ABSTRACT
Edible medicinal plants Curcuma longa, Kaempferia galanga and Zingiber officinale are considered to be suitable for pregnant mother and neonates. Their essential oils are claimed to have therapeutic effects, yet its immunomodulatory activities need to be investigated. To validate the immunomodulatory effect, cord blood monocyte cells (CBMCs) treated with different concentrations of essential oils ranging from 0 to 40 µg/ml. K. galanga oil at 40 µg/ml concentrations upregulated IFN-γ at 49.23 pg/ml, while C. longa oil downregulated the IL-10 at 10.45 pg/ml showing immunostimulant activity on CBMCs. However, Z. officinale oil showed no significant result at any concentration. K. galanga and C. longa oils also showed an increase in lymphocyte proliferation in a dose-dependent manner. The present study was aimed to evaluate the immunomodulatory potentialities of essential oils which will attract the interest of researchers and pharmaceutical industries for clinical studies and other applications in the therapy of diseases related to neonatal infections.
GRAPHICAL ABSTRACT
1. Introduction
Neonatal immune responses have been assumed to be less mature than adult immune responses; however, the mechanisms involved in these responses are not properly characterized. There is a shift from T helper 1 (Th1) response to a T helper 2 (Th2) bias during pregnancy (Wegmann, Lin, Guilbert, & Mosmann, Citation1993). The mechanism that regulates the Th1 and Th2 ratio is still to be clarified in order to understand the importance of maternal immune tolerance during pregnancy. T-lymphocytes exhibit a Th2 profile at birth and are characterized by a limited ability to produce cytokines. After birth, for few months, these Th2-skewed responses are modified into low-level immunity, predominantly expressing Th1-cytokines (Holt & Jones, Citation2000; Prescott et al., Citation1999). Cytokine is the major immunoregulator of the body. TH1 cytokine response leads towards cell-mediated humoral immune response. Interferon gamma (IFN-γ) is a TH 1 cytokine mainly displaying antiviral, anti-tumour and counter-autoimmune capacities (Schroder, Hertzog, & Ravasi, Citation2004). Activation and the increase in antigen presentation by macrophages increase leukocyte migration and upregulation of MHC expression, which are some prominent features of IFN-γ (Tayal & Kalra, Citation2008). Patients under clinical studies with recombinant IFN-γ show a vast spectrum of activities which include enhancement of antibody-dependent cell-mediated cytotoxicity and an increase in the oxidative metabolism of macrophages (Tayal & Kalra, Citation2008). IFN-γ expression on tumour growth induces CD8+ cytotoxic T-cells and thus shows potent tumour immunity against neuroblastoma, breast cancer and other tumour models (Young & Hardy, Citation1995). The damage of proliferating cells and depletion of peripheral blood lymphocytes result in immune suppression, leaving organisms vulnerable to various adaptable pathogens and even cause various infections that could become lethal (Goel, Prakash, Ali, & Bala, Citation2007). At present, IFN-γ therapy is active in the treatment of chronic granulomatous disease, idiopathic pulmonary fibrosis, osteoporosis and ovarian cancer (Cutler & Brombacher, Citation2005). On the other hand, the immunosuppressive cytokine IL-10 downregulates the cell-mediated immune response by inhibiting and suppressing various pro-inflammatory cytokines like IL-1, IL-2, IL-6, IL-8 and TNF-α (Niiro, Otsuka, & Kuga, Citation1994; de Waal Malefyt, Yssel, & de Vries, Citation1993). In various B-cell lymphoproliferative diseases, IL-10 may function as a growth factor for malignant B cells (Beatty, Krams, & Martinez, Citation1997); and elevated levels of IL-10 were found to be associated with the systemic infection, rheumatoid arthritis, multiple sclerosis, melanomas, leukaemia and lymphomas. Immunomodulation of the lymphocyte through dietary supplements either towards TH 1 or TH 2 pathway mainly determines the pattern of the immune response. There are several medicinal plants in India that are being used traditionally for the prevention and treatment of various diseases, but till date only few medicinal plants were being studied for the immunomodulatory effect of their derived materials. The rhizomes of Curcuma longa (Haldi), Kaempferia galanga (Aromatic ginger) and Zingiber officinale (Ginger) plants are dietary supplement in India. They are also used in aroma therapy and claim to have