on the allergenicity of CPP. This study was conducted with a questionnaire completed by a sera-bank, obtained from 500 patients recruited from Fez hospitals. These patients were questioned and their sera analyzed for specific IgE against CPP.
Results indicated that self-reported adverse reaction to fish and shellfish, evaluated in our population, was of 11.8%. By specific IgE measurement, we found that 11.8% of all patients tested had specific IgE to CPP level greater than 150 IU/ml. According to age, results showed a discrepancy between self-reported fish sensitivity and specific IgE evaluation. In this case, children (13.5%) presented a high specific IgE to CPP compared with adults (11.4%), whereas the prevalence of self-reported adverse reaction to fish and shellfish was higher in adults (12.4%) than in children (9%). This variation was also observed in others studies where researchers have used different methods to detect food sensitivity in patients, suggesting that the use of IgE measurements may be helpful and added value to the diagnosis of food sensitivity (Čelakovská, Ettlerová, Ettler, Vaněčková, & Bukač, Citation2015; Erwin et al., Citation2010; Lim et al., Citation2008; Perez-Gordo et al., Citation2013; Yang et al., Citation2010).
The sera with higher IgE levels were selected and used to estimate the binding variation of IgE to CPP under heating at 90°C and pepsin hydrolysis. Dot-blot and indirect ELISA were used to analyze the human IgE binding to CPP. Concerning the results of heat treatment (90°C), the SDS-PAGE profile showed a parvalbumin band in heated extract CPP, but with a slight decrease in their intensity compared with the original band. Previous studies with SDS-PAGE showed the presence of parvalbumin in heated extracts from various fish species (Kubota, Kobayashi, Kobayashi, Shiomi, & Hamada-Sato, Citation2016; Saptarshi, Sharp, Kamath, & Lopata, Citation2014). On the other hand, when IgE immunoreactivity to heated CPP was analyzed by ELISA and dot-blotting, we noted that 95% of patients showed a high reduction in the human IgE binding to CPP, with an average diminution of 41.9%. These results were similar to previous works studying the effect of heating (>80°C) on the IgE reactivity of parvalbumin extracted from various fish species (Cai et al., Citation2010; Li, Jiang, You, Luo, & Feng, Citation2014; Saptarshi et al., Citation2014; Shibahara, Uesaka, Wang, Yamada, & Shiomi, Citation2013). Interestingly, a recent study by Kubota et al. (Citation2016) found that the IgE reactivity of Pacific mackerel parvalbumin is linearly reduced with the increase in heating temperature, suggesting that more than 50% reduction of IgE reactivity was caused by heating at 80°C and almost complete loss of IgE reactivity by heating at 140°C. Furthermore, other studies by our laboratory showed that the IgE-binding activity to SP was decreased at 90°C in 18/20 patients tested with a maximum reduction of 54.5% (Mejrhit, Azdad, El Kabbaoui et al., Citation2017). These works confirm our finding and indicate that heating is effective to reduce the allergenicity of fish parvalbumin. This allowed us to consider that CPP undergoes conformational changes depending on the heating load, resulting in a decrease in the binding ability to the CPP antibody. This suggests that the CPP epitopes recognized by human IgE are almost conformational.
By treatment with pepsin hydrolysis, results of the SDS-PAGE profile have shown that pepsin hydrolysis altered the CPP protein, resulting in the absence of the CPP band after this treatment. Similar results have been obtained in the research of caviar parvalbumin in digestion conditions by pepsin at pH 2 for 30 s (Untersmayr et al., Citation2003). Concerning the effect of pepsin hydrolysis on the allergenicity of CPP, we noticed that 75% of patients showed a diminution in their IgE binding to CPP with an average reduction of 22.9%. These results were confirmed by dot-blot assay, showing that the intensity of spots became weak in 11 patients tested. Several studies are consistent with our findings which indicate the reduction of the allergenicity of fish parvalbumin by enzymatic digestion processing (Cai et al., Citation2010; De Jongh et al., Citation2013; Mejrhit, Azdad, El Kabbaoui et al., Citation2017; Untersmayr et al., Citation2003). Those results suggest that the CPP epitopes recognized by human IgE are partially conformational, while remaining binding of pepsin digested CPP to human IgE indicated that a large part of epitopes involved were sequential. However, 25% of patients showed a slight increase in their IgE binding to CPP digested by pepsin compared with native CPP, which is probably due to the development of new epitopes likely to be sequential sites and hence the increase in the allergenicity of CPP.
