ABSTRACT
The aim of the study was to evaluate changes in surface receptor expression of B and T lymphocytes and concentration of TGF-β in children who either developed tolerance to cow’s milk protein (CMP) or manifested persistent cow’s milk allergy (CMA). The study involved 30 patients with CMA who underwent an open food challenge after 12 months of milk-free diet. After the milk challenge, decreased concentration of CD19+CD23+ was observed in children who acquired tolerance to CMP, in comparison with the test before cow’s milk (CM) challenge (42.2% vs. 29.1%, p = .006). The same group demonstrated lower concentration of TGF-β than patients with persistent allergy (median 37.9 pg/ml vs. 52.8 pg/ml, p = .003, respectively). Moreover, before CM challenge, higher percentage of CD3+CD8+CD28+CD152+ cells (median 2.88% vs. 1.2%, p = .03) and CD3+CD4+CD25+CD62L+ (median 42.3% vs. 13.4%, p = .032) was noted in children who acquired tolerance to CMP, in comparison with subjects who remained allergic to CMP.
Introduction
The ability to maintain the internal integrity and adapt to the environment is a phenomenon called immune tolerance (Weiner, Citation2001). Food tolerance is a part of peripheral tolerance which averts an excessive immune response towards food allergens or microflora of the gastrointestinal (GI) tract (Hughes, Citation1999). The type of immune response depends on the dose of antigen and the frequency of its administration. High antigen doses promote the development of tolerance by deletion or clonal anergy, whereas low antigen doses usually induce active suppression involving regulatory T lymphocytes (Treg) and immunosuppressive cytokines produced by interleukin-10 (IL-10) and TGF-β (van Wijk & Knippels, Citation2007).
Cow’s milk allergy (CMA) is one of the most common food allergies observed in childhood (Sicherer, Citation2011). However, the majority of infants with CMA spontaneously develop tolerance to cow’s milk proteins (CMP) by school age (Savilahti & Savilahti, Citation2013; Wood et al., Citation2013). Little is known about the potential existence of a peripheral regulatory mechanism modulating the development of tolerance to CMP. Previously published reports have demonstrated that cow’s milk (CM)-specific T cells isolated from children with CMA display a Th2-skewed phenotype (Savilahti et al., Citation2010). It has been suggested that tolerance to cow’s milk allergens is associated with the suppression of the activity of proallergic innate effectors including tissue mast cells, basophils and eosinophils (Jo, Garssen, Knippels, & Sandalova, Citation2014). Children who outgrew their allergy to CMP had higher levels of circulating CD4+CD25+ regulatory T cells and decreased proliferative responses in vitro to bovine-lactoglobulin in peripheral blood mononuclear cells compared to children with persistent CMA (Karlsson, Rugtveit, & Brandtzaeg, Citation2004). In addition, reduction of TGF-β-producing T cells has been observed in the duodenal mucosa of children with food allergy as compared to non-allergic subjects (Pérez-Machado et al., Citation2003). Low concentrations of specific IgE to CM resulted in tolerance achievement more frequently than high levels (Wood et al., Citation2013). Other studies have suggested that Th1 responses underlie the immunological mechanism of food allergy resolution (Savilahti & Savilahti, Citation2013).
The aim of the study was to evaluate changes in the expression of surface receptors of peripheral blood lymphocyte populations (T and B) and the serum concentration of TGF-β in children with CMA who either developed tolerance to cow’s milk or manifested persistent allergy. In regard to T cells, we tested the concentrations of T-helper/inducer (CD3+CD4+) cells, T-cytotoxic/suppressor lymphocytes (CD3+CD8+) cells, regulatory T cells (CD3+CD4+CD25high), T cells expressing the receptor of activation (CD3+CD28+) and/or inhibitory receptor (CD3+CD152+/CTLA-4) and regulatory T cells expressing the central memory receptor (CD3+CD4+CD25+CD62L+). We also analysed subpopulations of B cells expressing CD19+, CD19+CD23+, CD19+CD40+ markers. The secondary aim of our study was to find the immunological predictor of CMP tolerance development in children.
