ABSTRACT
Immunoassay direct for 3-amino-5-morpholinomethyl-2-oxazolidone (AMOZ), the metabolite of furaltadone, based on a specific antibody was usually unavailable due to its small molecule weight. In this work, a hapten with a double bond and an active carboxyl group was designed and then conjugated to peptide dendrimer to obtain novel multiple haptens. The haptens and multiple haptens were coupled to bovine serum albumin as immunogens. Polyclonal antibodies showed binding to free AMOZ in a heterologous competitive indirect enzyme-linked immunosorbent assay were obtained. The assay showed IC50 (half maximal inhibitory concentration) of 4.1 μg/kg and limit of detection of 0.2 μg/kg for AMOZ. Recoveries of AMOZ from grass carp were tested from 83.1% to 117.0%, with the coefficient of variation below 12%. As no laborious derivatisation procedure was employed, the proposed assay should be useful for the screening of AMOZ for a large number of samples.
1. Introduction
Immunoassay based on antigen–antibody interaction has many excellent features, such as being accurate, quick, sensitive and high throughput (Gomaa & Boye, Citation2015; Kong et al., Citation2017; Shen et al., Citation2017; Tighe, Ryder, Todd, & Fairclough, Citation2015). It has been successfully applied in detecting low molecular mass organic contaminations in food, especially in screening large numbers of samples, playing an important role in food safety regulation (Anfossi, Di Nardo, Giovannoli, Passini, & Baggiani, Citation2015; Bolarinwa, Orfila, & Morgan, Citation2014; Gujral, Yoo, Bamdad, Suh, & Sunwoo, Citation2017; Xie, Kong, Liu, Song, & Kuang, Citation2017). However, due to the lack of epitope of haptens, the production of antibodies with high affinity and high specificity is still one of the bottlenecks of immunoassay for some small molecules (Luo et al., Citation2014; Luo et al., Citation2014; Mu et al., Citation2015; Romestand et al., Citation2010; Romestand et al., Citation2010; Zhang et al., Citation2017). Numerous studies have shown that if the molecular weight of the target analyte is less than 300 Da, an antibody with high quality will be difficult or even impossible to obtain (Chappey, Debray, Niel, & Scherrmann, Citation1994; Cooper, Caddell, Elliott, & Kennedy, Citation2004). Unfortunately, there are many harmful substances with very low molecular weight in food, posing a threat to human health (Escher & Fenner, Citation2011; Janči et al., Citation2017; Selvi, Sreenivasa, & Manonmani, Citation2011). Therefore, it is necessary to strengthen research on the antibody production for molecules with super-low molecular weight.
3-Amino-5-morpholinomethyl-2-oxazolidone (AMOZ), the metabolite of furaltadone antibiotic, revealed potential teratogenicity and carcinogenicity (McCracken, Blanchflower, Rowan, McCoy, & Kennedy, Citation1995; Points, Burns, & Walker, Citation2015; Vroomen et al., Citation1990; Yan, Hu, Zhang, Liu, & Wang, Citation2012). However, immunoassay for the direct determination of AMOZ was usually unavailable due to the difficulty to prepare a specific antibody. Thus far, most of the immunoassays established for AMOZ were based on antibodies showing specificity to the derivative of AMOZ (Jester, Abraham, Wang, El Said, & Plakas, Citation2014; Pimpitak, Putong, Komolpis, Petsom, & Palaga, Citation2009; Shen et al., Citation2012). For example, in the first immunoassay for AMOZ by Pimpitak et al., a monoclonal antibody was produced using the 3-carboxybenzaldehyde derivative of AMOZ (CPAMOZ) as the immunogen hapten and competitive indirect enzyme-linked immunosorbent assay (ciELISA) was performed for the 2-nitrobenzaldehyde derivative of AMOZ rather than AMOZ. The derivative procedure for AMOZ is always time-consuming and cumbersome (Jester et al., Citation2014; Pimpitak et al., Citation2009; Xu et al., Citation2013). Although antibodies produced against CPAMOZ sometimes showed good binding affinity to free AMOZ (cross-reactivity (CR)), the binding ability varied significantly for different reports, and sometimes it is hard to screen.
Multiple antigen peptides (MAPs), which are prepared by attaching target peptides to the surface of a dendritic peptidyl, first described by Tam in 1988, have been successfully applied for vaccine use (Joshi, Dighe, Thakuria, Malik, & Kumar, Citation2013; Manki, Ono, Uenaka, Seino, & Nakayama, Citation1998; Tam, Citation1996a). Thanks to high-density multimers, MAP could present target peptides to the immune system efficiently and thus induce a high-affinity immune reactivity, which could improve the quality of antibodies, such as higher affinity and better sensitivity in an immunoassay (Briand, Barin, van Regenmortel, & Muller, Citation1992; Boas & Heegaard, Citation2004; Heegaard, Boas, & Sorensen, Citation2010; Tam, Citation1996b). However, there is no report of a similar strategy used in the field of antibody production for organic small molecular compounds.