When the two treatments were combined (heating followed by pepsin), the SDS-PAGE profile showed a parvalbumin band in treated CPP, but their intensity appeared less prominent in comparison with the native and heated one. Whereas when CPP was treated by pepsin alone, the CPP band disappeared completely and faded away as explained above. It can be assumed that the aggregation of denatured particles or intermolecular association during heating processing, as a route toward refolding to the native form, prevents the access of pepsin to CPP cleavage sites and protect CPP against degradation by pepsin. Therefore, the aggregation formation might explain the resistance of CPP against pepsin digestion after heating. These suggestions are in agreement with those reported recently by Zhao et al. (Citation2017) and others authors (De Jongh, Taylor, & Koppelman, Citation2011) who work on the structural and immunological properties of parvalbumin. Furthermore, previous works studying the effect of heating on ovalbumin protein and whey proteins found that heat induces denaturation and aggregation of proteins (Oldfield, Singh, Taylor, & Pearce, Citation1998; Weijers, Barneveld, Stuart, Martien, & Visschers, Citation2003). On the other hand, results of ELISA demonstrated that the IgE recognition of CPP was strongly diminished for all patients, with an average diminution of 50.5%. The same decrease was obtained with dot-blot assay, whose results showed that the intensity of spots almost disappeared in all patients. These results suggest that the combination of treatments may cause an addition of the inhibitory effects of temperature and enzymatic digestion in nine patients (107, 180, 249, 124, 222, 227, 81, 119 and 171), which indicate that heating followed by pepsin hydrolysis may destroy more antibody conformational epitopes of CPP. While in other patients, we noted that the combination of treatments showed an increase in their IgE reactivity compared with pepsin alone or heating-alone effects, which may due to the generation of new epitopes, as a result of aggregation of pepsin/heating generated peptides during combination treatments leading to an increase in IgE reactivity.
In conclusion, these results demonstrate that the Moroccan population reported sensitivity to CPP was confirmed by specific IgE measurement. Our study has shown that CPP showed a reduction in their immunoreactivity with human IgE under heating and pepsin hydrolysis with an action higher with temperature than enzymatic digestion processing.
Acknowledgements
We would like to thank the University Hospital Center of Fez as well as the laboratories of Fez for their assistance in finding patients. This work was supported by grants of the Moroccan National Center for Scientific and Techniques Research (CNRST) to Najlae Mejrhit.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Najlae Mejrhit
Najlae Mejrhit is a PhD student in biotechnology and food safety, she is student at the Faculty of Sciences & Techniques of the University Sidi Mohamed Ben Abdellah, Fez, Morocco, Laboratory of Bioactive Molecules (LMBSF).
Ouarda Azdad
Ouarda Azdad is a PhD student in biotechnology and food safety, she is student at the Faculty of Sciences & Techniques of the University Sidi Mohamed Ben Abdellah, Fez, Morocco, Laboratory of Bioactive Molecules (LMBSF).
Lotfi Aarab
Lotfi Aarab is a PhD in immunology and food safety. Presently, he is working as a professor and researcher at the Faculty of Sciences & Techniques of the University Sidi Mohamed Ben Abdellah, Fez, Morocco, Laboratory of Bioactive Molecules (LMBSF). His research interest includes food allergy and food safety.