Methods
Patient selection
The study was performed in The Department of Pediatrics, Gastroenterology and Allergology, Medical University of Bialystok in the years 2010–14. The study design is presented in of Supplementary Material. The investigation involved 30 patients with CMA (18 females/12 males), aged 1–13 years at the beginning of the study. Criteria for CMA diagnosis included clinical manifestations, a positive oral cow’s milk challenge test result, a positive skin prick test result and positive cow’s milk-specific IgE in serum. Exclusion criteria included a history of an anaphylactic reaction, an allergy to inhalant allergens, known immunodeficiency syndromes and infections of the GI tract. The control group consisted of 20 children (9 females/11 males), aged 2–13 years, without CMA confirmed with sIgE tests. A cow’s milk-free diet was introduced in all subjects with CMA 12 months prior to study commencement. A CM open challenge test was performed at the hospital following the recommended protocol during the first appointment (Nowak-Wegrzyn et al., Citation2009). After a 6-week follow-up period, the patients were reassessed during the second appointment. Subjects who manifested allergy symptoms during the CM challenge were included in the group of persistent CMA, while those who did not display allergy symptoms were assigned to the group with CMP tolerance.
The study protocol was approved by the Bioethics Committee of the Medical University in Bialystok. Parental written consent for the children’s participation in the follow-up phase of the study was obtained prior to study enrolment. The initial evaluation included an interview with the child’s parents, clinical examination and blood sampling. A questionnaire regarding the presence of allergic diseases (i.e. asthma, atopic dermatitis – AD or allergic rhinitis – AR) was completed.
Blood sample collection and blood cell count
Blood samples were collected before the CM challenge (first appointment) and 6 weeks after the CM challenge (second appointment). The samples were obtained by venipuncture, under standard conditions, after a 12-hour overnight fast. The samples of 2 ml whole blood were obtained in EDTA and processed within 4 hours of collection.
Flow cytometry
All samples were processed using the ImmunoPrep whole blood lysis system (ImmunoPrep Work Station, Beckman Coulter). Briefly, 100 μl of whole blood was stained with 10 μl of the following antibodies: anti-CD3 (phycoerythrin-cyanin 5 PECy5-conjugated, UCHT1 clone), anti-CD4 (phycoerythrin-cyanin 7 PECy7-conjugated, SFCI12T4D11 clone), anti-CD8 (phycoerythrin-cyanin 5 PECy5-conjugated, B9.11 clone), anti-CD25 (phycoerythrin-Texas Red ECD-conjugated, B1.49.9 clone), anti-CD69 (phycoerythrin-conjugated, TP1.55.3 clone), anti-CD62L (fluorescein isothiocyanate-conjugated, DREG56 clone), anti-CD28 (fluorescein isothiocyanate, CD28.2 clone), anti-CD152 (phycoerythrin-conjugated, BMI3 clone), anti-CD19 (phycoerythrin-cyanin 5.1-conjugated, clone J3-119), anti-CD23 (phycoerythrin-conjugated, clone 9P25) purchased from Beckman Coulter (Brea, CA, USA), Beckton Dickinson (San Jose, CA, USA) and eBioscience (San Diego, CA, USA). Respective isotype control antibodies were used. The samples were analysed using the five-colour flow cytometer Beckman Cytomics FC 500 MPL (Beckman Coulter). A minimum of 105 events were acquired for each analysis.
Measurement of serum TGF-β
TGF-β concentrations were determined by ELISA kit (R&D Systems) according to the manufacturer’s instructions. Concentration values were presented in pg/ml.
Statistical analysis
Data were analysed using the Statistica10.0 software (StatSoft Inc., USA). The significance of difference was evaluated by the Mann–Whitney U-test and p < .05 was considered as statistically significant. Cut-off levels, specificity and sensitivity were calculated using the receiver operating characteristic (ROC) analysis.
Results
Background of study group
contains the demographic and clinical characteristics of the study groups at the time of CMA diagnosis. There were no significant differences between the CMA and the control group regarding age and sex. Among the children with CMA, 10 patients with a median age of 2 years (7 females/3 males) demonstrated skin lesions (AD), 10 children with a median age of 4.5 years (5 females/5 males) manifested respiratory tract symptoms (recurrent bronchitis, recurrent rhinitis) and 10 children with a median age of 3.7 years (6 females/4 males) reported GI tract symptoms. No association between the CM tolerance acquisition rate and clinical manifestations of CMA was established ().