In this study, AMOZ was chosen as a model and five haptens and two multiple haptens were designed and synthesised in attempts to obtain an antibody with high specificity and binding activity to free AMOZ. After antigen preparation and animal immunity, a polyclone antibody was obtained and a competitive indirect ELISA was developed for the direct determination of AMOZ residue in fish samples.
2. Material and methods
2.1. Reagents, chemicals, buffers and solutions
AMOZ (99%), glyoxylic acid monohydrate, maleic anhydride, succinic anhydride, 2-morpholinoethylamine, 2-morpholin-4-ylacetic acid, glutaraldehyde (50% in H2O), n-octanoic acid, ammonium sulfate, N,N-dimethylformamide, dimethyl sulfoxide and 6-maleimidohexanoic acid N-hydroxysuccinimide ester (EMCS) were purchased from Aladdin (Shanghai, China). N-hydroxysuccinimide, dicyclohexyl carbodiimide, bovine serum albumin (BSA), ovalbumin (OVA), complete and incomplete Freund’s adjuvant, 3,3′,5,5′-tetramethylbenzidine (TMB) and horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (HRP-goat anti-rabbit IgG) were obtained from Sigma (St. Louis, MO, USA). Polystyrene ELISA plates were purchased from Jiete Biotech Co., Ltd (Guangzhou, China). New Zealand male white rabbits were provided by the Guangdong Medical Laboratory Animal Center. Fish samples for this assay were bought from a local supermarket. Strata-X cartridge solid phase extraction cartridges were purchased from Phenomenex Science Apparatus Co. (Guangzhou, China). All other reagents used in this study were of analytical grade and obtained from a local chemical supplier (Yunhui Trade Co., Ltd., Guangzhou, China).
The buffers used in this study were as follows: 0.01 mol/L phosphate-buffered saline (PBS, pH 7.4), 0.06 mol/L acetate-buffered saline (pH 4.8) for antibody purification, 0.1 mol/L carbonate-buffered solution (pH 9.6) for coating, 0.01 mol/L PBST buffer (0.01 mol/L PBS containing 0.05% Tween-20, pH 7.4) for antibody dilution and washing, 0.01 mol/L citrate and sodium phosphate (pH 5.5) for substrate buffer and 2.0 mol/L H2SO4 as the stopping reagent. The TMB solution was a mixture of substrate buffer, TMB-DMF (dimethylformamide) (1%, w/v) and H2O2 (6%, w/v), and their volumes were 10 mL, 150 μL and 2.5 μL, respectively.
2.2. Instruments
Electrospray ionisation tandem mass spectrometry (ESI-MS) with an Agilent HP1100 series (Agilent, Palo Alto, CA) and nuclear magnetic resonance (NMR) with both the DRX-400 and DRX-600 NMR spectrometers (Bruker, Germany) were used to analyse the fine structures of haptens. Ultraviolet–visible (UV–visible) spectra of immunogens were obtained with a UV-160A Shimadzu spectrophotometer (Kyoto, Japan). Sodium dodecyl sulfate-polyacrylamide gelelectrophoresis (SDS-PAGE) analysis was performed on a Mini-Protean Tetra cell (Bio-Rad, USA). ELISA plates were washed with a Multiskan MK2 microplate washer (Thermo Scientific, USA). The absorbance value at 450 nm of wavelength was recorded on a multiskan MK3 microplate reader (Thermo Scientific, USA). The 1200 serious LC system (Agilent Technologies, USA) equipped with the Agilent 6410 Triple Quad LC-MS system (Agilent Technologies, USA) was used for validating the analysis.
2.3. Hapten synthesis and characterisation
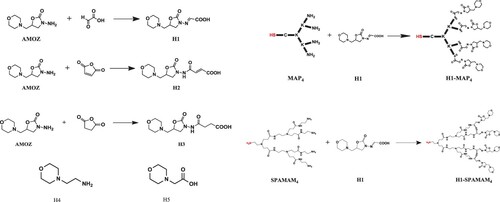
Seven haptens, including two multiple haptens, were designed and synthesised as shown in . H1 was designed by introducing a double bond and an active carboxyl group in the amide group of AMOZ. Multiple hapten H1-MAP4 was designed by H1 coupled to a four-branched dendron constructed by lysine and cysteine; multiple hapten H1-SPAMAM4 was designed by coupling H1 to a four-branched dendron constructed by ethylenediamine and methyl acrylate. H2 and H3 were designed by introducing an active carboxyl group in the amide group of AMOZ; H4 and H5, commercial 2-morpholinoethylamine and 2-morpholin-4-ylacetic acid that contain a morpholine ring same as AMOZ were adopted directly. The synthetic methods and characterisation data have been shown in Supporting Information Sections 1 and 2.
