ABSTRACT
Polyclonal antibodies against Pythium aphanidermatum and Fusarium oxysporum proteins were developed for the detection of rhizome rot in ginger using serological assays. Under optimal experimental conditions, the detection limit of P. aphanidermatum by indirect ELISA was 10 µg/ml with a linear working range from 5 to 100 µg/ml (R2 = 0.994). In case of F. oxysporum, the linear working range was 5–100 µg/ml (R2 = 0.991) and the limit of detection was 25 µg/ml. The developed antibodies showed the highest titer in ELISA at 1:2000 dilutions. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis identified proteins ranging from molecular weights 15–97 kDa and 14–116 kDa of P. aphanidermatum and F. oxysporum isolates, respectively. In Western blot analysis, the developed antisera gave positive reactions against the isolated antigens of the fungi. The antibodies revealed immune-reactive bands of molecular weights 59 and 65 kDa in P. aphanidermatum and 44 and 75 kDa in F. oxysporum. The results suggest that the developed antibodies could be successfully applied for the specific immunodetection of P. aphanidermatum and F. oxysporum at an early stage of rhizome rot disease.
1. Introduction
Ginger (Zingiber officinale Rosc.), a tropical perennial herb of Zingiberaceae is an important commercial crop grown for its aromatic rhizomes which are used as a spice and a medicine (Sharma, Vijayvergia, & Singh, Citation2010). India is the largest producer of ginger accounting for about one-third of total world output. In 2016, Spice Board India estimated that the country produced 683,160 tons of ginger from an area of 38,200 hectares. Indian ginger is exported mainly in the form of whole and dry. Cochin and Calicut ginger are the two popular Indian ginger varieties in the world market. The export demand of Indian ginger is high due to its superior quality. India dominates the world trade in ginger oil and oleoresin by occupying 50% market share (Karthick, Alagumani, & Anbarassan, Citation2015). Countries such as China, Nigeria and Thailand are competing with India to make a position in the global market. The refreshing aroma, biting taste and carminative property of Indian ginger makes it an indispensable ingredient of food processing throughout the world.
Although ginger is a high return crop, however, the productivity is quite low, as it is affected by the diseases caused by fungi, bacteria, viruses, mycoplasma and nematodes. Among the diseases, rhizome rot poses a persistent threat to the cultivation and storage of ginger. Crop loss begins in the field and continues in the packing house, during transportation and till storage. Rhizome rot is caused mostly by Pythium aphanidermatum during cultivation and Fusarium oxysporum during postharvest stages. About 50–80% yield loss during storage has been reported due to this disease (Joshi & Sharma, Citation1980). Over the past few years, rhizome rot has affected nearly all states of India, resulting in decline of rhizome yield (Chaithra et al., Citation2013). The disease ultimately results in the partial or total loss of affected clumps. So an early detection of the disease can prevent the crop loss caused by the pathogens.
Traditional methods of studying the population of Pythium and Fusarium species have involved culture plating technique onto a selective medium (Al-Sheikh, Citation2010; Banks et al., Citation1996; Cha et al., Citation2007) and the observation of morphological features. However, the accuracy and reliability of these techniques depends on the experience and skill of the person making the diagnosis (McCartney, Foster, Fraaije, & Ward, Citation2003). Moreover, morphological identification is time consuming and difficult and thus is not suitable for rapid pathogen diagnosis (Meirelles et al., Citation2006). The results obtained by this method also may be misleading due to the presence of nonpathogenic species of Pythium and Fusarium or pathogenic species that grow too slowly to be counted (Schroeder, Okubara, Tambong, Lévesque, & Paulitz, Citation2006).
