?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Vitamin B2 (riboflavin) is a water-soluble vitamin that has important roles in human health. In this study, we developed a sensitive and specific monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) and lateral-flow immunochromatographic assay (ICA) strip for the rapid detection of vitamin B2. Following routine fusion and selection, the optimum monoclonal antibody against vitamin B2 was obtained. The 50% inhibitory concentration and limit of detection of ic-ELISA were 8.18 and 1.80 ng/mL, respectively. The cut-off value of the lateral-flow ICA strip was 50 ng/mL. The results revealed that our developed methods are suitable for the on-site detection and mass screening of vitamin B2 in food and pharmaceutical products.
Introduction
Vitamin B2, or riboflavin, is one of the most widely distributed B vitamins, which participates in several cellular processes. Vitamin B2 plays vital roles in biological systems such as cellular respiration and metabolism of fats, carbohydrates, and proteins (Basaranoglu et al., Citation2017; Henriques, Olsen, Bross, & Gomes, Citation2010; Lewicka et al., Citation2017; Powers, Citation2003). Vitamin B2 is present in a wide variety of animal and plant foods, such as liver, dairy products, and leafy vegetables. Low intake of dairy products, excessive alcohol consumption, and certain diseases may contribute to vitamin B2 deficiency, characterized by anemia, angular cheilitis, cataracts, seborrheic dermatitis, and growth retardation (Kennedy, Citation2016; MacMillan et al., Citation2017; Mazur-Bialy & Pocheć, Citation2017; Mazur-Bialy, Pochec, & Plytycz, Citation2015; Powers et al., Citation2011; Qi, Kniazeva, & Han, Citation2017; Thomas & Mirowski, Citation2010). Therefore, vitamin B2 supplementation is particularly important for individuals with vitamin B2 deficiency. In the US, the recommended daily allowance for vitamin B2 is 1.7 mg/day for pregnant women (Fankhanel & Gassmann, Citation1998), 0.3–0.4 mg/day for infants, and 0.6–1.8 mg/day for adults. To reduce the risk of vitamin B2 deficiency, vitamin B2 is commonly added to several foods. Therefore, a highly sensitive and specific vitamin B2 analytical method is critical for assessing the nutritional quality and safety of foods.
Currently, the available methods to determine vitamin B2 include microbiological assays (Barton-Wright & Booth, Citation1943; Hyma, Citation1945), high-performance liquid chromatography (HPLC); (Hampel, York, & Allen, Citation2012; Marti-Andres, Escuder-Gilabert, Martin-Biosca, Sagrado, & Medina-Hernandez, Citation2015; Schmidt, Schreiner, & Mayer, Citation2017), electrochemical methods (Kowalczyk, Sadowska, Krasnodebska-Ostrega, & Nowicka, Citation2017), and fluorescence spectroscopy (Chen, Li, Cui, Yu, & Zhai, Citation2015; Gliszczyńska-Świglo & Rybicka, Citation2015; Privitera & Lozano, Citation2017). Microbiological assays, which are based on Lactobacillus rhamnosus, are highly sensitive. A commercial kit (VitaFast® Vitamin B2, R-Biopharm Co., Ltd., Darmstadt, Germany) has recently been developed for vitamin B2 determination with a limit of detection (LOD) of 1.8 µg vitamin B2/100 g (mL). However, the incubation time is lengthy (44–48 h), and the operation process is time-consuming. HPLC allows the quantification of vitamin B2 in foods and biological samples. However, due to the complexity of food matrices, HPLC requires tedious sample pre-treatment steps, sophisticated equipment, and trained personnel (Geng et al., Citation2017; Nurit, Lyan, Piquet, Branlard, & Pujos-Guillot, Citation2015). These determination methods cannot be adapted for high-throughput screening or on-site detection. Electrochemical methods and fluorescence spectroscopy have been used to determine vitamin B2; however, they are non-reproducible, have poor sensitivity for complex food matrices, and are not suitable for on-site testing. Biosensors based on electrochemistry methods and fluorescence spectroscopy have been developed to improve detection sensitivity (Farzin & Shamsipur, Citation2017; Puangjan, Chaiyasith, Taweeporngitgul, & Keawtep, Citation2017; Sá, da Silva, Jost, & Spinelli, Citation2015); however, biosensors require complex sample preparation steps and expensive instruments.