2.4. Immunogen and coating antigen
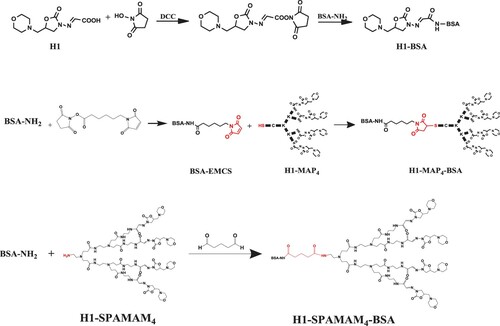
Three haptens (H1, H1-MAP4 and H1-SPAMAM4) were coupled to BSA for immunogens. Five haptens (H1–H5) were coupled to OVA for coating antigens. The active ester method was adopted to conjugate H1, H2, H3 and H5 to the carrier protein according to Wang et al. (Citation2017). H4 and H1-SPAMAM4 were connected with BSA via glutaraldehydes according to Romestand et al. (Citation2010). H1-MAP4 was connected with BSA via a heterobifunctional reagent EMCS. Briefly, BSA was modified with EMCS to make it a site for binding sulfhydryl presented in the hapten. 1.8 mmol EMCS in 0.2 mL DMF was added to a stirred solution of 20 mg BSA in 2-mL PBS. The mixture was left to be stirred overnight at 4°C. After dialysing against PBS, BSA-EMCS (BSA modified with EMCS) was obtained. Next, the solution of BSA-EMCS was added in 6.5-mg H1-MAP4 and stirred for 6 h at 4°C. After dialysing against PBS, the immunogen H1-MAP4-BSA was obtained. All hapten-protein conjugates were stored at −20°C after sub-packaging. The conjugates were confirmed by full wavelength UV–visible scanning and SDS-PAGE. The synthetic routes of three immunogens are shown in . The absorption spectra and electrophoretic patterns have been illustrated in Supporting Information Section 3.
2.5. Production of pAb
The Guangdong Medical Laboratory Animal Center was charged with the animal experiment. For each immunogen, two New Zealand male rabbits weighting 1.5–2.0 kg were immunised. After emulsifying with an equal volume of complete Freund’s adjuvant, immunogens were injected into rabbits hypodermically for the initial immunisation. Four weeks later, booster injections were given intraperitoneally every 3 weeks 4 times with the same amount of immunogen emulsified with incomplete Freund’s adjuvant. Every rabbit was injected with 0.5 mg coupled protein for each immunisation. Ten days later after the final immunisation, blood samples were collected and antiserums were obtained after low- and high-speed twice centrifugations. The antiserums were separated into aliquots and stored at −20°C until purification by saturated ammonium sulfate precipitation (Xu et al., Citation2013).
2.6. Characterisation of pAb
All the serum samples prepared were screened for their titres and binding activities to AMOZ via ciELISA with all coating antigens. For ciELISA, the plates were coated with coating antigen in carbonate buffer (1 mg/L, 100 μL/well) overnight at 37°C. The plates were washed 2 times with washing buffer, and then 5% skimmed milk in double-distilled water (200 μL/well) was added to block the excess binding sites. After removal of the liquid inside, the plates were dried for 1 h at 37°C. To each well of the plates 50 μL of AMOZ in PBS and 50 μL of antiserum in PBST were added, and then the plates were left to stand for 40 min at 37°C. After washing 5 times, diluted HRP-goat anti-rabbit IgG in PBST (1:5000, 100 μL/well) was added. The plates were incubated for 40 min at 37°C, and then washed 5 times. The wells were added to the TMB solution (100 μL/well) and incubated for 10 min at 37°C. The enzyme-catalysed reaction was stopped by the addition of H2SO4 (2 mol/L, 100 μL/well). At last, the absorbance values were recorded at 450 nm.
2.7. Development of ciELISA
To improve the ciELISA performance, several influential physicochemical parameters were optimised. The concentration of coating antigen and antibody was screened via a chessboard titration and other factors were optimised according to Luo et al. (Citation2014). Using a serial concentrations of AMOZ standard with different concentration was added to construct inhibition curves, and Amax and IC50 were obtained. A higher ratio of Amax to IC50 means better sensitivity under such conditions.
2.8. Cross-reactivity (CR)
The AMOZ solution in the ciELISA procedure was replaced with its analogues for determining the CR of the antibody. CR was calculated by the following formula: CR (%) = (AMOZ IC50 × 100)/IC50 of analogues tested.