Likewise, although molecular techniques are very sensitive and capable of separating many species of Pythium and Fusarium, the techniques are not quantitative or designed for identification directly from soil or plant samples (Li, Senda, Komatsu, Suga, & Kageyama, Citation2010). A solution to this is serological assays, which have been demonstrated to be highly specific, sensitive, simple, rapid and cost-effective and can be automated for large-scale applications. Research also suggests that plants secrete defense proteins in response to pathogenic fungi secretions during host–pathogen interactions (Gupta et al., Citation2015). Study of these polypeptides could be utilized to develop a rapid and early detection method for fungal infection based on immunological assay. Polyclonal antibodies have been used previously to detect multiple isolates (Biazon et al., Citation2006; Fleurat-Lessard, Luini, Berjeaud, & Roblin, Citation2010; Gautam, Cahill, & Hardham, Citation1999). The present study was designed (i) to develop the polyclonal antibodies against the antigens isolated from P. aphanidermatum and F. oxysporum at different stages of disease development and (ii) to investigate the specificity and sensitivity of the developed antibodies and to determine the feasibility of ELISA test for rapid detection of the pathogens using the developed antibodies at different stages of infection.
2. Materials and methods
2.1. Collection of infected rhizomes, isolation and identification of pathogens
Infected ginger rhizomes were collected from different regions of Odisha, Telangana and Tamil Nadu. The rhizomes were washed with water to remove adhering soil and then cut into 2- to 3-mm-thick pieces. The pieces were surface sterilized by immersing the sample in 70% ethanol for 30 s and then rinsed three times with sterile water followed by drying on sterile filter paper for 1 min. The pieces were grown on potato dextrose agar (PDA) plates containing streptomycin (100 µg/µl) for 7 days at 27°C. All the fungal colonies were again re-cultured on PDA for isolation of pure cultures.
The morphological and cultural characterizations of the cultures grown on PDA were studied. Microscopic slides were prepared from these isolates and stained using lacto phenol cotton blue according to Parija and Prabhakar (Citation1995). The slides were examined under a light microscope. Morphological features of P. aphanidermatum were examined and identification of the fungal isolates was done using the key provided by Van der Plaats-Niterink (Citation1981). Keys and descriptions by Waterhouse (Citation1968) and Middleton (Citation1943) were also consulted for confirmation of identifications. Similarly, F. oxysporum was identified using the descriptions of Leslie and Summerell (Citation2008).
2.2. Assessment of mycelial growth
For mycelial growth, P. aphanidermatum and F. oxysporum were aseptically transferred to sterilized conical flasks containing 50 ml potato dextrose broth (PDB) and incubated in a rotary shaker at 150 rpm at 28 ± 2°C for 7 days. Mycelia of each of the isolates were harvested by filtration through Whatman No. 1 filter paper and repeatedly washed with sterile distilled water.
2.3. Extraction and estimation of fungal antigens
Fungal antigens were extracted from 7-day-old mycelia grown in PDB. The collected mycelia were homogenized (1 g/ml) in pre-chilled mortar and pestle with 50 mM Tris-HCl buffer (pH 7.5) containing 0.1 mM ethylenediaminetetraacetic acid. The extracts were centrifuged at 10,000 rpm for 20 min at 4°C and the supernatant was collected. Protein isolated from healthy ginger rhizomes were used as positive control. Protein contents were estimated following Bradford (Citation1976) using bovine serum albumin as the standard.
Ginger rhizomes infected by P. aphanidermatum and F. oxysporum (Pa1–Pa7 and Fo1–Fo4) were categorized into three different stages of infection based on the antigen loads. The concentrations of proteins in the isolates are mentioned in .
Table 1. Sample-wise collection from different states of India and content of protein (mg/g fr. wt) in P. aphanidermatum and F. oxysporum – infected ginger rhizomes at various stages of disease development.
2.4. Separation of fungal proteins by sodium dodecyl sulfate–polyacrylamide gel electrophoresis
Analysis of fungal proteins was carried out by polyacrylamide gel electrophoresis (PAGE) using 12% (w/v) gels with 5% (w/v) stacking gels, containing sodium dodecyl sulfate (SDS) (Laemmli, Citation1970). Based on antigen loads, 10 µg (mild) to 100 µg (chronic) proteins in sample buffer were loaded into single wells of multi-welled gels. A standard protein marker (Sigma) was used for molecular weight estimation. Protein separation was carried out in a Bio-Rad Mini-PROTEAN vertical electrophoresis system under a constant voltage of 100 V for 1 h. After electrophoresis, the gels were stained with Coomassie Brilliant Blue R-250 (Sigma) to visualize the fungal proteins and molecular weight markers.