therapeutic effects like anti-oxidative, anti-cancer, anti-inflammatory and antimicrobial properties (Bagad, Joseph, Bhaskaran, & Agarwal, Citation2013). Surpluses of plant-derived materials (aromatic compounds, proteins, lectins, polysaccharides, etc.) have been shown to stimulate the immune system (Tzianabos, Citation2000). Some of the plants with established immunomodulatory activity are Asparagus racemosus, Azadirachta indica, Tinospora cordifolia, Polygala senega and Ocimum santum (SaiRam, Sharma, Ilavazhagan, Kumar, & Selvamurthy, Citation1997; Estrada, Katselis, Laarveld, & Barl, Citation2000; Mediratta, Sharma, & Singh, Citation2002). There are several essential oil yielding plants like Salvia officinales, Syzgium aromaticum, Mentha sps., Eucalyptus sps. and Ocimum basilicum that have been found to stimulate immune cell proliferation, cytokines expression and natural killer cells cytotoxicity, both in vitro and in vivo (Carrasco et al., Citation2009; Yousofi, Daneshmandi, Soleimani, Bagheri, & Karimi, Citation2012; Premanathan et al., Citation2000). The immunomodulating activity of the essential oil yielding medicinal plants like C. longa, K. galanga and Z. officinale which are edible spices and have been reported to have anti-oxidative, anti-cancer, anti-inflammatory and antimicrobial properties (Craig, Citation1997; Newman, Cragg, & Snader, Citation2003; Murakami, Takahashi, Jiwajinda, Koshimizu, & Ohigashi, Citation1999) is yet underexplored. Till date, there are very little reports whether these essential oils are capable of direct effects on cytokine production and activation of cord blood mononuclear cells (CBMCs). The present study has been designed to find out the immunomodulatory role of rhizome oils of three edible medicinal plants C. longa, K. galanga and Z. officinale in the stimulation of Th1 and Th2 cytokines of CBMCs. The findings of this study could help us to understand how neonates raise different Th1 and Th2 responses in human CBMCs and the influence of plant products (oils) in the development of the neonatal immune system.
2. Materials and methods
2.1. Collection of rhizomes
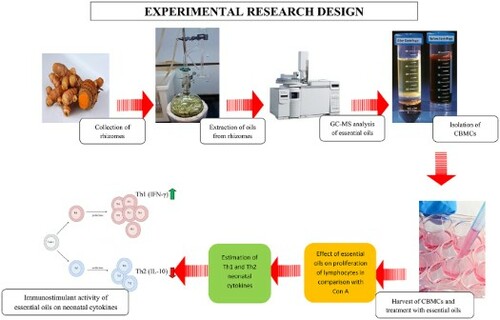
The rhizomes of C. longa, K. galanga and Z. officinale () were collected from different parts of Odisha and the specimen was authenticated by Dr P.C. Panda, Senior Scientist, Taxonomy and Conservation Division of Regional Plant Resource Centre, Bhubaneswar. The collected rhizomes were planted in the greenhouse of Centre of Biotechnology and after complete growth, the rhizomes were collected from these plants. The specimens are retained in the herbarium of COPT vide Voucher no. 1803.
2.2. Extraction of essential oil
The oils were extracted by steam distillation of fresh rhizomes in a Clevenger’s apparatus (Guenther, Citation1972). The fresh rhizomes of each plant were washed, peeled and sliced. Sliced rhizomes (100 g) were mixed with distilled water. Flasks containing the sliced rhizomes were heated for 3–4 h and the condensed vapours were separated throughout an auto-oil/water separator. The oils were collected in the collecting tubes. Each essential oil extraction was run in triplicate. Yield percentage was recorded on a fresh weight basis. The distilled essential oils were dried over anhydrous sodium sulphate and stored at –4°C in airtight containers for further use.
2.3. GC–MS analysis of oils
GC–MS analysis of the oils were done using a 6890 series instrument (Agilent Technologies, Palo Alto, CA, USA), provided with an FID and HP-5 fused silica capillary column (30 m × 0.25 mm) (internal diameter: film thickness 0.25 m). The oven temperature was maintained from 50°C to 240°C at 4°C/min; from 240°C to 270°C at 15°C/min; held isothermal at 50°C for 1 min and at 270°C for 15 min. Both auto-injector and detector’s temperatures were kept at 280°C; sample injection volume was 1 µl and the split ratio was 100:1. Nitrogen was used as the carrier gas having a flow rate of 1.2 ml/min. GC–MS (70 eV) data were calculated on the same gas chromatograph coupled with an MSD 5973. MS source temperature was 230°C; MS quadrapole temperature was 150°C; interface temperature was 290°C; mass scan, 20–600 amu and helium was the carrier gas with a flow rate of 1.0 ml/min. Identification of the compounds was done by comparing the retention indices and mass spectra with the data given in the literature, National Institute of Standards and Technology (NIST), Wiley and our own created library (Adams, Citation2007).