References
- Beale, J. E., Jeebhay, M. F., & Lopata, A. L. (2009). Characterisation of purified parvalbumin from five fish species and nucleotide sequencing of this major allergen from Pacific Pilchard, Sardinops sagax. Molecular Immunology, 46(15), 2985–2993. doi: https://doi.org/10.1016/j.molimm.2009.06.018
- Bousfiha, A., & Aarab, L. (2013). Effect of heat and enzymatic treatments on human IgE and rabbit IgG sensitivity to white bean allergens. Iranian Journal of Allergy, Asthma and Immunology, 12(4), 304–311.
- Bugajska-Schretter, A., Elfman, L., Fuchs, T., Kapiotis, S., Rumpold, H., Valenta, R., & Spitzauer, S. (1998). Parvalbumin, a cross-reactive fish allergen, contains IgE-binding epitopes sensitive to periodate treatment and Ca2+ depletion. Journal of Allergy and Clinical Immunology, 101(1), 67–74. doi: https://doi.org/10.1016/S0091-6749(98)70195-2
- Cabanillas, B., Maleki, S. J., Rodríguez, J., Burbano, C., Muzquiz, M., Jiménez, M. A., … Crespo, J. F. (2012). Heat and pressure treatments effects on peanut allergenicity. Food Chemistry, 132(1): 360–366.
- Cai, Q. F., Liu, G. M., Li, T., Hara, K., Wang, X. C., Su, W. J., & Cao, M. J. (2010). Purification and characterization of parvalbumins, the major allergens in red stingray (Dasyatis akajei). Journal of Agricultural and Food Chemistry, 58(24), 12964–12969. doi: https://doi.org/10.1021/jf103316h
- De Jongh, H. H., Robles, C. L., Timmerman, E., Nordlee, J. A., Lee, P. W., Baumert, J. L., … Koppelman, S. J. (2013). Digestibility and IgE-binding of glycosylated codfish parvalbumin. BioMed Research International, 2013, 1–10. doi: https://doi.org/10.1155/2013/756789
- De Jongh, H. H., Taylor, S. L., & Koppelman, S. J. (2011). Controlling the aggregation propensity and thereby digestibility of allergens by Maillardation as illustrated for cod fish parvalbumin. Journal of Bioscience and Bioengineering, 111(2), 204–211. doi: https://doi.org/10.1016/j.jbiosc.2010.09.015
- de Martino, M., Novembre, E., Galli, L., de Marco, A., Botarelli, P., Marano, E., & Vierucci, A. (1990). Allergy to different fish species in cod-allergic children: In vivo and in vitro studies. Journal of Allergy and Clinical Immunology, 86(6), 909–914. doi: https://doi.org/10.1016/S0091-6749(05)80154-X
- Čelakovská, J., Ettlerová, K., Ettler, K., Vaněčková, J., & Bukač, J. (2015). Evaluation of allergy to soy in patients with atopic dermatitis older than 14 years of age. Food and Agricultural Immunology, 26(1), 60–70. doi: https://doi.org/10.1080/09540105.2013.864604
- Erwin, E. A., James, H. R., Gutekunst, H. M., Russo, J. M., Kelleher, K. J., & Platts-Mills, T. A. (2010). Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Annals of Allergy, Asthma & Immunology, 104(6), 496–502. doi: https://doi.org/10.1016/j.anai.2010.03.018
- Girija, N., & Rehbein, H. (1988). Comparison of parvalbumin patterns from different fish species by isoelectric focusing of muscle extracts. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 91(4), 723–728. doi: https://doi.org/10.1016/0305-0491(88)90199-X
- Hamada, Y., Nagashima, Y., Shiomi, K., Shimojo, N., Kohno, Y., Shibata, R., … Ikezawa, Z. (2003). Reactivity of IgE in fish-allergic patients to fish muscle collagen. Allergology International, 52(3), 139–147. doi: https://doi.org/10.1046/j.1440-1592.2003.00293.x