2.5. Development of polyclonal antibodies
Polyclonal antisera were produced against pooled antigens of P. aphanidermatum and F. oxysporum isolates separately in two male New Zealand white rabbits. The rabbits were injected subcutaneously with 1 mg of fungal antigens in complete Freund’s adjuvant. Normal (pre-immune) sera were collected from rabbits for negative controls before the first immunization. Three subsequent intramuscular booster injections of 500 µg antigens in incomplete Freund’s adjuvant were given at an interval of 15 days. The rabbits were bleeding 1 week after the second and fourth immunization. All animals were kept in proper conditions according to standards required by the University Bioethics Committee. The collected antisera were employed for ELISA.
2.6. Screening of antibodies by indirect ELISA
Indirect ELISA as described by Ray et al. (Citation2016) was performed with slight modifications to test the titers of both the developed antisera. Microtiter plate was coated with 100 µl of P. aphanidermatum and F. oxysporum antigens (10–100 µg/ml) diluted in 50 mM carbonate coating buffer (pH 9.6) and incubated overnight at 4°C. The plate was washed three times with phosphate buffer saline-tween 20 (PBS-T) and incubated for 1 h at room temperature by blocking the wells with 200 ml of 2% skimmed milk in PBS. After washing thrice with PBS-T, 100 µl of antisera against respective antigens were diluted in 1% skimmed milk in PBS and added to the wells. Again the plate was incubated for overnight at room temperature. After washing three times with PBS-T, 100 µl of horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma) at a dilution of 1:5000 was added to each well and incubated for 1 h at room temperature. The plate was washed thrice with PBS-T and incubated with 100 µl of H2O2/TMB (Hydrogen peroxide/3,3′, 5,5′-Tetramethybenzidine) substrate per well for 10 min in dark for color development. The reaction was stopped by adding 50 µl of 2 N sulfuric acid (H2SO4) and absorbance was measured at 450 nm in a micro plate reader (Bio-Rad).
2.7. Assessment of specificity of antibodies by Western blotting
Fungal proteins used in the qualitative assessment were prepared by pooling equal volumes of samples from each stage. The proteins separated by electrophoresis were transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore) using Mini-Trans Blot Cell Module (Bio-Rad). Electroblotting was performed for 90 min at a constant voltage of 50 V. The membranes were stained with Amido black (Sigma) and destained with destaining solution (10% methanol, 10% acetic acid and dist. H2O). The transferred membrane strips were blocked for 30 min at room temperature in the blocking buffer (Tris-buffered saline Tween 20 (TBST): 0.02 M Tris-HCl with 0.15 M NaCl, 0.05% Tween-20, pH 8) containing 3% skimmed milk with slight shaking (50 rpm). After extensive washing in TBST buffer, the strips were incubated with 1:1000 dilutions of the respective polyclonal antibodies for 3 h at room temperature. After incubation, the strips were again washed for three times and incubated for 1 h at room temperature in 1:5000 dilution of horseradish peroxidase-conjugated anti-rabbit IgG (Sigma). Bands were developed after washing vigorously with TBST and immersing the treated strips in chemiluminescent substrate solution (Pierce ECL Western Blotting Substrate, Thermo Scientific). Specific bands were depicted on Hyper-Film (Kodak) by developing in the darkroom using developer and fixer.
2.8. Validation of ELISA technique
Detection sensitivity, specificity and accuracy of the technique for diagnosis of the infected ginger rhizomes were calculated using Microsoft Excel 2013 software. Diagnostic sensitivity [true positive (TP)/(false negative (FN) + TP)], specificity [(true negative (TN)/(false positive (FP) + TN)] and accuracy [(TP + TN)/(TP + TN + FP + FN)], the results of the calculations, were expressed in percentages.