2.4. Isolation of CBMCs
Human umbilical cord blood from randomly selected full-term healthy infants (37 week of gestation) were obtained by venipuncture of the umbilical vein immediately after delivery and placed in sterile sodium heparin tubes. The heparinized whole blood was centrifuged for 20 min at 2000 RPM at a temperature of 15°C to obtain leukocyte ring. The ring was extracted, transferred to a Falcon tube, and added to phosphate-buffered saline (PBS) to complete 10 ml. Subsequently, to obtain the CBMCs, the homogenate was applied to the medium density gradient using Ficoll-PaqueTM PLUS (GE Healthcare). This solution was centrifuged for 20 min at 1000 RPM at a temperature of 15°C to obtain leukocyte cloud. The cells from the interphase were extracted with the help of a pipette and washed three times in PBS (10 min, 1000 RPM, 15°C). After the last wash, cells were maintained in RPMI 1640 medium with 20 mm Hepes supplemented with 10% foetal bovine serum (FBS), HiMedia. The number of CBMCs was counted by using a haemocytometer.
2.5. Cell proliferation assays
These assays were performed by using the e-Bioscience MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit as per the manufacturer’s instruction. Briefly, human CBMCs suspensions were resuspended (5 × 106cells/ml) in RPMI-1640, Roswell Park Memorial Institute medium in the presence of ConA (Concanavalin A). Then 100 μl of different concentrations (0, 10, 20, 30 and 40 μg/ml) of each oil in RPMI-1640 was added. The plates were incubated for 48 h under 5% CO2 at 37°C temperature. After incubation, 10 μl of MTT (2.5 mg/ml) solution was added to each well and plates were wrapped to avoid exposure to light and incubated for 4 h. One hundred microlitres of the solubilizing reagent was added to each well and the absorbance was measured at 570 nm using a Mindray MR-96A microplate absorbance reader.
2.6. Flow cytometry analysis for lymphocyte proliferation
CBMCs were isolated from cord blood samples by density gradient centrifugation. Then the purified CBMC were reconstituted with PBS + 5% FBS and stained with 5, 6-carboxyfluorescein diacetatesuccinimidyl ester (CFSE) (Sigma, USA) at a concentration of 5 μM for 5 min. The staining was stopped by adding RPMI media + 10% FBS and centrifuging them at 1000 RPM for 10 min. CBMCs were washed with RPMI media + 10% FBS for two times and reconstituted to 1 × 105 cells/ml. The stained cells were cultured for 48 h under stimulation in vitro with essential oil of C. longa and K. galanga both at the concentration of 40 µl/ml and mitogen (Concanavalin A) for studying the lymphocyte proliferative responses. Then the cultured cells were stained with propidium iodide to exclude the dead cells and proliferation was measured by using FACS (BD FACSCalibur Flow Cytometer, BD Biosciences, France). Cytokine levels were measured by using ELISA Kits and expressed in pg/ml by interpolation from the standard curve as described by the manufacturer’s instruction. Histogram was designed with the software FlowJo Version 7.6.5.
2.7. Estimation of cytokine
Fresh CBMCs were cultured in triplicate at a concentration of 5 × 106cells/ml. C. longa, K. galanga and Z. officinale oil at the concentration of 5 μg/ml were added separately into a 96 wells (U-bottom) tissue culture plate. ConA (10 ng/ml) was used as the positive control for stimulation of IL-10 and IFN-γ, respectively. The control group of cells was cultured without any oil. The plates were incubated in a CO2 incubator (5% carbon dioxide) at 37°C for 48 h. A separate set of wells was used for harvesting culture supernatants for cytokine production. The culture supernatants were harvested, centrifuged and the supernatants were stored at –70°C for estimation of cytokine. Later on cytokines were quantified by using IFN-γ and IL-10 sandwich ELISA kits (e-Bioscience) as per the manufacturer’s instruction and were expressed in picograms per millilitre by interpolation from standard curves.