Table 1. Background data of paediatric patients with cow’s milk allergy (CMA) and control group.
Table 2. Results of the open challenge test with cow’s milk (second visit).
A significantly greater number of patients demonstrated IgE-mediated CMA (p = .039). The CM open challenge test result was positive in 13 (43.4%) patients, who re-developed symptoms. The test result was more likely to be positive in the subjects with IgE-mediated CMA (69.2%, p = .049) and high-specific IgE levels (4.85–81.2 kUA/L, class 3–5, respectively) (). Out of the 10 children with a previous, IgE-mediated allergy who developed tolerance to CMP, the level of specific IgE ranged from 0.46 to 1.19 kUA/L (class 1–2). No patient presented with severe manifestations such as an anaphylactic shock during the CM open challenge.
Differences in lymphocyte subpopulations in children with persistent CMA, tolerant to CM and controls
In all the study participants, different subpopulations of T (CD3+, CD3+CD4+, CD3+CD8+, CD3+CD4+CD28+, CD3+CD4+CD152+, CD3+CD8+CD28+, CD3+CD8+CD152+, CD3+CD4+CD25+CD62L+, CD3+CD4+CD25high, CD3+CD4+CD69+, CD3+CD8+CD69+) and B (CD19+, CD19+CD23+, CD19+CD40+) cells in blood were tested prior to and following the CM challenge. Following the CM challenge, a decrease in the percentage of CD19+ cells co-expressing CD23+, the “low-affinity” receptor for IgE, was observed both in the children with a persistent CMA and those tolerant to CMP. However, a significant post-CM challenge decline was observed in the children who developed tolerance to CMP (42.2% vs. 29.1%, p = .006) ((A)). Regarding T cell subpopulations, significant differences in CD3+CD4+CD28+CD152+, CD3+CD4+CD28+, CD3+CD8+CD28+CD152+ and CD3+CD4+CD25+CD62L+ were noted between the study groups (, ). Only the children with a persistent CMA demonstrated a higher percentage of CD3+CD4+CD28+CD152+T cells before the CM challenge than after this procedure (median 3.1% vs. 1.04%, p = .038) (). The same group demonstrated a significantly lower percentage of CD3+CD4+CD28+cells, both prior to and following the CM challenge, when compared to the controls (88.8% vs. 94.9%, p = .01; and 87.2% vs. 94.9%, p = .03, respectively) (). In turn, children who became tolerant to CMP had a higher percentage of CD3+CD8+CD28+CD152+ cells (median 2.88% vs. 1.2%, p = .03; (C)) and CD3+CD4+CD25+CD62L+, central memory T cells (median 42.3% vs. 13.4%, p = .032; (D)) prior to the CM challenge in comparison with the subjects who remained allergic to CM.
Figure 1. Differences in the expression level of CD19+CD23+ (A), CD3+CD4+CD28+CD152+ (B), CD3+CD8+CD28+CD152+ (C) and CD3+CD4+CD25+CD62L+ (D) cells in blood obtained from children with a persistent cow’s milk allergy (CMA), those who acquired tolerance to cow’s milk protein (CMP) and controls before and after CM challenge test.
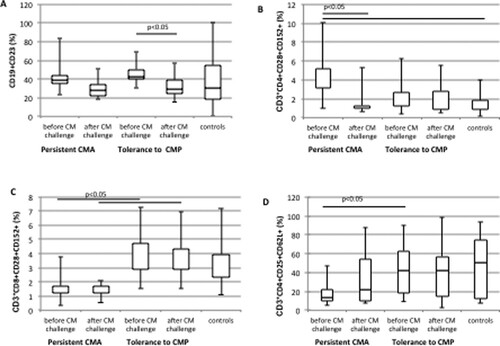
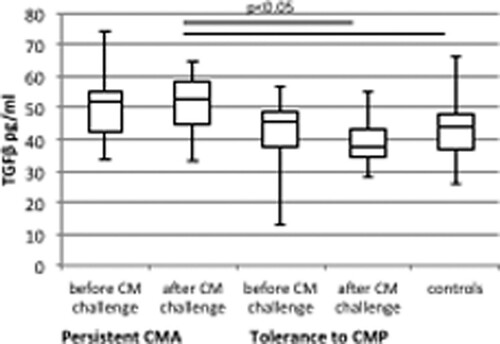
Table 3. Serum TGF-β and peripheral blood lymphocyte subsets of children with persistent cow’s milk allergy (CMA) and controls.