3. Results and discussion
3.1. SDS-PAGE analysis
Protein banding pattern of fungal isolates allowed identification of multiple protein bands as shown in . The molecular weights of the P. aphanidermatum proteins ranged from approximately 15 to 95 kDa (Anusuya & Sathiyabama, Citation2014; Ray et al., Citation2016) and of F. oxysporum proteins from 14 to 116 kDa (Bhuvanendra et al., Citation2010; Dong & Wang, Citation2011). Analysis of fungal proteins showed several specific protein bands which were absent in healthy ginger rhizomes. The number and intensity of bands in the antigenic protein isolated from stage III infected ginger rhizomes were higher in comparison to stage I and II rhizomes.
Figure 1. Protein banding pattern of P. aphanidermatum and F. oxysporum isolates separated in a 12% SDS-PAGE. (A) Proteins bands of P. aphanidermatum isolated from infected ginger rhizomes [M: marker; Lanes: a: healthy ginger rhizomes, b–h: P. aphanidermatum isolates (Pa1–Pa7)]; and (B) Proteins bands of F. oxysporum isolates [M: marker; Lanes: a: healthy ginger rhizomes, b–e: F. oxysporum isolates (Fo1–Fo4)]. Molecular weight of standard protein is indicated on left margin.
![Figure 1. Protein banding pattern of P. aphanidermatum and F. oxysporum isolates separated in a 12% SDS-PAGE. (A) Proteins bands of P. aphanidermatum isolated from infected ginger rhizomes [M: marker; Lanes: a: healthy ginger rhizomes, b–h: P. aphanidermatum isolates (Pa1–Pa7)]; and (B) Proteins bands of F. oxysporum isolates [M: marker; Lanes: a: healthy ginger rhizomes, b–e: F. oxysporum isolates (Fo1–Fo4)]. Molecular weight of standard protein is indicated on left margin.](/cms/asset/0b2d6b46-92f0-4bd8-a4a0-759892d9af6b/cfai_a_1365820_f0001_ob.jpg)
3.2. ELISA analysis
By ELISA assay, the developed antisera recognized the antigens from the corresponding titration in concentrations of 1:500, 1:1000, 1:2000, 1:3000 and 1:5000. Under optimal experimental conditions, the assay developed against P. aphanidermatum showed a good sensitivity with limit of detection (LOD) of 10 µg/ml and linear range from 5 to 100 µg/ml (R2 = 0.994). Similarly, LOD for F. oxysporum was determined as 25 µg/ml with linear range from 5 to 100 µg/ml (R2 = 0.991) (). As shown in , the results indicated that antisera used at a dilution 1:2000 provided optimal reactivity with both the antigens when the absorbance value was approximately 0.5–0.7 units.
Figure 2. Standard curve of ELISA under optimized conditions. The values plotted were mean ± SD based on three repeats.
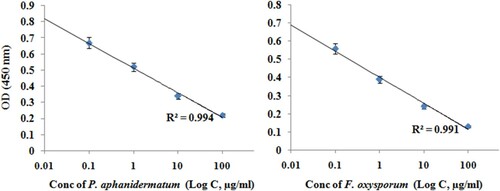
Figure 3. Optimization of (i) P. aphanidermatum and (ii) F. oxysporum antigens and developed antibodies dilution of indirect ELISA.
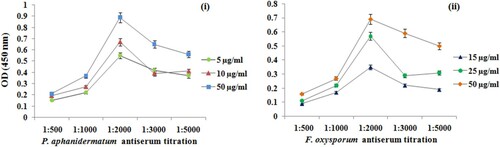
Reactions were detected 15 min after application of the substrate and no change in readings for the negative control was observed even after incubation for 30 min. The reactivity of the fungal isolates to the developed antibodies is summarized in . The developed antibodies also showed immunological reaction with proteins isolated from different infected rhizome samples and the titration profile of these antibodies are illustrated in .
Figure 4. (A) Data of ELISA showing the variation in absorbance at 450 nm monitored in the presence of fungal proteins at different stages of disease development. (B) ELISA titration of (i) antiserum against P. aphanidermatum proteins and (ii) antiserum against F. oxysporum proteins isolated at stage I of disease development. Mean ± SD; n = 3.