The enzyme-linked immunosorbent assay (ELISA) based on antibody–antigen interactions has received considerable attention due to its high sensitivity, specificity, and throughput (Berlina, Zherdev, Xu, Eremin, & Dzantiev, Citation2017; Guo et al., Citation2015; Kuang, Liu, et al., Citation2013). An indirect competitive ELISA (ic-ELISA) based on polyclonal antibodies against vitamin B2 has been used for the determination of vitamin B2 in foods and pharmaceuticals (Wang et al., Citation2013). Even though this method is sensitive and specific, polyclonal antibodies have great limitations in commercial applications because they cannot be reused and have poor reproducibility. Therefore, a highly sensitive and specific monoclonal (mAb)-based ic-ELISA for vitamin B2 determination is required. The lateral-flow immunochromatographic assay (ICA) strip, which is based on antigen–antibody interactions, have been widely used in the toxins, heavy metals, and chemicals analysis (Kong, Xie, Liu, Song, Kuang, Cui, et al., Citation2017; Kuang, Xing, et al., Citation2013; Peng et al., Citation2017). The lateral-flow ICA strip is simple, rapid (results can be obtained with the naked eye within 10 min), effective, and suitable for semi-quantitative detection, qualitative detection, and mass sample screenings.
In this study, we produced a mAb against vitamin B2. Highly sensitive and specific mAb-based ic-ELISA and lateral-flow ICA strip were developed for the determination of vitamin B2 in food and pharmaceutical products.
Materials and methods
Reagents and materials
Vitamin B2, N,N′-carbonyldiimidazole (CDI), bovine serum albumin (BSA), ovalbumin (OVA), Freund’s complete adjuvant, Freund’s incomplete adjuvant, and enzyme immunoassay-grade horseradish peroxidase (HRP)-labeled goat anti-mouse immunoglobulin were purchased from Sigma-Aldrich (Shanghai, China). Gelatin and 3, 3′, 5, 5′-tetramethylbenzidine (TMB) were obtained from Aladdin Chemistry Co., Ltd. (Shanghai, China). Other reagents, which were of analytical grade, were acquired from the National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China). Energy drink and compound vitamin B tablet were purchased from a local supermarket. Nitrocellulose (NC) high-flow-plus membranes (Pura-bind RP) were supplied by Whatman-Xinhua Filter Paper Co. (Hangzhou, China), and polynylchloride (PVC) backing materials, absorbance pad, and sample pad were obtained from Goldbio Tech Co. (Shanghai, China).
Solutions
The buffers used in this study included (1) PBS buffer (0.01 M phosphate-buffered saline, pH 7.4), (2) coating buffer (0.01 M sodium carbonate buffer, pH 9.6), (3) blocking buffer (0.2% w/v gelatin in coating buffer), (4) washing buffer (PBS buffer containing 0.05% v/v Tween-20), (5) antibody dilution buffer (PBS buffer containing 0.1% w/v gelatin and 0.05% v/v Tween-20), (6) stop buffer (2 M sulfuric acid), and (7) substrate solution (2 mL of 0.06% w/v TMB in glycol mixed with 10 mL of 0.1 M citrate phosphate buffer, pH 5.0 containing 1.8 µL of 30% hydrogen peroxide).
Antigen preparation
Vitamin B2 antigens were synthesized by conjugating vitamin B2 with carrier proteins (BSA or OVA). We prepared vitamin B2-BSA-1, vitamin B2-BSA-2, and vitamin B2-BSA-3 at reaction ratios 50:1, 100:1, and 150:1, respectively. Similarly, we prepared vitamin B2-OVA-1, vitamin B2-OVA-2, and vitamin B2-OVA-3 at reaction ratios 50:1, 100:1, and 150:1, respectively. Briefly, BSA/OVA were dissolved in 0.1 M sodium carbonate–bicarbonate buffer (CB, pH 9.6) at 5 mg/mL. Vitamin B2 (8.42 mg) and CDI (18.15 mg) were dissolved in 1 mL of DMF, heated to 40°C, and stirred in the dark for 1 h. Subsequently, the reaction mixture was slowly added to 2 mL of carrier protein solution and stirred overnight at room temperature. The conjugate was dialyzed against 0.01 M PBS for 3 days. Antigens were characterized by UV-Vis spectroscopy.
MAb preparation
Vitamin B2-BSA-1, vitamin B2-BSA-2, and vitamin B2-BSA-3 were used as immunogens. Female BALB/c mice (8–10 weeks of age) were immunized by continuous multipoint subcutaneous injections with the immunogens to generate polyclonal antibodies against vitamin B2. The mouse with the highest serum antibody titer and lowest 50% inhibitory concentration (IC50) was sacrificed, and its spleen was fused with Sp2/0 murine myeloma cells (Kong, Liu, Song, Kuang, & Xu, Citation2017). The target cells were selected by ic-ELISA and obtained by the limiting dilution method. MAbs were purified by the caprylic acid-ammonium sulfate precipitation method.