2.8. Statistical analysis
Data were analysed for statistical significance using Student’s t-test.. T-cell proliferative response was compared by using Fischer’s exact test. The unpaired t-test was used for comparison of cytokine secretion among oil treated and untreated samples. A P value <.05 was considered as significant.
3. Results
3.1. GC–MS analysis of oils
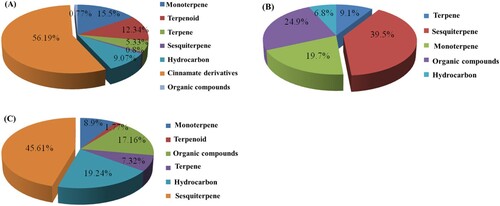
The GC–MS analysis of the rhizome oils of C. longa, K. galanga and Z. officinale species revealed the presence of monoterpenes, sesquiterpenes, terpenoids, hydrocarbons and few traces of organic compounds that were identified by comparing the fragmentation patterns in the resulting mass spectra with the published literature (Adams, Citation2012) and using the NIST mass spectral database of the gas chromatograph’s computer. The rhizome oil of C. longa contains a high proportion of sesquiterpenes (39.5%) in which ar-turmerone, α-turmerone and β-turmerone were the major constituents. The remaining compounds were reported to be monoterpenes (19.7%), terpenes (9.1%), hydrocarbon (6.8%) and few organic compounds (24.9%) in which 1,8-cineole was the major constituent (). The rhizome oil of K. galanga showed the dominance of cinnamate derivatives (56.19%) in which ethyl-p-methoxycinnamate (32%) and methyl cinnamate (15.07%) were the major constituents. The other compounds found were monoterpenes (15.5%) in which eucalyptol (9.64%) was the major component, terpenoids (12.34%) in which carvone (8.31%) was the major component. The remaining consisted of few traces of terpenes (5.33%), sesquiterpenes (0.8%), hydrocarbons (9.07%) and other organic compounds (0.77%). The rhizome oil of Z. officinale contained high sesquiterpenes (45.61%) in which zingiberene (29.13%) was the major compound followed by β-sesquiphellandrene (10%). The other groups of compound found were monoterpenes (8.9%), terpenoides (1.77%), terpenes (7.32%), hydrocarbons (19.24%) and other organic compounds (17.16%) ().
3.2. Proliferation assay
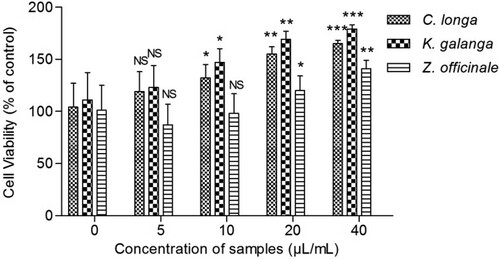
CBMCs cell count was increased with the treatment of different oils in comparison to control (without oil) which indicates that CBMC proliferation occurs in response to essential oil of these plants. The essential oil from C. longa and K. galanga rhizome was found to significantly simulate the proliferation of lymphocytes at all concentrations (0–40 μg/ml). At 40 μg/ml, the percentage of cell viability was 165 ± 2.99% for C. longa and 179 ± 3.89% for K. galanga which is much higher than that of the control. Also rhizome oil of Z. officinale showed a significant increase in lymphocyte proliferation at higher concentrations ().
Figure 3. Effects of various rhizome oils on the proliferation of CBMCs. All data are presented as the mean ± SD of five measurements. CBMCs (5 × 106 cells/ml) were cultured in vitro with Con A (1 µl/ml) along with different doses of various oils (0–40 µl/ml). After 48 h of incubation, the CBMCs viability was measured by MTT method. NS: non-significant (P > .05); **P < .01; ***P < .001 compared with 0 µl/ml.
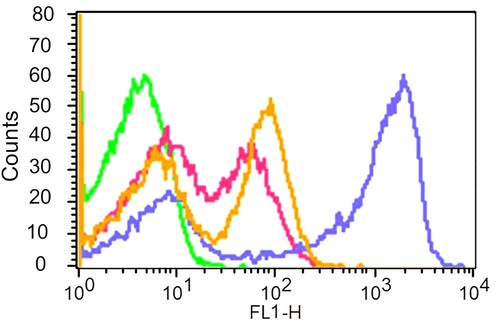
3.3. Flow cytometry analysis for lymphocyte proliferation
The effect of the essential oil of C. longa and K. galanga was tested on CBMC proliferation by using CFSE combined with flow cytometry. To test whether these oils are able to induce CBMC proliferation in the absence of Con A stimulation, unstimulated CFSE-labeled CBMCs were incubated with the oils at the dose of 40 µl/ml for 48 h. Our result showed that the rhizome oil of K. galanga has a significantly high lymphocyte proliferative effect at 40 µl/ml as compared with the oil of C. longa ().