2.9. Preparation of sample
Fish samples were confirmed to have no AMOZ residue by LC-MS/MS, which was completed by the Guangzhou Marine and Fisheries Environment Monitoring Center. Before homogenisation, the scale, skin and bone were taken out of the fish. Samples were fortified with AMOZ (in methanol) at the final levels of 1.0, 2.5 and 10 μg/kg, respectively. 2.5 g of the spiked sample was weighed into a 50-mL polypropylene centrifuge tube followed by the addition of 10 mL of 0.2 M HCl. After thorough mixing, the tube was subjected to ultrasound for 15 min to release the bond AMOZ from the tissue. The pH value of the mixture solution was adjusted to 7.0 with sodium hydroxide solution (1 M). The mixture was centrifuged (4000 r/min, 5 min), and then the supernatant was added in 1 g of aluminium oxide to remove parts of fat. After thorough mixing for 2 min, the mixture was centrifuged (4000 r/min, 5 min) again and the supernatant was collected. The supernatant was pushed through a Strata-X SPE cartridge which was preconditioned with 2 mL of acetonitrile and 2 mL of water. The cartridge was subsequently rinsed with 2 mL water, and then eluted with 3 mL acetonitrile twice. The collected eluent was dried under nitrogen and then redissolved in a 0.5-mL assay buffer. The extract was filtered with a microporous membrane (0.22 µm), and then used for ELISA analysis.
2.10. Assay validation
The LC-MS/MS method was employed to validate the ELISA analysis results, which was also completed by the Guangzhou Marine and Fisheries Environment Monitoring Center. A Shiseido Capcell PAK MG C18 (2.0 mm Å–150 mm, 3.0 μm particle size) column was used. Mobile phase A included water containing 0.1% formic acid and 1 mmol/L ammonium acetate, and mobile phase B included acetonitrile, and it was used in the following gradient profile: 0.2 min, 20% B; 9.0 min, 95% B and 9.1 min, 20% B. The flow rate of the mobile phase was 0.25 mL/min, and an aliquot of 10 μL of each sample was injected into the LC system. Analyses were determined by ESI-MS/MS in positive mode. The gas temperature was 600°C and the capillary voltage was 5.5 kV. High-purity nitrogen (>99.99%) served as the nebuliser and collision gas.
3. Results and discussion
3.1. Hapten design, synthesis and characterisation
In the development of immunoassays for small organic molecules, as well known, hapten design plays a significant role. However, for some molecules with super-low molecular weight or simple structure, the desired effect cannot be always achieved simply by altering the structure of hapten (Isanga et al., Citation2017; Luo et al., Citation2014; Romestand et al., Citation2010; Xu et al., Citation2013). At these cases, antibodies were always produced against the derivative but not the small analyte, which means that a pre-derivatisation of the small analyte is necessary prior to analysis. The derivative strategy is always time-consuming and cumbersome. For AMOZ, a derivative strategy was adopted in most immunoassays (Pimpitak et al., Citation2009; Shen et al., Citation2012). It would take at least 16 h to perform only the derivative step, which restricts the wide application of the established immunoassays.
In this present work, the research effort was focused on the hapten design. Three different types of immunising haptens were synthesised for AMOZ, trying to produce high-quality antibodies. For H1, the double bond was expected to expose the hapten parts of the immunogen to the surface of the carrier protein and the carboxyl group could react with primary amino groups presented in carrier proteins or dendrons. Based on the inspiration of the use of MAP to enhance the immunogenicity of a vaccine (Briand et al., Citation1992; Heegaard et al., Citation2010; Joshi et al., Citation2013; Manki et al., Citation1998; Tam, Citation1996a), H1 was coupled to dendrimers to obtain multiple haptens. H1-MAP4 was prepared by attaching H1 to a four-branched dendron constructed by lysine and cysteine, which was similar to the one described in Gómara et al. (Citation2000), with the only difference lying in the amino acid residue that cysteine instead of alanine was introduced in the carboxyl terminus. H1-SPAMAM4 was prepared by coupling H1 with a four-branched dendron that a single branch of a second-generation polyamidoamine (PAMAM) constructed with ethylenediamine and methyl acrylate. The spectra of ESI-MS for H1-MAP4 and H1-SPAMAM4 proved that both multiple haptens were prepared successfully (see Supporting Information Section 4). It was hoped that these two multiple haptens will enhance the immune response of the animal to hapten with the increase of the hapten ratio on the carrier protein. Several heterologous coating haptens () were also designed and synthesised since the heterologous coating has been proven useful for the improvement of assay sensitivity.
3.2. Production and characterisation of antisera
All the antisera against each immunogen were harvested and screened for their titre and binding ability to free AMOZ via ciELISA with all coating antigens. The results are shown in . The titre of antiserum H1-MAP4 and antiserum H1-SPAMAM4 was 4–10 times higher than that of antiserum H1-BSA, but all the antisera showed no or only little (11% inhibition of antiserum H1-SPAMAM4 at 0.5 mg/L of AMOZ) binding ability to free AMOZ. It seemed that the application of dendrimer to enhance the ratio of haptens on the carrier could only improve the titre of the antiserum, but not improve the binding ability to free AMOZ. Interestingly, the following screening of heterologous coating found that all the antisera showed good binding ability to AMOZ while using H2-OVA as the coating antigen, the inhibition of 0.5-mg/L AMOZ towards antiserum H1 in ciELISA was about 69%, and it was 52% and 89% towards antiserum H1-MAP4 and antiserum H1-SPAMAM4, respectively. The antiserum H1 showed higher titre and binding ability than that of antiserum H1-MAP4 and H1-SPAMAM4 when H4-OVA and H5-OVA were used as coating antigens, indicating the different specificities of antisera using different immunising haptens.