Development of ic-ELISA
In this experiment, 96-well microplates were coated with coating antigen diluted in coating buffer (100 µL/well) and incubated at 37°C for 2 h. Subsequently, the wells were washed three times with washing buffer. After washing the plates, 200 µL blocking buffer was added to each well and incubated at 37°C for 2 h. After washing, 50 µL anti-vitamin B2 mAb and 50 µL vitamin B2 standard were added to each well and incubated for 0.5 h at 37°C. After washing, 100 µL HRP-labeled goat anti-mouse IgG was added to each well, and the plates were incubated for 0.5 h at 37°C. After washing the plates three times, 100 µL substrate solution was added to each well and incubated for 15 min at 37°C in the dark. The reaction was stopped with the addition of 2 M sulfuric acid (50 µL/well). Absorbance was measured at 450 nm (Ding, Liu, Song, Kuang, & Xu, Citation2017). IC50 and LOD were calculated by a standard curve generated by plotting optical density at 450 nm (OD450) on the y-axis against vitamin B2 concentration on the x-axis.
Cross-reactivity
MAb specificity was evaluated by measuring cross-reactivity (CR). Vitamins B1, B3, B5, B6, and B12, biotin, and folic acid were analyzed by ic-ELISA. CR was calculated using the following equation:
Preparation of the gold nanoparticle (GNP)-labeled mAb
GNPs and GNP-labeled mAb were synthesized in our laboratory. For the synthesis of GNP-labeled mAb, the pH value of a GNP solution was adjusted to 8.0 with 0.1 M K2CO3. Subsequently, 0.2 mg anti-vitamin B2 mAb was slowly added to 10 mL GNP solution and maintained at room temperature for 1 h. BSA (0.5% w/v, 1 mL) was added dropwise. Following a 2-h incubation, the solution was centrifuged (7000g for 30 min at 4°C). Unconjugated gold nanoparticle and anti-vitamin B2 mAb in the supernatant were discarded. The resulting precipitate was washed three times with 0.02 M PBS (containing 5% sucrose, 1% BSA, and 0.5% polyethylene glycol 6000, pH 7.4), dissolved in 5 mL of 0.02 M PBS (containing 0.02% NaN3), and stored at 4°C (Kong, Xie, Liu, Song, Kuang, & Xu, Citation2017).
Preparation of the lateral-flow ICA strip
The lateral-flow ICA strip was developed in our laboratory as previously reported (Xing et al., Citation2015). PVC backing card, NC membrane, sample pad, and absorption pad were assembled in layers. The NC membrane was pasted onto the center of the PVC backing card. The sample and absorption pads were glued to the bottom and upper section of the PVC backing card, respectively, with a 2-mm overlap of the NC membrane. Using a membrane dispenser, goat anti-mouse IgG and coating antigen were sprayed onto the NC membrane at 1 µL/cm to form the control line and test line (C line and T line, respectively). After drying at 37°C, the card was cut into 3-mm wide individual test strips and stored in a desiccator for further experiments.
Principle of the lateral-flow ICA strip
The detection principle of the lateral-flow ICA strip is based on a competitive interaction between vitamin B2 present in the sample and the coating antigen sprayed on the T line for GNP-labeled mAb. Sample solution (100 µL) was mixed with 50 µL of GNP-labeled mAb in the wells of a microtiter plate and allowed to react at room temperature for 5 min. The ends of the strips with sample pad were inserted into the mixture. By capillary action, the mixture migrates from the sample pad to the NC membrane and reacts with the coating antigen on the T line and the goat anti-mouse IgG on the C line, finally reaching the absorption pad. The results are visualized by the naked eye within 5 min. In vitamin B2-negative samples, red T and C lines are obtained because GNP-labeled mAbs are captured by the coating antigen. In vitamin B2-positive samples, only a red C line is obtained. Vitamin B2 present in the sample combines with GNP-labeled mAbs; therefore, less GNP-labeled mAbs are available to combine with the coating antigen on the T line, resulting in a weak red color on the T line. With increasing vitamin B2 concentrations, the T line color intensity decreases. When the concentration of vitamin B2 in the sample exceeds a certain value, no color appears on the T line. This critical concentration is defined as the cut-off value of the strip (Isanga et al., Citation2017). GNP-labeled mAbs or vitamin B2-GNP-labeled mAbs continue to migrate and react with the goat-anti-mouse IgG antibody, resulting in a deep red color on the C line. The sensitivity of the lateral-flow ICA strip is determined by the detection of a series of vitamin B2 standards. In this study, each test was repeated seven times.