3.4. Estimation of cytokines
The levels of IFN-γ and IL-10 cytokines after co-incubation of the rhizome oil of these edible medicinal plants with human CBMCs showed that the increase in the secretion of these cytokines was different in different oils. For instance after 48 h of incubation, significant (P < .05) dose-dependent upregulation of IFN-γ and downregulation of IL-10 as compared with the control (0 μg/ml) was observed in C. longa and K. galanga oil (). However, the rhizome oil of Z. officinale did not show a statistically significant result as compared with the control. At 40 μg/ml, the concentration of IFN-γ (37.98 pg/ml) was significantly higher (P < .001) than that of the control (16.57 pg/ml) in C. longa rhizome oil. Similarly with K. galanga rhizome oil at 40 μg/ml, the concentration of IFN-γ was (49.23 pg/ml) higher as compared with control (19.32 pg/ml). The level of IFN-γ did not show a significant increase or decrease on treatment with Z. officinale oil at 0–40 µg/ml concentrations. Similarly, C. longa and K. galanga oil considerably downregulated IL-10 secretion (). At the concentration of 40 µg/ml, the level of IL-10 in the culture supernatant was 10.45 pg/ml and 9.08 pg/ml of C. longa and K. galanga, respectively, and this was significantly (P < .001) lower than that of the control group. However, the oil of Z. officinale did not show any significant effect on IL-10 secretion as compared with the control ().
Table 1. Effects of different rhizome oils on expression pattern of Th1 and Th2 cytokine IFN-γ and IL-10 at various concentrations after 48 h of incubation
4. Discussion
Natural immunomodulators that can obtain continuous immune activation are urgently required for the prevention of various diseases. Medicinal plant products like essential oils which activate the host defence mechanism in the presence of an impaired immune responsiveness can provide supportive therapy to conventional chemotherapy (Wagner & Bladt, Citation1996). The complex interactions of the immune system are chiefly governed by the cytokines. Among them, IFN-γ cytokine possesses cell-mediated immune responses (Stevens, Bossie, & Sanders, Citation1988) and IL-10 mediated humoral immune response as well as also promotes allergic reactions by increasing IgE formation (Coffman & Carty, Citation1986). C. longa, K. galanga and Z. officinale and its oil are widely consumed as food additive and medicine, which possess anti-inflammatory, anti-oxidant, anti-cancer and immunomodulatory properties (Aggarwal, Sundaram, Malani, & Ichikawa, Citation2007; Umar et al., Citation2014; Craig, Citation1997; Newman et al., Citation2003; Murakami et al., Citation1999). However, scientific investigations to compare their pharmacological effects are rarely reported. In the present study, the essential oils of three important edible medicinal plants C. longa, K. galanga and Z. officinale were examined for immunomodulatory activity emphasizing on TH1 and TH2 cytokine response. The oil of C. longa and K. galanga showed high immunostimulant activity which is added importance to the previously reported anti-inflammatory and anti-cancerous activities of these oils (Craig, Citation1997; Newman et al., Citation2003; Murakami et al., Citation1999). The rhizome of C. longa oil contains α-turmerone and β-turmerone as the major constituents. Both α-turmerone and aromatic turmerone were reported to show stimulatory effects on CBMC proliferation and cytokine production (Yue et al., Citation2010). Our results of flow cytometry also support that both α-turmerone and ar-turmerone may stimulate CBMCs proliferation and cytokine production. Our result showed that 40 μl/ml of essential oil of both the plants C. longa and K. galanga exhibited a stimulatory effect on CBMCs proliferation as shown by flow cytometry. Our results also showed that these oils were not as significant as mitogen ConA which is consistent with data reported by others (Pranoto, Salokhe, & Rakshit, Citation2005). Other data revealed that the major compound of C. longa ar-turmerone and methoxycinnamate of K. galanga might stimulate lymphocyte proliferation (Yue et al., Citation2010). Alpha-turmerone and ar-turmerone were shown for the first time that it might exert modulatory activities in human CBMCs with