- Hamada, Y., Tanaka, H., Ishizaki, S., Ishida, M., Nagashima, Y., & Shiomi, K. (2003). Purification, reactivity with IgE and cDNA cloning of parvalbumin as the major allergen of mackerels. Food and Chemical Toxicology, 41(8), 1149–1156. doi: https://doi.org/10.1016/S0278-6915(03)00074-7
- Klimogianni, A., Koumoundouros, G., Kaspiris, P., & Kentouri, M. (2004). Effect of temperature on the egg and yolk-sac larval development of common pandora, Pagellus erythrinus. Marine Biology, 145(5), 1015–1022. doi: https://doi.org/10.1007/s00227-004-1382-y
- Kubota, H., Kobayashi, A., Kobayashi, Y., Shiomi, K., & Hamada-Sato, N. (2016). Reduction in IgE reactivity of Pacific mackerel parvalbumin by heat treatment. Food Chemistry, 206, 78–84. doi: https://doi.org/10.1016/j.foodchem.2016.03.043
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. doi: https://doi.org/10.1038/227680a0
- Li, Z., Jiang, M., You, J., Luo, Y., & Feng, L. (2014). Impact of Maillard reaction conditions on the antigenicity of parvalbumin, the major allergen in grass carp. Food and Agricultural Immunology, 25(4), 486–497. doi: https://doi.org/10.1080/09540105.2013.838943
- Lim, D. L. C., Neo, K. H., Yi, F. C., Chua, K. Y., Goh, D. L. M., Shek, L. P. C., … Lee, B. W. (2008). Parvalbumin the major tropical fish allergen. Pediatric Allergy and Immunology, 19(5), 399–407. doi: https://doi.org/10.1111/j.1399-3038.2007.00674.x
- Mejrhit, N., Azdad, O., Chda, A., El Kabbaoui, M., Bousfiha, A., Bencheikh, R., … Aarab, L. (2017). Evaluation of the sensitivity of Moroccans to shrimp tropomyosin and effect of heating and enzymatic treatments. Food and Agricultural Immunology, 1–12.
- Mejrhit, N., Azdad, O., El Kabbaoui, M., Ouahidi, I., Tazi, A., & Aarab, L. (2017). Sensitivity of Moroccans to sardine parvalbumin and effect of heating and enzymatic treatments. Food and Agricultural Immunology, 1–12.
- Oldfield, D. J., Singh, H., Taylor, M. W., & Pearce, K. N. (1998). Kinetics of denaturation and aggregation of whey proteins in skim milk heated in an ultra-high temperature (UHT) pilot plant. International Dairy Journal, 8(4), 311–318. doi: https://doi.org/10.1016/S0958-6946(98)00089-2
- Ouahidi, I., Aarab, L., & Dutau, G. (2010). Influence des traitements thermiques et acides sur l’allergénicité des protéines d’arachide au niveau de la population de la région Fès-Meknès au Maroc. Revue Française D’Allergologie, 50(1), 15–21. doi: https://doi.org/10.1016/j.reval.2009.08.008
- Ouahidi, I., El Hamsas, A. E. Y., & Aarab, L. (2011). Modulation of egg white protein allergenicity under physical and chemical treatments. Food and Agricultural Immunology, 22(1), 57–68. doi: https://doi.org/10.1080/09540105.2010.526202
- Pascual, C. Y., Reche, M., Fiandor, A., Valbuena, T., Cuevas, T., & Esteban, M. M. (2008). Fish allergy in childhood. Pediatric Allergy and Immunology, 19(7), 573–579. doi: https://doi.org/10.1111/j.1399-3038.2008.00822.x