Sample analysis
An energy drink and compound vitamin B tablet were analyzed. Following pH adjustment to 7–7.5, the energy drink (vitamin B2 concentration: 4 mg/100 mL) was passed through a 0.2-µm filter membrane. The filtrate was diluted to a suitable concentration for analysis. Two pieces of compound vitamin B tablets (vitamin B2 concentration: 1.25 mg/piece) were dissolved in pure water to generate a stock solution. The solution was centrifuged at 6500g for 25 min at 4°C. The lipid layer was removed, and the supernatant was collected. The solution was diluted to a suitable concentration for analysis.
Results and discussion
UV spectroscopic characterization
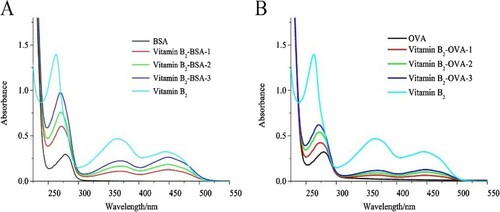
Vitamin B2-BSA and vitamin B2-OVA conjugates were used as immunogen and coating antigen, respectively. Vitamin B2-BSA and vitamin B2-OVA conjugates were characterized by UV absorption spectroscopy (). The carrier proteins (BSA and OVA) had a characteristic absorption peak at 278 nm. Vitamin B2-BSA and vitamin B2-OVA conjugates had absorption peaks at 364 and 445 nm, and vitamin B2 had absorption peaks at 263, 364, and 445 nm. A significant blue shift was observed between the antigens and carrier proteins, probably due to vitamin B2 coupling at 263 nm. These results confirmed that the antigens were successfully conjugated to the carrier proteins.
Development and characterization of ic-ELISA
Vitamin B2-BSA was used as immunogen in mice immunization, and vitamin B2-OVA was used as coating antigen in ic-ELISA. The mouse with the highest serum antibody titer and lowest IC50 was selected for cell fusion. Following cell infusion, the hybridoma cell line 3H8 was obtained, and vitamin B2 antibody was purified from ascites by the caprylic acid-ammonium sulfate precipitation method.
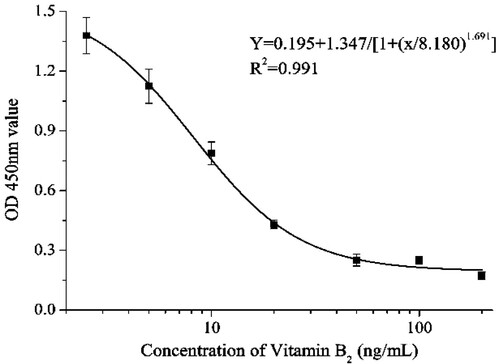
The standard curve () of mAb 3H8 against vitamin B2 concentration (y = 0.195 + 1.347/[1 + (x/8.180) × 1.691]) had a linear regression correlation coefficient (R2) of 0.991. The IC50 value was 8.18 ng/mL, and the LOD defined as IC10 value, was 1.80 ng/mL with a linear range of 3.60–18.56 ng/mL. The results revealed that our developed method was sensitive for the detection of vitamin B2. MAb specificity was evaluated by ic-ELISA using other B vitamins (). The results showed that vitamin B2 mAb did not significantly react with other B vitamins (CR values <0.1%). Therefore, the developed ic-ELISA was specific to vitamin B2.
Table 1. The CR value of related analytes by the Ic-ELISA method.
Optimization and characterization of the lateral-flow ICA strip
The analytical performance and sensitivity of the lateral-flow ICA strip may be affected by a series of parameters, including coating antigen and suspension buffer. To determine the appropriate reaction ratio of vitamin B2-OVA, three kinds of coating antigens (reaction ratios 50:1, 100:1, and 150:1) were sprayed onto the NC membrane. The lateral-flow ICA strip was used to analyze a vitamin B2-negative sample (0 ng/mL) and a vitamin B2-positive sample (50 ng/mL). The results are presented in (A). No color on T line was obtained with the vitamin B2-positive sample at any reaction ratio. A reaction ratio of 50:1 contributed to the deepest color on the T line with the vitamin B2-negative sample. Therefore, the coating antigen with reaction ratio 50:1 was used for subsequent experiments.