respect to TH1 and TH2 cytokines. Numerous experiments reported that the immunomodulatory activity of sesquiterpenes (Berges, Fuchs, Opelz, Daniel, & Naujokat, Citation2009; Mathema, Koh, Thakuri, & Sillanpaa, Citation2012; Gertsch, Sticher, Schmidt, & Heilmann, Citation2003) induces immunomodulatory effect through apoptosis of NF-Κb inactivation. Anti-inflammatory and anti-cancerous activities of sesquiterpenes through immunomodulation were also reported in different studies (Lee, Huang, Piantadosi, Pagano, & Geissman, Citation1971). In C. longa plant, the rhizome oil contains about 89.14% of sesquiterpene, which might be possible for their high immunostimulatory activities on human CBMCs with respect to TH1 (IFN-γ) and TH2 (IL-10) cytokine, respectively. A profound increase in TH1 cytokine (IFN-γ) level and a decrease in TH2 cytokine (IL-10) level were also evident by the stimulation of K. galanga rhizome oil (). Similar results are also reported (Yamada, Tokunaga, & Ikeda, Citation2003; Ko, Rho, & Lee, Citation2004) in other plant materials. In the present study, a concentration-dependent increase in the human CBMCs was evident and the proliferation was much higher in the ConA treated sample along with K. galanga rhizome oil than ConA alone. This might be due to K. galanga rhizome oil inducing IFN-γ cytokine which is natural human CBMC stimulating factor or the rhizome oil of K. galanga itself may possess mitogenic effect. Even though several studies showed the biological and therapeutic properties of the essential oils of these plant species, such as antimicrobial, anti-cancerous, anti-inflammatory, anti-oxidant, antimutagenic and others (Aggarwal et al., Citation2007; Umar et al., Citation2012, Citation2014), our study for the first time exhibited the immunomodulatory activity of essential oil of these edible plant of neonatal cytokines.
Table 2. Effects of different rhizome oils on expression pattern of Th1 (IFN-γ) and Th2 (IL-10) cytokine at 40 µl/ml after 48 h of incubation.
5. Conclusion
The essential oil of these edible plants (C. longa and K. galanga) can be used as dietary intake by pregnant mothers to enhance the immune system of the neonates. The oil exerted an immunostimulant effect on cord blood monocytes, without affecting cell viability. Further assays should investigate the immunomodulatory activity of the individual bioactive compound of oils after incubation with cord blood monocyte cells. Better comprehension of the mechanisms of action of these bioactive compounds can also be studied.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adams, R. P. (2007). Identification of essential oil components by gas chromatography/mass spectrometry. Illinois,IL: Allured Publishing Corp.
- Adams, R. P. (2012). Identification of essential oils by ion trap-mass spectroscopy. New York, NY: Academic Press.
- Aggarwal, B. B., Sundaram, C., Malani, N., & Ichikawa, H. (2007). Curcumin: The Indian solid gold. Advances in Experimental Medicine and Biology, 595, 1–75. doi: https://doi.org/10.1007/978-0-387-46401-5_1
- Bagad, A. S., Joseph, J. A., Bhaskaran, N., & Agarwal, A. (2013). Comparative evaluation of anti-inflammatory activity of curcuminoids, turmerones, and aqueous extract of Curcuma longa. Advances in Pharmacological Sciences, 1–7. doi: https://doi.org/10.1155/2013/805756
- Beatty, P. R., Krams, S. M., & Martinez, O. M. (1997). Involvement of IL-10 in the autonomous growth of EBV-transformed B cell lines. The Journal of Immunology, 15, 4045–4051.
- Berges, C., Fuchs, D., Opelz, G., Daniel, V., & Naujokat, C. (2009). Helenalin suppresses essential immune functions of activated CD4+ T cells by multiple mechanisms. Molecular Immunology, 46, 2892–2901. doi: https://doi.org/10.1016/j.molimm.2009.07.004
- Carrasco, F. R., Schmidt, G., Romero, A. L., Sartoretto, J. L., Caparroz-Assef, S. M., & Amado, C. A. B. (2009). Immunomodulatory activity of Zingiber officinale Roscoe, Salvia officinalis L. and Syzygium aromaticum L. essential oils: Evidence for humor- and cell-mediated responses. Journal of Pharmacy and Pharmacology, 61, 961–967. doi: https://doi.org/10.1211/jpp.61.07.0017
- Coffman, R. L., & Carty, J. A. (1986). T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. The Journal of Immunology, 136, 949–954.