Table 1. Characterisation of antiserum against free AMOZ under homologous and heterologous ciELISA.
There are several possible reasons for the observed increase in the antibody specificity of antiserum H1-SPAMAM4. It is claimed that the characteristic high-density haptens obtained by this method have a favourable immunogenicity. It has been proved that the increase in hapten density could lead to an increase in immunogenicity of the antigen. Another alternative is that the SPAMAM construct allows the AMOZ part (the epitope) to be processed and presented by antigen presenting cells in a more efficient manner. Thanks to the poor flexibility, the SPAMAM dendron provides a rigid support for exposing epitopes sufficiently, leading to a stronger immune response. The special conformation of the immunogen may also be a possible reason. The multiple hapten part attains a somewhat unnatural polymeric structure which may aid in resistance to proteolytic degradation resulting in a longer immunogen halftime, which contributes to a longer lasting response.
3.3. Optimisation of ciELISA
For further comparing the difference in the sensitivity, both antiserum H1-SPAMAM4 and antiserum H1 were applied to develop ciELISA, using H2-OVA as the coating antigen. The concentrations of coating antigen and antibody, reacting buffer system, Tween-20 concentration, ionic strength and pH were optimised. The effect of physicochemical parameters on ELISA performances has been shown in Supporting Information Section 5.
Finally, the optimum operation conditions of two antisera were determined and are summarised in . Under the optimal conditions, the dose–response curves for AMOZ were constructed and are shown in . On comparison with the traditional hapten, the strategy of using the novel non-amino acid multiple hapten as the immunising hapten was able to obtain a two- to three-fold improvement in sensitivity. The IC50 for AMOZ is 20.7 μg/L, the limit of detection (LOD) is 0.9 μg/L and the linear range was from 2.9 to 145.2 μg/L for the antiserum H1-SPAMAM4-based ciELISA.
Figure 3. Standard curves and calibration curves in the linear range (inset) of two antiserums for AMOZ.
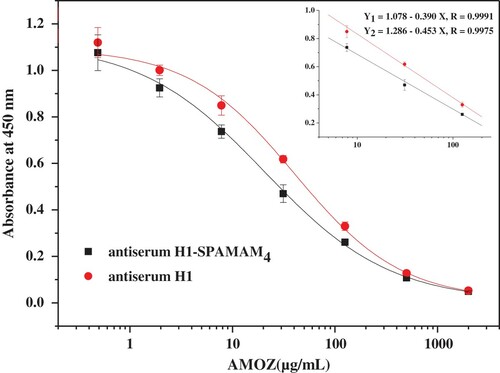
Table 2. The condition and sensitivity to AMOZ for antiserum H1- and antiserum H1-SPAMAM4-based ciELISA.
3.4. Assay specificity
For evaluating the applicability in AMOZ screening, the selectivity of the obtained polyclonal antisera was tested by examining the CR to parent nitrofurans and other nitrofuran metabolites in the optimised ciELISA condition. The test result is summarised in . When the reactivity to AMOZ was set as 100%, both antisera showed significant CR with their parent drug furaltadone; the values were 188% and 165%, respectively. There was a negligible cross-reaction with other tested compounds, less than 0.1%. This result was in agreement with the works of other researchers and may be explained by the strikingly similar structure of AMOZ and furaltadone. Both of them contain a sub-structure, 3-imidogen-5-morpholinomethyl-2-oxazolidone. Thanks to its instability, furaltadone is rapidly metabolised into AMOZ in vivo. Therefore, no residue of furaltadone in samples is always observed. On the other hand, the high CR with furaltadone provides a possibility for the antibody exploited to detect furaltadone abuse in the feed.
Table 3. Cross-reactivity of obtained pAbs with AMOZ and its analogues.
3.5. Sample preparation and recovery test
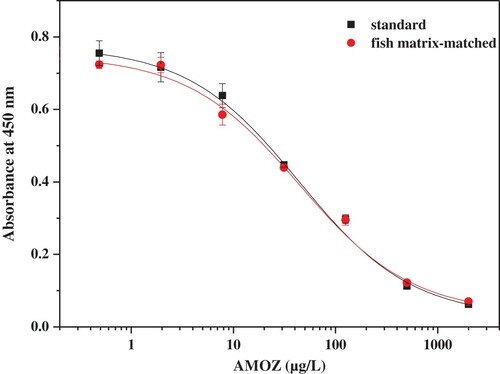
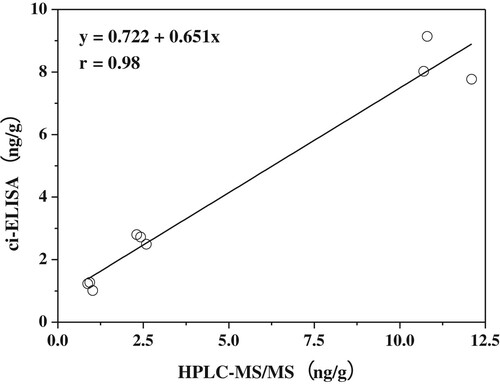
The accuracy of the developed ciELISA was investigated by carrying out recovery tests. Before the analysis of spiked samples, the interference from the matrix was evaluated by comparing the standard cure with the matrix-matched cure (). The two cures were very similar, indicating that there was no interference to the sensitivity of ciELISA and this extraction was satisfactory. Samples were spiked with AMOZ at levels of 1.0, 2.5 and 10.0 μg/kg. An appropriate recovery ranging from 83.1% to 117.0% was obtained, with the coefficients of variation (CV) ranging from 6.0% to 11.9% (). The results of ciELISA were further confirmed by standard HPLC-MS (high-performance liquid chromatography mass spectrometry)/MS. As shown in , the squared coefficient of correlation was 0.98 between the results of ciELISA and HPLC-MS-MS using spiked fish samples, which indicated good reliability and accuracy of the proposed ciELISA.