Figure 3. The optimization of the lateral-flow ICA strip. (A) Strip with three kinds of coating antigens at 0.5 mg/mL: (1) coating antigen with reaction ratio 50:1; (2) coating antigen with reaction ratio 100:1; (3) coating antigen with reaction ratio 150:1. (B) Optimization of suspension solution for sample pad: (1) basic suspension buffer; (2) 1% PVP; (3) 1% PEG; (4) 1% PVA; (5) 1% BSA; (6) 1% Casein; (7) 1% sucrose; (8) 1% trehalose; (9) 1% sorbitol; (10) 1% mannitol; (11) 1% brij 35; (12) 1% triton X-100; N, vitamin B2-negative sample (0 ng/mL); P, vitamin B2-positive sample (50 ng/mL). (C) Optimization of two different surfactants in basic suspension buffer: (1) 1% brij 35; (2) 1% triton X-100; N, vitamin B2-negative sample (0 ng/mL); P, vitamin B2-positive sample (25 ng/mL).
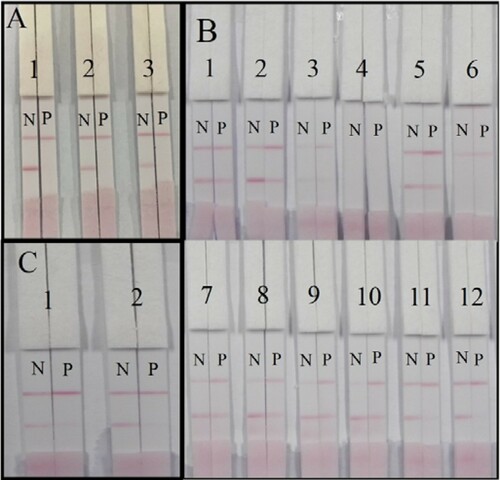
The composition of the suspension buffer affects the flow rate, background color, sharpness, and intensity of the T line. The basic suspension buffer contained 20 mM Tris (pH 8.2), 0.1% PEG, 0.1% Tween, 5% sucrose, 5% trehalose, and 0.2% BSA. Different surfactants were subsequently added. The vitamin B2-negative sample and the vitamin B2-positive sample were analyzed ((B)). When the basic suspension buffer containing 1% PEG, 1% PVA, or 1% casein were used, we obtained no color on the T line with the vitamin B2-negative sample. The basic suspension buffer and suspension buffer containing 1% PVP, 1% BSA, 1% sucrose, 1% trehalose, 1% sorbitol, or 1% mannitol contributed to a weak T color with the vitamin B2-positive sample. The basic suspension buffer containing brij 35 or triton X-100 contributed to a deep red T line color with the vitamin B2-negative sample. Therefore, subsequent experiments were performed using suspension buffer that contained brij 35 and triton X-100.
(C) shows that when the vitamin B2 concentration was decreased from 50 to 25 ng/mL, no red line on T line could be observed for both two kinds the suspension buffer. However, for vitamin B2-negative sample, the suspension buffer containing brij 35 contributed to a weak red T line color, while the basic suspension buffer containing triton X-100 contributed to a distinct red T line color. Therefore, the optimum conditions of the developed lateral-flow ICA strip consisted of a reaction ratio of 50:1 and a suspension buffer containing 1% triton X-100.
PBS samples spiked with different concentrations of vitamin B2 (0, 2.5, 5, 10, 25, and 50 ng/mL) were analyzed by the lateral-flow ICA strip (). The cut-off value for vitamin B2 was 50 ng/mL.
Sample analysis
An energy drink and compound vitamin B2 tablet were analyzed. The energy drink and compound vitamin B2 tablet samples were analyzed at 6400-, 3200-, 1600-, 800-, 400-, 200-, and 100-fold dilution (). Ultrapure water was used as the negative control. For the energy drink sample, a lighter color was observed on the T line with the 1600-fold diluted sample, and the T line disappeared with the 800-fold diluted sample (50 ng/mL). For the compound vitamin B tablet, a lighter color was observed on the T line with the 3200-fold diluted sample, and the T line disappeared with the 1600-fold diluted sample (78.13 ng/mL). The results obtained from the energy drink sample were more sensitive compared with the results obtained from the compound vitamin B tablet sample, which is probably due to substrate conditions and matrix interference effects. More sensitive results could be obtained by repeated measurements of different dilution ratios. The results revealed that our developed lateral-flow ICA strip assay was sensitive, accurate, and suitable for the detection and screening of vitamin B2 in real samples.