- Craig, W. J. (1997). Phytochemicals: Guardians of our health. The Journal of the American Dietetic Association, 97, S199–S204. doi: https://doi.org/10.1016/S0002-8223(97)00765-7
- Cutler, A., & Brombacher, F. (2005). Cytokine therapy. Annals of the New York Academy of Sciences, 1056, 16–29. doi: https://doi.org/10.1196/annals.1352.002
- de Waal Malefyt, R., Yssel, H., & de Vries, J. E. (1993). Direct effects of IL-10 on sub-sets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. The Journal of Immunology, 50, 4754–4765.
- Estrada, A., Katselis, G. S., Laarveld, B., & Barl, B. (2000). Isolation and evaluation of immunological adjuvant activities of saponins from Polygala senega L., comparative immunology. Diagnostic Microbiology and Infectious Disease, 23, 27–43.
- Gertsch, J., Sticher, O., Schmidt, T., & Heilmann, J. (2003). Influence of helenanolide-type sesquiterpene lactones on gene transcription profiles in Jurkat T cells and human peripheral blood cells: Anti-inflammatory and cytotoxic effects. Biochemical Pharmacology, 66, 2141–2153. doi: https://doi.org/10.1016/j.bcp.2003.08.006
- Goel, H. C., Prakash, H., Ali, A., & Bala, M. (2007). Podophyllum hexandrum modulates gamma radiation-induced immunosuppression in Balb/c mice: Implications in radioprotection. Molecular and Cellular Biochemistry, 295, 93–103. doi: https://doi.org/10.1007/s11010-006-9277-5
- Guenther, E. (1972). The essential oils: Histort-orogin in plants production-analysis (voll), Robert E. kriger publishing Co., Malabar, Florida, 427pp. in tissue preparations. Ethiopian Medical Journal, 45, 371–376.
- Holt, P. G., & Jones, C. A. (2000). The development of the immune system during pregnancy and early life. Allergy, 55, 688–697. doi: https://doi.org/10.1034/j.1398-9995.2000.00118.x
- Ko, E., Rho, S., & Lee, E. J. (2004). Traditional Korean medicines (SCRT) modulate TH1/TH2 specific cytokine production in mice CD4+ T cell. The Journal of Ethnopharmacology, 92, 121–128. doi: https://doi.org/10.1016/j.jep.2004.02.008
- Lee, H. K., Huang, E. S., Piantadosi, C., Pagano, J. S., & Geissman, T. A. (1971). Cytotoxicity of sesquiterpene lactones. Cancer Research, 31, 1649–1654.
- Mathema, V. B., Koh, Y. S., Thakuri, B. C., & Sillanpaa, M. (2012). Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation, 35, 560–565. doi: https://doi.org/10.1007/s10753-011-9346-0
- Mediratta, P. K., Sharma, K. K., & Singh, S. (2002). Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. The Journal of Ethnopharmacology, 80, 15–20. doi: https://doi.org/10.1016/S0378-8741(01)00373-7
- Murakami, A., Takahashi, M., Jiwajinda, S., Koshimizu, K., & Ohigashi, H. (1999). Identification of zerumbone in Zingiber zerumbet Smith as a potent inhibitor of 12-O-tetradeconyolphorbol-13-acetate-induced Epstein-Barr virus activation. Bioscience, Biotechnology, and Biochemistry, 63, 1811–1812. doi: https://doi.org/10.1271/bbb.63.1811
- Newman, D. J., Cragg, G. M., & Snader, K. M. (2003). Natural products as sources of new drugs over the period 1981−2002. Journal of Natural Products, 66, 1022–1037. doi: https://doi.org/10.1021/np030096l
- Niiro, H., Otsuka, T., & Kuga, S. (1994). IL-10 inhibits prostaglandin E2 production by lipopolysaccharide-stimulated monocytes. International Immunology, 6, 661–664. doi: https://doi.org/10.1093/intimm/6.4.661
- Pranoto, Y., Salokhe, V. M., & Rakshit, S. K. (2005). Physical and antibacterial properties of alginate-based edible film incorporated with garlic oil. Food Research International, 38, 267–272. doi: https://doi.org/10.1016/j.foodres.2004.04.009
- Premanathan, M., Rajendran, S., Ramanathan, T., Kathiresan, K., Nakashima, H., & Yamamoto, N. (2000). A survey of some Indian medicinal plants for anti-human immunodeficiency virus (HIV) activity. Indian Journal of Medical Research, 112, 73–77.