Table 4. Recoveries of spiked fish samples by ciELISA (n = 6, μg/kg).
Given an enrichment factor of five, the LOD of the developed ciELISA for AMOZ in fish samples was corrected to 0.2 μg/kg, and the linear range was corrected to 0.6–29.0 μg/kg. The sensitivity of this assay can satisfy the detecting need; the minimum required performance limit for AMOZ had been set at 1.0 μg/kg in the EU.
4. Conclusion
Due to the unique molecular characteristics of dendrimers (multimericity, definability and derivatisability), they demonstrated the ability to enhance the immunogenicity of vaccine antigen for immunoassay. However, in this work, no significant improvement in the immune response, especially the specific response to the hapten epitope, was observed when using AMOZ as a model case. The immune response of the animal to immunogens was complicated and it was hard to say why the dendrimers did not work in our study. Further application of dendrimers in the field of hapten immunoassay can be made since they had been used as carrier molecules to enhance immunogenicity of antigens for vaccine purposes (Heegaard et al., Citation2010), as molecular adjuvants (Shukla et al., Citation2012; Tam, Citation1996a). A recent work showed that the use of self-synthesised third generation dendri-graft-L-lysine (DGL-G3) can act as an efficient carrier for raising antibodies directed against small molecules of histamine (Romestand et al., Citation2010). The potential of dendrimers in the improvement of immunoassay characteristics can be further studied.
In conclusion, an immunising multiple hapten was provided for producing antibodies specific to AMOZ and a pAb-based competitive indirect ELISA without derivatisation was developed for determining AMOZ residue. For AMOZ, the IC50 value was 4.1 μg/kg and the LOD was 0.2 μg/kg in fish samples. The recovery from spiked fish samples ranged from 83.1% to 117.0%, with the CV below 12%. Good consistencies were obtained between the results of ciELISA analysis and standard LC-MS/MS analysis. Meanwhile, without the step of derivatisation, which is necessary in the current immunoassays and standard chromatographic analysis, the sample pre-treatment in the ciELISA was much easier.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anfossi, L., Di Nardo, F., Giovannoli, C., Passini, C., & Baggiani, C. (2015). Enzyme immunoassay for monitoring aflatoxins in eggs. Food Control, 57, 115–121. doi: https://doi.org/10.1016/j.foodcont.2015.04.013
- Boas, U., & Heegaard, P. M. H. (2004). Dendrimers in drug research. Chemical Society Reviews, 33, 43–63. doi: https://doi.org/10.1039/b309043b
- Bolarinwa, I. F., Orfila, C., & Morgan, M. R. A. (2014). Development and application of an enzyme-linked immunosorbent assay (ELISA) for the quantification of amygdalin, a cyanogenic glycoside, in food. Journal of Agricultural and Food Chemistry, 62, 6299–6305. doi: https://doi.org/10.1021/jf501978d
- Briand, J. P., Barin, C., van Regenmortel, M. H., & Muller, S. (1992). Application and limitations of the multiple antigen peptide (MAP) system in the production and evaluation of antipeptide and anti-protein antibodies. Journal of Immunological Methods, 156, 255–265. doi: https://doi.org/10.1016/0022-1759(92)90033-P
- Chappey, O., Debray, M., Niel, E., & Scherrmann, J. M. (1994). Association constants of monoclonal antibodies for hapten: Heterogeneity of frequency distribution and possible relationship with hapten molecular weight. Journal of Immunological Methods, 172, 219–225. doi: https://doi.org/10.1016/0022-1759(94)90109-0