Figure 5. The sample analysis with lateral-flow ICA strip. (A) Energy drink sample: (1) ultrapure water; (2) 6400 times dilution; (3) 3200 times dilution; (4) 1600 times dilution; (5) 800 times dilution; (6) 400 times dilution; (7) 200 times dilution; (8) 100 times dilution. (B) Compound vitamin B tablet sample: (1) ultrapure water; (2) 6400 times dilution; (3) 3200 times dilution; (4) 1600 times dilution; (5) 800 times dilution; (6) 400 times dilution; (7) 200 times dilution; (8) 100 times dilution.

Conclusions
In this study, we developed a highly sensitive and specific mAb-based ic-ELISA and lateral-flow ICA strip for the detection and screening of vitamin B2 in food and pharmaceutical products. The IC50 and LOD of ic-ELISA was 8.18 and 1.80 ng/mL, respectively, with a linear dynamic range of 3.60–18.56 ng/mL. The cut-off value of the lateral-flow ICA strip was 50 ng/mL. These methods were effective for the analysis of both simple and complex matrices. Therefore, the developed mAb-based ic-ELISA and lateral-flow ICA strip are effective for on-site detection and mass sample screening of food and pharmaceutical products.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Lu Zeng
Lu Zeng got her bachelor degree from Zhejiang Chinese Medical University, Hangzhou, China in 2015 and then she began to study in Jiangnan University (Wuxi, China) for her Master Degree in food science. Her research interests are immunoassay applications in food.
Wei Jiang
Wei Jiang got her bachelor degree from Yangzhou University, Yangzhou, China in 2015 and then she began to study in Jiangnan University (Wuxi, China) for her Master Degree in food science. Her research interest includes immunoassay development for food safety.
Liqiang Liu
Liqiang Liu got his Ph. D in Food science in 2014 from Jiangnan University, Wuxi, China and then became a faculty in the college of Food science and technology of Jiangnan University. His research interests are immunochromatographic strip design and application.
Shanshan Song
Shanshan Song got her Master degree in Food science in 2012 from Jiangnan University, Wuxi, China and then became a research assistant in the college of Food science and technology of Jiangnan University. Her research interests are monoclonal antibody development.
Hua Kuang
Hua Kuang got her Ph. D from China Agricultural University in 2009 and then began to work as a faculty in the college of Food science and technology of Jiangnan University. She is currently a full professor in food safety. Her research interest is biosensor development.
References
- Barton-Wright, E. C., & Booth, R. G. (1943). The assay of riboflavin in cereals and other products: 1. Microbiological assay. 2. Fluorometric assay. The Biochemical Journal, 37(1), 25–30. doi: https://doi.org/10.1042/bj0370025
- Basaranoglu, S., Agacayak, E., Ucmak, F., Tunc, S. Y., Deregozu, A., Akkurt, Z. M., … Gul, T. (2017). The role of vitamin B1-B2 and plasma lipid profile in intrahepatic cholestasis of pregnancy. Journal of Perinatal Medicine, 45(4), 461–465. doi: https://doi.org/10.1515/jpm-2015-0337
- Berlina, A. N., Zherdev, A. V., Xu, C., Eremin, S. A., & Dzantiev, B. B. (2017). Development of lateral flow immunoassay for rapid control and quantification of the presence of the colorant Sudan I in spices and seafood. Food Control, 73, 247–253. doi: https://doi.org/10.1016/j.foodcont.2016.08.011
- Chen, J., Li, B. Q., Cui, Y. Q., Yu, E., & Zhai, H. L. (2015). A fast and effective method of quantitative analysis of VB1, VB2 and VB6 in B-vitamins complex tablets based on three-dimensional fluorescence spectra. Journal of Food Composition and Analysis, 41, 122–128. doi: https://doi.org/10.1016/j.jfca.2015.02.003
- Ding, X., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Rapid and ultrasensitive detection of 3-amino-2-oxazolidinone in catfish muscle with indirect competitive enzyme-linked immunosorbent and immunochromatographic assays. Food and Agricultural Immunology, 28(3), 463–475. doi: https://doi.org/10.1080/09540105.2017.1297778
- Fankhanel, S., & Gassmann, B. (1998). Dietary Reference Intakes, Report 2 - Vitamins B-1, B-2, B-6, B-12, niacin, folic acid, pantothenic acid, biotin, choline. Ernahrungs-Umschau, 45(8), 298–299.