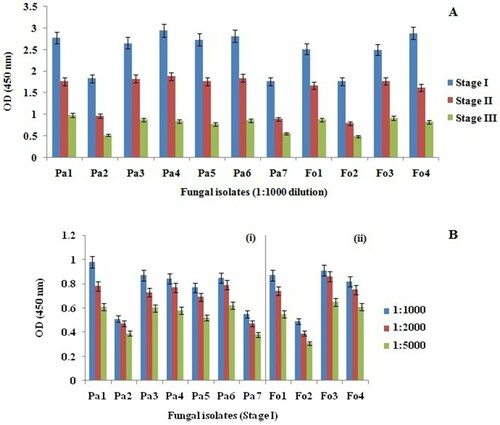
Table 2. Sensitivity of P. aphanidermatum and F. oxysporum isolates to developed antibodies by indirect ELISA.
3.3. Western blot analysis
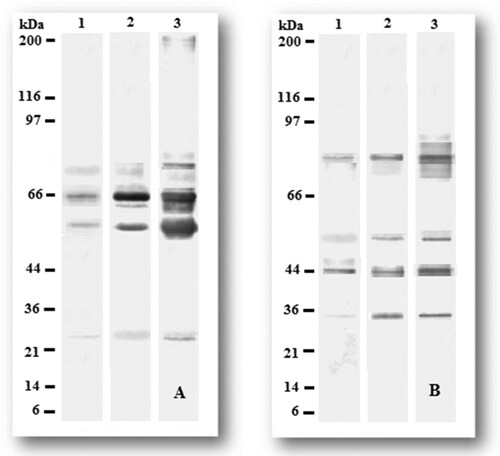
The Western blot analysis of both the developed polyclonal antibodies against the antigens at various stages of disease development showed a remarkable difference in the recognition profile. The polyclonal antibodies reacted with several bands ranging from molecular weight of 15–97 kDa and 14–116 kDa in P. aphanidermatum and F. oxysporum, respectively, in all three stages of infection. The antibody raised against P. aphanidermatum showed the highest response against the antigens with molecular weight of 59 and 65 kDa, and additional bands of reactivity, including 25 and 72 kDa, were seen (). Similarly, the antibody raised against F. oxysporum detected two immune-dominant antigens with molecular weight of 44 and 75 kDa, including two other bands at 33 and 97 kDa. Immunogenic bands with similar molecular weights also have been detected in past reports (Ghosh, Datta, & Purkayastha, Citation2006; Peschen, Li, Fischer, Kreuzaler, & Liao, Citation2004; Ray et al., Citation2016).
Figure 5. Western blot analysis on pooled (A) P. aphanidermatum and (B) F. oxysporum antigens from different stages of infection. Proteins were electrophoresed and blotted onto PVDF membranes and incubated with sera (1:1000 dilution). Band pattern for the isolates are as follows: Lane 1: pooled antigens isolated at stage I infection; Lane 2: pooled antigens isolated at stage II infection; Lane 3: pooled antigens isolated at stage III infection. Molecular weight of standard protein is indicated on left margin.
3.4. Validation of ELISA technique
The sensitivity, specificity and accuracy of the assay were calculated using a hypothetical set of results from infected and non-infected isolates. The test was conducted on a sample size of 115, consisting of both infected and healthy rhizome samples and the results of the calculations are summarized in . Hence, it was concluded that the obtained antibodies against P. aphanidermatum and F. oxysporum were highly sensitive, specific and accurate thus permitting detection of signals in rhizome rot-affected ginger rhizomes.
Table 3. Diagnostic performance of ELISA for detection of P. aphanidermatum and F. oxysporum antibodies in protein samples isolated from infected and non-infected populations.
4. Conclusions
Indian ginger has a high demand because of its food and medicinal uses; hence, early diagnosis of diseases can improve its quality, yield and shelf life by reducing disease losses in turn leading to a rise in the export value. The present study described the development of specific, sensitive and reliable immunoassays for early detection of P. aphanidermatum and F. oxysporum in rhizome rot-affected ginger. In these serological approaches, we have shown that the detection is independent of antigenic load or the stages of infections. The results indicated that antigens even at the initial stage of disease development can be used for early detection. The findings of this study suggest that the concentration of antigens and appropriate dilutions of antisera are important factors that affect early detection of pathogens. Furthermore, the system has excellent potential for direct quantification of the fungus in plant tissues and, therefore, can be used as rapid tool for diagnosis of the disease.