- Cooper, K. M., Caddell, A., Elliott, C. T., & Kennedy, D. G. (2004). Production and characterisation of polyclonal antibodies to a derivative of 3-amino-2-oxazolidinone, a metabolite of the nitrofuran furazolidone. Analytica Chimica Acta, 520, 79–86. doi: https://doi.org/10.1016/j.aca.2004.05.074
- Escher, B. I., & Fenner, K. (2011). Recent advances in environmental risk assessment of transformation products. Environmental Science and Technology, 45, 3835–3847. doi: https://doi.org/10.1021/es1030799
- Gomaa, A., & Boye, J. (2015). Simultaneous detection of multi-allergens in an incurred food matrix using ELISA, multiplex flow cytometry and liquid chromatography mass spectrometry (LC-MS). Food Chemistry, 175, 585–592. doi: https://doi.org/10.1016/j.foodchem.2014.12.017
- Gomara, M. J., Riedemann, S., Vega, I., Ibarra, H., Ercilla, G., & Haro, I. (2000). Use of linear and multiple antigenic peptides in the immunodiagnosis of acute hepatitis A virus infection. Journal of Immunological Methods, 234, 23–34. doi: https://doi.org/10.1016/S0022-1759(99)00196-9
- Gujral, N., Yoo, H., Bamdad, F., Suh, J. W., & Sunwoo, H. (2017). Sensitive double antibody sandwich elisa for the quantification of phosvitin. Food and Agricultural Immunology, 28, 834–847. doi: https://doi.org/10.1080/09540105.2017.1313821
- Heegaard, P. M. H., Boas, U., & Sorensen, N. S. (2010). Dendrimers for vaccine and immunostimulatory uses. A review. Bioconjugate Chemistry, 21, 405–418. doi: https://doi.org/10.1021/bc900290d
- Isanga, J., Mukunzi, D., Chen, Y., Suryoprabowo, S., Liu, L., Kuang, H., & Xu, C. (2017). Development of a monoclonal antibody assay and a lateral flow strip test for the detection of paromomycin residues in food matrices. Food and Agricultural Immunology, 28(3), 355–373. doi: https://doi.org/10.1080/09540105.2016.1272551
- Janči, T., Valinger, D., Gajdoš, K. J., Mikac, L., Vidaček, S., & Ivanda, M. (2017). Determination of histamine in fish by surface enhanced Raman spectroscopy using silver colloid SERS substrates. Food Chemistry, 224, 48. doi: https://doi.org/10.1016/j.foodchem.2016.12.032
- Jester, E. L. E., Abraham, A., Wang, Y. S., El Said, K. R., & Plakas, S. M. (2014). Performance evaluation of commercial ELISA kits for screening of furazolidone and furaltadone residues in fish. Food Chemistry, 145, 593–598. doi: https://doi.org/10.1016/j.foodchem.2013.08.090
- Joshi, V. G., Dighe, V. D., Thakuria, D., Malik, Y. S., & Kumar, S. (2013). Multiple antigenic peptide (MAP): a synthetic peptide dendrimer for diagnostic, antiviral and vaccine strategies for emerging and re-emerging viral diseases. Indian Journal of Virology, 24, 312–320. doi: https://doi.org/10.1007/s13337-013-0162-z
- Kong, D. Z., Xie, Z. J., Liu, L. Q., Song, S. S., Kuang, H., & Xu, C. L. (2017). Development of ic-ELISA and lateral-flow immunochromatographic assay strip for the detection of vancomycin in raw milk and animal feed. Food and Agricultural Immunology, 28(3), 414–426. doi: https://doi.org/10.1080/09540105.2017.1293014
- Luo, L., Xu, Z. L., Yang, J. Y., Xiao, Z. L., Li, Y. J., Beier, R. C., … Shen, Y. D. (2014). Synthesis of novel haptens and development of an enzyme-linked immunosorbent assay for quantification of histamine in foods. . Journal of Agricultural and Food Chemistry, 62, 12299–12308. doi: https://doi.org/10.1021/jf504689x
- Manki, A., Ono, T., Uenaka, A., Seino, Y., & Nakayama, E. (1998). Vaccination with multiple antigen peptide as rejection antigen peptide in murine leukemia. Cancer Research, 58, 1960–1964.