- Farzin, L., & Shamsipur, M. (2017). Separation and preconcentration of riboflavin from human plasma using polythionine coated magnetite/hydroxyapatite nanocomposite prior to analysis by surfactant-enhanced fluorimetry. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 184, 109–118. doi: https://doi.org/10.1016/j.saa.2017.04.087
- Geng, C., Guo, X., Liu, J., Gao, M., Yuan, G., Bu, F., … Guo, R. (2017). LC-MS/MS for the determination of four water-soluble vitamins: Method development, validation and comparison to EC method. Chromatographia, 80(2), 259–264. doi: https://doi.org/10.1007/s10337-016-3232-8
- Gliszczyńska-Świglo, A., & Rybicka, I. (2015). Simultaneous determination of caffeine and water-soluble vitamins in energy drinks by HPLC with photodiode array and fluorescence detection. Food Analytical Methods, 8(1), 139–146. doi: https://doi.org/10.1007/s12161-014-9880-0
- Guo, J., Liu, L., Xue, F., Xing, C., Song, S., Kuang, H., & Xu, C. (2015). Development of a monoclonal antibody-based immunochromatographic strip for cephalexin. Food and Agricultural Immunology, 26(2), 282–292. doi: https://doi.org/10.1080/09540105.2014.907242
- Hampel, D., York, E. R., & Allen, L. H. (2012). Ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS) for the rapid, simultaneous analysis of thiamin, riboflavin, flavin adenine dinucleotide, nicotinamide and pyridoxal in human milk. Journal of Chromatography B, 903, 7–13. doi: https://doi.org/10.1016/j.jchromb.2012.06.024
- Henriques, B. J., Olsen, R. K., Bross, P., & Gomes, C. M. (2010). Emerging roles for riboflavin in functional rescue of mitochondrial beta-oxidation flavoenzymes. Current Medicinal Chemistry, 17(32), 3842–3854. doi: https://doi.org/10.2174/092986710793205462
- Hyma, A. M. (1945). A synthetic medium for microbiological assay of riboflavin, pantothenic acid, biotin, nicotinic acid, pyridoxine and polic acid. Dissertation Abstracts International, 6(2), 10.
- Isanga, J., Mukunzi, D., Chen, Y., Suryoprabowo, S., Liu, L., Kuang, H., & Xu, C. (2017). Development of a monoclonal antibody assay and a lateral flow strip test for the detection of paromomycin residues in food matrices. Food and Agricultural Immunology, 28(3), 355–373. doi: https://doi.org/10.1080/09540105.2016.1272551
- Kennedy, D. O. (2016). B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients, 8(2), 68. doi: https://doi.org/10.3390/nu8020068
- Kong, D., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of sensitive, rapid, and effective immunoassays for the detection of vitamin B-12 in fortified food and nutritional supplements. Food Analytical Methods, 10(1), 10–18. doi: https://doi.org/10.1007/s12161-016-0543-1
- Kong, D., Xie, Z., Liu, L., Song, S., Kuang, H., Cui, G., & Xu, C. (2017). Development of indirect competitive ELISA and lateral-flow immunochromatographic assay strip for the detection of sterigmatocystin in cereal products. Food and Agricultural Immunology, 28(2), 260–273. doi: https://doi.org/10.1080/09540105.2016.1263985
- Kong, D., Xie, Z., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of ic-ELISA and lateral-flow immunochromatographic assay strip for the detection of vancomycin in raw milk and animal feed. Food and Agricultural Immunology, 28(3), 414–426. doi: https://doi.org/10.1080/09540105.2017.1293014
- Kowalczyk, A., Sadowska, M., Krasnodebska-Ostrega, B., & Nowicka, A. M. (2017). Selective and sensitive electrochemical device for direct VB2 determination in real products. Talanta, 163, 72–77. doi: https://doi.org/10.1016/j.talanta.2016.10.087
- Kuang, H., Liu, L., Xu, L., Ma, W., Guo, L., Wang, L., & Xu, C. (2013). Development of an enzyme-linked immunosorbent assay for dibutyl phthalate in liquor. Sensors, 13(7), 8331–8339. doi: https://doi.org/10.3390/s130708331
- Kuang, H., Xing, C., Hao, C., Liu, L., Wang, L., & Xu, C. (2013). Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors, 13(4), 4214–4224. doi: https://doi.org/10.3390/s130404214
- Lewicka, A., Lewicki, S., Klos, A., Debski, B., Kuryl, T., & Bertrandt, J. (2017). Influence of protein deficient diet, vitamin B-2 supplementation and physical training on serum composition of polyunsaturated fatty acids (PUFAs) in rats. Annals of Agricultural and Environmental Medicine, 24(2), 185–189.
- MacMillan, L., Lamarre, S. G., dasilva, R. P., Jacobs, R. L., Brosnan, M. E., & Brosnan, J. T. (2017). Riboflavin deficiency in rats decreases de novo formate production but does not affect plasma formate concentration. Journal of Nutrition, 147(3), 346–352.