- Perez-Gordo, M., Pastor-Vargas, C., Lin, J., Bardina, L., Cases, B., Ibáñez, M. D., … Sampson, H. A. (2013). Epitope mapping of the major allergen from Atlantic cod in Spanish population reveals different IgE-binding patterns. Molecular Nutrition & Food Research, 57(7), 1283–1290. doi: https://doi.org/10.1002/mnfr.201200332
- Saptarshi, S. R., Sharp, M. F., Kamath, S. D., & Lopata, A. L. (2014). Antibody reactivity to the major fish allergen parvalbumin is determined by isoforms and impact of thermal processing. Food Chemistry, 148, 321–328. doi: https://doi.org/10.1016/j.foodchem.2013.10.035
- Shibahara, Y., Uesaka, Y., Wang, J., Yamada, S., & Shiomi, K. (2013). A sensitive enzyme-linked immunosorbent assay for the determination of fish protein in processed foods. Food Chemistry, 136(2), 675–681. doi: https://doi.org/10.1016/j.foodchem.2012.08.066
- Thomas, K., Herouet-Guicheney, C., Ladics, G., Bannon, G., Cockburn, A., Crevel, R., … Vieths, S. (2007). Evaluating the effect of food processing on the potential human allergenicity of novel proteins: International workshop report. Food and Chemical Toxicology, 45(7), 1116–1122. doi: https://doi.org/10.1016/j.fct.2006.12.016
- Untersmayr, E., Schöll, I., Swoboda, I., Beil, W. J., Förster-Waldl, E., Walter, F., … Boltz-Nitulescu, G. (2003). Antacid medication inhibits digestion of dietary proteins and causes food allergy: A fish allergy model in BALB/c mice. Journal of Allergy and Clinical Immunology, 112(3), 616–623. doi: https://doi.org/10.1016/S0091-6749(03)01719-6
- Wang, Z., Li, L., Yuan, D., Zhao, X., Cui, S., Hu, J., & Wang, J. (2014). Reduction of the allergenic protein in soybean meal by enzymatic hydrolysis. Food and Agricultural Immunology, 25(3), 301–310. doi: https://doi.org/10.1080/09540105.2013.782268
- Weijers, M., Barneveld, P. A., Stuart, C., Martien, A., & Visschers, R. W. (2003). Heat-induced denaturation and aggregation of ovalbumin at neutral pH described by irreversible first-order kinetics. Protein Science, 12(12), 2693–2703. doi: https://doi.org/10.1110/ps.03242803
- Xu, Q., Shi, J., Yao, M., Jiang, M., & Luo, Y. (2016). Effects of heat treatment on the antigenicity of four milk proteins in milk protein concentrates. Food and Agricultural Immunology, 27(3), 401–413. doi: https://doi.org/10.1080/09540105.2015.1117059
- Yang, A. C., Arruda, L. K., Santos, A. B. R., Barbosa, M. C., Chapman, M. D., Galvão, C. E., … Morato-Castro, F. F. (2010). Measurement of IgE antibodies to shrimp tropomyosin is superior to skin prick testing with commercial extract and measurement of IgE to shrimp for predicting clinically relevant allergic reactions after shrimp ingestion. Journal of Allergy and Clinical Immunology, 125(4), 872–878. doi: https://doi.org/10.1016/j.jaci.2009.11.043
- Zhao, Y. J., Cai, Q. F., Jin, T. C., Zhang, L. J., Fei, D. X., Liu, G. M., & Cao, M. J. (2017). Effect of maillard reaction on the structural and immunological properties of recombinant silver carp parvalbumin. LWT – Food Science and Technology, 75, 25–33. doi: https://doi.org/10.1016/j.lwt.2016.08.049
- Zheng, L. N., Lin, H., Pawar, R., Li, Z. X., & Li, M. H. (2011). Mapping IgE binding epitopes of major shrimp (Penaeus monodon) allergen with immunoinformatics tools. Food and Chemical Toxicology, 49(11), 2954–2960. doi: https://doi.org/10.1016/j.fct.2011.07.043