Acknowledgments
The authors are grateful to Prof. (Dr) S.C. Si, Dean, Centre of Biotechnology and Prof. (Dr) M.R. Nayak, President, Siksha “O” Anusandhan University for the generous financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Monalisa Ray
Monalisa Ray is working as a PhD research scholar at Centre for Biotechnology under the supervision of Dr. Shikha Singh.
Swagatika Dash
Swagatika Dash is also working as a PhD research scholar at Centre for Biotechnology under the supervision of Dr. Shikha Singh.
K. Gopinath Achary
Dr. K. Gopinath Achary is the head of monoclonal division at Imgenex India Pvt Ltd., Bhubaneswar.
Sanghamitra Nayak
Prof. Sanghamitra Nayak is the head of the department of Centre for Biotechnology.
Shikha Singh
Dr. Shikha Singh is working as an Assistant Professor at Centre for Biotechnology.
References
- Al-Sheikh, H. (2010). Two pathogenic species of Pythium: P. aphanidermatum and P. diclinum from a wheat field. Saudi Journal of Biological Sciences, 17(4), 347–352. doi: https://doi.org/10.1016/j.sjbs.2010.05.001
- Anusuya, S., & Sathiyabama, M. (2014). Preparation of β-d-glucan nanoparticles and its antifungal activity. International Journal of Biological Macromolecules, 70, 440–443. doi: https://doi.org/10.1016/j.ijbiomac.2014.07.011
- Banks, J. N., Rizvi, R. H., Barker, I., Turner, J. A., Rahman, S., & Northway, B. J. (1996). Specific monoclonal antibodies to Fusarium species and Microdochium nivale. Food and Agricultural Immunology, 8(4), 249–268. doi: https://doi.org/10.1080/09540109609354924
- Bhuvanendra, K. H., Udaya, S. A., Nayaka, C., Kini, S. R., Shetty, H. S., & Prakash, H. S. (2010). Biochemical characterization of Fusarium oxysporum f. sp. cubense isolates from India. African Journal of Biotechnology, 9(4), 523–530.
- Biazon, L., Meirelles, P. G., Ono, M. A., Itano, E. N., Taniwaki, M. H., Sugiura, Y., … Ono, E. Y. (2006). Development of polyclonal antibodies against Fusarium verticillioides exoantigens. Food and Agricultural Immunology, 17(1), 69–77. doi: https://doi.org/10.1080/09540100600621458
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. doi: https://doi.org/10.1016/0003-2697(76)90527-3
- Cha, S. D., Jeon, Y. J., Ahn, G. R., Han, J. I., Han, K. H., & Kim, S. H. (2007). Characterization of Fusarium oxysporum isolated from Paprika in Korea. Mycobiology, 35(2), 91–96. doi: https://doi.org/10.4489/MYCO.2007.35.2.091
- Chaithra, M., Shobha, K. S., Pallavi, S., Sachidananda Swamy, H. C., Vivek, M. N., Manasa, M. Asha, M. M. (2013). In vitro screening of natural products on Fusarium oxysporum from rots in ginger. Journal of Biological and Scientific Opinion, 1(3), 182–185. doi: https://doi.org/10.7897/2321–6328.01309
- Dong, Z., & Wang, Z. (2011). Isolation and characterization of an exopolygalacturonase from Fusarium oxysporum f. sp. cubense race 1 and race 4. BMC Biochemistry, 12(1), 51. doi: https://doi.org/10.1186/1471-2091-12-51
- Fleurat-Lessard, P., Luini, E., Berjeaud, J. M., & Roblin, G. (2010). Diagnosis of grapevine esca disease by immunological detection of Phaeomoniella chlamydospora. Australian Journal of Grape and Wine Research, 16(3), 455–463. doi: https://doi.org/10.1111/j.1755-0238.2010.00106.x
- Gautam, Y., Cahill, D. M., & Hardham, A. R. (1999). Development of a quantitative immunodipstick assay for Phytophthora nicotianae. Food and Agricultural Immunology, 11(3), 229–242. doi: https://doi.org/10.1080/09540109999753
- Ghosh, R., Datta, M., & Purkayastha, R. P. (2006). Intraspecific strains of Pythium aphanidermatum induced disease resistance in ginger and response of host proteins. Indian Journal of Experimental Biology, 44, 68–72.