- Prescott, S. L., Macaubas, C., Smallacombe, T., Holt, B. J., Sly, P. D., & Holt, P. G. (1999). Development of allergen-specific T-cell memory in atopic and normal children. The Lancet, 353, 196–200. doi: https://doi.org/10.1016/S0140-6736(98)05104-6
- SaiRam, M., Sharma, S. K., Ilavazhagan, G., Kumar, D., & Selvamurthy, W. (1997). Immunomodulatory effects of NIM-76 a volatile fraction from neem oil. The Journal of Ethnopharmacology, 55, 133–139. doi: https://doi.org/10.1016/S0378-8741(96)01487-0
- Schroder, K., Hertzog, P. J., & Ravasi, T. (2003). Interferon-gamma: An overview of signals, mechanisms and functions. Journal of Leukocyte Biology, 75, 163–189. doi: https://doi.org/10.1189/jlb.0603252
- Stevens, T. L., Bossie, A., & Sanders, V. M. (1988). Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature, 334, 255–258. doi: https://doi.org/10.1038/334255a0
- Tayal, V., & Kalra, B. S. (2008). Cytokines anti-cytokines as therapeutics – An update. European Journal of Pharmacology, 579, 1–12. doi: https://doi.org/10.1016/j.ejphar.2007.10.049
- Tzianabos, A. O. (2000). Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biological function. Clinical Microbiology Reviews, 13, 523–533. doi: https://doi.org/10.1128/CMR.13.4.523
- Umar, M. I., Asmawi, M. Z., Sadikun, A., Atangwho, I. J., Yam, M. F., Altaf, R., & Ahmed, A. (2012). Bioactivity-guided isolation of ethyl-p-methoxycinnamate, an anti-inflammatory constituent, from Kaempferia galanga L. extracts. Molecules, 17, 8720–8734. doi: https://doi.org/10.3390/molecules17078720
- Umar, M. I., Asmawi, M. Z., Sadikun, A., Majid, A. M. S. A., Al-Suede, F. S. R., Hassan, L. E. A., … ,Ahamed, M. B. K. (2014). Ethyl-p-methoxycinnamate isolated from Kaempferia galanga inhibits inflammation by suppressing interleukin-1, tumor necrosis factor-α and angiogenesis by blocking endothelial functions. Clinics, 69, 134–144.
- Wagner, H., & Bladt, S. (1996). Plant drug analysis: a thin layer chromatography atlas. Berlin: Springer Science & Business Media.
- Wegmann, T. G., Lin, H., Guilbert, L., & Mosmann, T. R. (1993). Bidirectional cytokine interactions in the maternal–fetal relationship: Is successful pregnancy a TH2 phenomenon. Immunology Today, 14, 353–356. doi: https://doi.org/10.1016/0167-5699(93)90235-D
- Yamada, K., Tokunaga, Y., & Ikeda, A. (2003). Effect of dietary fiber on the lipid metabolism and immune function of aged Sprague–Dawley rats. Bioscience, Biotechnology, and Biochemistry, 67, 429–433. doi: https://doi.org/10.1271/bbb.67.429
- Young, H. A., & Hardy, K. J. (1995). Role of interferon-γ in immune cell regulation. Journal of Leukocyte Biology, 58, 373–381. doi: https://doi.org/10.1002/jlb.58.4.373
- Yousofi, A., Daneshmandi, S., Soleimani, N., Bagheri, K., & Karimi, M. H. (2012). Immunomodulatory effect of Parsley (Petroselinum crispum) essential oil on immune cells: Mitogen-activated splenocytes and peritoneal macrophages. Immunopharmacology and Immunotoxicology, 34, 303–308. doi: https://doi.org/10.3109/08923973.2011.603338
- Yue, G. G., Chan, B. C., Hon, P. M., Lee, M. Y., Fung, K. P., Leung, P. C., & Lau, C. B. (2010). Evaluation of in vitro anti-proliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food and Chemical Toxicology, 48, 2011–2020. doi: https://doi.org/10.1016/j.fct.2010.04.039