- McCracken, R. J., Blanchflower, W. J., Rowan, C., McCoy, MA,, & Kennedy, D. G. (1995). Determination of furazolidone in porcine tissue using thermospray liquid chromatography-mass spectrometry and a study of the pharmacokinetics and stability of its residues. The Analyst, 120, 2347–2351. doi: https://doi.org/10.1039/AN9952002347
- Mu, H. T., Wang, B. L., Xu, Z. L., Sun, Y. M., Huang, X. A., Shen, Y. D., … Lei, H. T. (2015). Stereospecific recognition and quantitative structure-activity relationship between antibodies and enantiomers: Ofloxacin as a model hapten. The Analyst, 140, 1037–1045. doi: https://doi.org/10.1039/C4AN02155J
- Pimpitak, U., Putong, S., Komolpis, K., Petsom, A., & Palaga, T. (2009). Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for detection of the furaltadone metabolite, AMOZ, in fortified shrimp samples. Food Chemistry, 116, 785–791. doi: https://doi.org/10.1016/j.foodchem.2009.03.028
- Points, J., Burns, T. D., & Walker, M. J. (2015). Forensic issues in the analysis of trace nitrofuran veterinary residues in food of animal origin. Food Control, 50, 92–103. doi: https://doi.org/10.1016/j.foodcont.2014.08.037
- Romestand, B., Rolland, J. L., Commeyras, A., Coussot, G., Desvignes, I., Pascal, R., & Vandenabeele-Trambouze, O. (2010). Dendrigraft poly-L-lysine: A Non-immunogenic synthetic carrier for antibody production. Biomacromolecules, 11, 1169–1173. doi: https://doi.org/10.1021/bm9012056
- Selvi, A. A., Sreenivasa, M. A., & Manonmani, H. K. (2011). Enzyme-linked immunoassay for the detection of glyphosate in food samples using avian antibodies. Food and Agricultural Immunology, 22(3), 217–228. doi: https://doi.org/10.1080/09540105.2011.553799
- Shen, X., Chen, J. H., Li, X. M., Lei, H. T., Xu, Z. L., & Liu, Y. J. (2017). Monoclonal antibody-based homogeneous immunoassay for three banned agonists and molecular modeling insight. Food and Agricultural Immunology, 31, 1–12. doi: https://doi.org/10.1080/09540105.2017.1347149
- Shen, Y. D., Xu, Z. L., Zhang, S. W., Wang, H., Yang, J. Y., Lei, H. T., … Sun, Y. M. (2012). Development of a monoclonal antibody-based competitive indirect enzyme-linked immunosorbent assay for furaltadone metabolite AMOZ in fish and shrimp samples. Journal of Agricultural and Food Chemistry, 60, 10991–10997. doi: https://doi.org/10.1021/jf302913h
- Shukla, N. M., Salunke, D. B., Balakrishn, R., Mutz, C. A., Malladi, S. S., & David, S. A. (2012). Potent adjuvanticity of a pure TLR7-agonistic imidazoquinoline dendrimer Plos One, 7, e43612. doi: https://doi.org/10.1371/journal.pone.0043612
- Tam, J. P. (1996a). Multiple antigen peptide system having adjuvant properties, vaccines prepared therefrom and methods of use thereof (U.S. Patent No. 5,580,563). Retrieved from http://www.google.co.in/patents/US5580563
- Tam, J. P. (1996b). Recent advances in multiple antigen peptides. Journal of Immunological Methods, 196, 17–32. doi: https://doi.org/10.1016/0022-1759(96)00066-X
- Tighe, P. J., Ryder, R. R., Todd, I., & Fairclough, L. C. (2015). ELISA in the multiplex era: Potentials and pitfalls. Proteomics – Clinical Applications, 9, 406–422. doi: https://doi.org/10.1002/prca.201400130
- Vroomen, L. H., Berghmans, M. C., van Bladeren, P. J., Groten, J. P., Wissink, C. J., & Kuiper, H. A. (1990). In vivo and in vitro metabolic studies of furazolidone: A risk evaluation. Drug Metabolism Reviews, 22, 663–676. doi: https://doi.org/10.3109/03602539008991460
- Wang, Y., Yang, J. Y., Shen, Y. D., Sun, Y. M., Xiao, Z. L., & Lei, H. T. (2017). Novel haptens synthesis and development of a monoclonal antibody-based enzyme-linked immunosorbent assay for leuco-malachite green in fish. Food and Agricultural Immunology, 43, 1–17. doi: https://doi.org/10.1080/09540105.2017.1348490
- Xie, Z. J., Kong, D. Z., Liu, L. Q., Song, S. S., & Kuang, H. (2017). Development of ic-ELISA and lateral-flow immunochromatographic assay strip for the simultaneous detection of avermectin and ivermectin. Food and Agricultural Immunology, 28(3), 439–451. doi: https://doi.org/10.1080/09540105.2017.1293016
- Xu, Z. L., Shen, Y. D., Sun, Y. M., Campbell, K., Tian, Y. X., Zhang, S. W., … Jiang, Y. M. (2013). Novel hapten synthesis for antibody production and development of an enzyme-linked immunosorbent assay for determination of furaltadone metabolite 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ). Talanta, 103, 306–313. doi: https://doi.org/10.1016/j.talanta.2012.10.059
- Yan, X. D., Hu, X. Z., Zhang, H. C., Liu, J., & Wang, J. P. (2012). Direct determination of furaltadone metabolite, 3-amino-5-morpholinomethyl-2-oxazolidinone, in meats by a simple immunoassay. Food and Agricultural Immunology, 23(3), 203–215. doi: https://doi.org/10.1080/09540105.2011.615060
- Zhang, Y., Yang, J., Lu, Y., Ma, D. Y., Qi, M. G., & Wang, S. (2017). A competitive direct enzyme-linked immunosorbent assay for the rapid detection of deoxynivalenol: Development and application in agricultural products and feedstuff. Food and Agricultural Immunology, 28(3), 516–527. doi: https://doi.org/10.1080/09540105.2017.1306491