- Gupta, R., Lee, S. E., Agrawal, G. K., Rakwal, R., Park, S., Wang, Y., & Kim, S. T. (2015). Understanding the plant-pathogen interactions in the context of proteomics-generated apoplastic proteins inventory. Frontiers in Plant Science, 6(352), 1–7.
- Joshi, L. K., & Sharma, N. D. (1980). Diseases of ginger and turmeric. In M.K. Nair, T. Premkumar, P.N. Ravindran, & Y.R. Sarma (Eds.), Proceedings of National Seminar on Ginger Turmeric (pp. 104–119). Calicut, CPCRI.
- Karthick, V., Alagumani, T., & Anbarassan, A. (2015). Growth and export performance of ginger in India – An economic analysis. Economic Affairs, 60(2), 207–214. doi: https://doi.org/10.5958/0976-4666.2015.00030.3
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685. doi: https://doi.org/10.1038/227680a0
- Leslie, J. F., & Summerell, B. A. (2008). The Fusarium laboratory manual. Iowa, USA: Blackwell Publishing.
- Li, M., Senda, M., Komatsu, T., Suga, H., & Kageyama, K. (2010). Development of real-time PCR technique for the estimation of population density of Pythium intermedium in forest soils. Microbiological Research, 165(8), 695–705. doi: https://doi.org/10.1016/j.micres.2009.11.010
- McCartney, H. A., Foster, S. J., Fraaije, B. A., & Ward, E. (2003). Molecular diagnostics for fungal plant pathogens. Pest Management Science, 59(2), 129–142. doi: https://doi.org/10.1002/ps.575
- Meirelles, P. G., Ono, M. A., Ohe, M. C. T., Maroneze, D. M., Itano, E. N., Garcia, G. T., … Ono, E. Y. (2006). Detection of Fusarium sp. contamination in corn by enzyme-linked immunosorbent assay. Food and Agricultural Immunology, 17(2), 79–89. doi: https://doi.org/10.1080/09540100600688754
- Middleton, J. T. (1943). The taxonomy, host range and geographic distribution of the genus Pythium. Memoirs of the Torrey Botanical Club, 20, 1–171.
- Parija, W., & Prabhakar, P. K. (1995). Evaluation of lacto-phenol cotton blue for wet mount preparation of feces. Journal of Clinical Microbiology, 33, 1019–1021.
- Peschen, D., Li, H. P., Fischer, R., Kreuzaler, F., & Liao, Y. C. (2004). Fusion proteins comprising a Fusarium-specific antibody linked to antifungal peptides protect plants against a fungal pathogen. Nature Biotechnology, 22(6), 732–738. doi: https://doi.org/10.1038/nbt970
- Ray, M., Dash, S., Shahbazi, S., Achary, K. G., Nayak, S., & Singh, S. (2016). Development and validation of ELISA technique for early detection of rhizome rot in golden spice turmeric from different agroclimatic zones. LWT-Food Science and Technology, 66, 546–552. doi: https://doi.org/10.1016/j.lwt.2015.10.071
- Schroeder, K. L., Okubara, P. A., Tambong, J. T., Lévesque, C. A., & Paulitz, T. C. (2006). Identification and quantification of pathogenic Pythium spp. from soils in eastern Washington using real-time polymerase chain reaction. Phytopathology, 96(6), 637–647. doi: https://doi.org/10.1094/PHYTO-96-0637
- Sharma, S., Vijayvergia, R., & Singh, T. (2010). Evaluation of antimicrobial efficacy of some medicinal plants. Journal of Chemical and Pharmaceutical Research, 2(1), 121–124.
- Van der Plaats-Niterink, A. J. (1981). Monograph of the genus Pythium. Studies in Mycology, 21, 1–244.
- Waterhouse, G. M. (1968). The genus Pythium Pringsheim. Mycology Papers, 110, 1–50.