- Marti-Andres, P., Escuder-Gilabert, L., Martin-Biosca, Y., Sagrado, S., & Medina-Hernandez, M. J. (2015). Simultaneous determination of pyridoxine and riboflavin in energy drinks by high-performance liquid chromatography with fluorescence detection. Journal of Chemical Education, 92(5), 903–906. doi: https://doi.org/10.1021/ed500544h
- Mazur-Bialy, A. I., & Pocheć, E. (2017). Vitamin B2 deficiency enhances the pro-inflammatory activity of adipocyte, consequences for insulin resistance and metabolic syndrome development. Life Sciences, 178, 9–16. doi: https://doi.org/10.1016/j.lfs.2017.04.010
- Mazur-Bialy, A. I., Pochec, E., & Plytycz, B. (2015). Immunomodulatory effect of riboflavin deficiency and enrichment - Reversible pathological response versus silencing of inflammatory activation. Journal of Physiology and Pharmacology, 66(6), 793–802.
- Nurit, E., Lyan, B., Piquet, A., Branlard, G., & Pujos-Guillot, E. (2015). Development of a LC-MS/MS method for the simultaneous screening of seven water-soluble vitamins in processing semi-coarse wheat flour products. Analytical and Bioanalytical Chemistry, 407(12), 3471–3479. doi: https://doi.org/10.1007/s00216-015-8553-1
- Peng, J., Liu, L., Xu, L., Song, S., Kuang, H., Cui, G., & Xu, C. (2017). Gold nanoparticle-based paper sensor for ultrasensitive and multiple detection of 32 (fluoro)quinolones by one monoclonal antibody. Nano Research, 10(1), 108–120. doi: https://doi.org/10.1007/s12274-016-1270-z
- Powers, H. J. (2003). Riboflavin (vitamin B-2) and health. American Journal of Clinical Nutrition, 77(6), 1352–1360. doi: https://doi.org/10.1093/ajcn/77.6.1352
- Powers, H. J., Hill, M. H., Mushtaq, S., Dainty, J. R., Majsak-Newman, G., & Williams, E. A. (2011). Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). American Journal of Clinical Nutrition, 93(6), 1274–1284. doi: https://doi.org/10.3945/ajcn.110.008409
- Privitera, M. L., & Lozano, V. A. (2017). Development of a second-order standard addition fluorescence method for the direct determination of riboflavin in human urine samples without previous clean up and separation steps. Microchemical Journal, 133, 60–66. doi: https://doi.org/10.1016/j.microc.2017.02.033
- Puangjan, A., Chaiyasith, S., Taweeporngitgul, W., & Keawtep, J. (2017). Application of functionalized multi-walled carbon nanotubes supporting cuprous oxide and silver oxide composite catalyst on copper substrate for simultaneous detection of vitamin B2, vitamin B6 and ascorbic acid. Materials Science and Engineering: C, 76, 383–397. doi: https://doi.org/10.1016/j.msec.2017.03.040
- Qi, B., Kniazeva, M., & Han, M. (2017). A vitamin-B2-sensing mechanism that regulates gut protease activity to impact animal’s food behavior and growth. Elife, 6.
- Sá, E. S., da Silva, P. S., Jost, C. L., & Spinelli, A. (2015). Electrochemical sensor based on bismuth-film electrode for voltammetric studies on vitamin B-2 (riboflavin). Sensors and Actuators B: Chemical, 209, 423–430. doi: https://doi.org/10.1016/j.snb.2014.11.136
- Schmidt, A., Schreiner, M. G., & Mayer, H. K. (2017). Rapid determination of the various native forms of vitamin B6 and B2 in cow’s milk using ultra-high performance liquid chromatography. Journal of Chromatography A, 1500, 89–95. doi: https://doi.org/10.1016/j.chroma.2017.04.009
- Thomas, D. M., & Mirowski, G. W. (2010). Nutrition and oral mucosal diseases. Clinics in Dermatology, 28(4), 426–431. doi: https://doi.org/10.1016/j.clindermatol.2010.03.025
- Wang, P., Yin, Y., Eremin, S. A., Rybakov, V. B., Zhang, T., Xu, Z., … Xi, R. (2013). Indirect competitive immunoassay for detection of vitamin B2 in foods and pharmaceuticals. Journal of Agricultural and Food Chemistry, 61(29), 7048–7054. doi: https://doi.org/10.1021/jf401078t
- Xing, C., Liu, L., Song, S., Feng, M., Kuang, H., & Xu, C. (2015). Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosensors and Bioelectronics, 66, 445–453. doi: https://doi.org/10.1016/j.bios.2014.12.004