No changes in the percentage of CD3+CD4+CD152+, CD3+CD8+CD152+ and CD3+CD8+CD28+, CD3+CD4+CD25high and expression of the activation marker (CD69+) were found in the study groups ().
Differences in TGF-β in children with persistent CMA and tolerant to CM and controls
Prior to the CM challenge, no differences in TGF-β level were found between the study groups. Following the CM challenge test, significantly higher serum concentrations of TGF-β were noted in the children with a persistent CM allergy in comparison with the subjects tolerant to CMP and the controls (median 52.8 vs. 37.9 pg/ml, p = .003 and 52.8 vs. 44.2 pg/ml, p = .03, respectively) (, ).
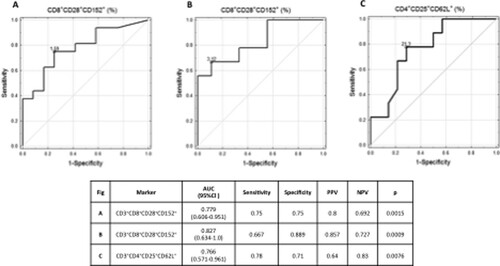
CD3+CD8+CD28+CD152+ lymphocyte subpopulation as a predictor of CMP tolerance development
An increased CD3+CD8+CD28+CD152+ lymphocyte subpopulation in serum prior to the CM challenge discriminated children who developed tolerance to CMP with area under the ROC curve (AUC) of 0.779 (95%CI 0.606–0.951, p = .0015), a cut-off level of 1.59% and a specificity of 75% ((A)). In the children with a previous, IgE-mediated CMA, a cut-off level was 3.12% with a specificity of 88.9% (AUC of 0.827; 95%CI 0.634–1, p = .0015) ((B)). Similar results were obtained for CD3+CD4+CD25+CD62L+, the marker of central memory on regulatory T cells. Its enhanced value differentiated patients who acquired tolerance to CMP from those with a persistent CMA, at a cut-off level of 21.3% with a 71% specificity (AUC of 0.766; 95%CI 0.571–0.96, p = .0076) ((C)).
Figure 3. Increased percentage of CD3+CD8+CD28+CD152+ (A, B) and CD3+CD4+CD25+CD62L+ in serum before CM challenge discriminates children who developed tolerances to CMP (A, C), particularly those with a previous, IgE-mediated CMA (B); AUC: area under the ROC curve; PPV: positive predicative values; NPV: negative predicative values; CI: confidents interval.
Discussion
The majority of children with CMA develop tolerance to CMP with age and therefore, indications for the continuation of the elimination diet require periodic re-evaluation. Understanding the mechanisms of a persistent CMA might help to establish accurate management guidelines for CMA patients. There is a need to determine a specific marker for CMP tolerance development. The concentration of specific IgE to CM has been suggested as a predictor of the development of CMP tolerance (Wan et al., Citation2012; Wood et al., Citation2013). In a study by Wood et al., 72% of children in whom milk allergy resolved had low levels of specific IgE to CM (less than 2 kUA/L), compared with only 23% of those with the level greater than 10 kUA/L (Wood et al., Citation2013). In our study, the level of specific CM IgE was lower than 1.2 kUA/L in all the children with CMP tolerance whereas the subjects with a persistent CMA demonstrated far higher levels of specific CM IgE (ranging from 4.85 to 81.2 kUA/L), which is in agreement with previous reports.
It has been reported that T cells displaying a Th2-skewed phenotype are mostly responsible for allergic reactions (Tiemessen et al., Citation2004). Lymphocyte distribution may change significantly in response to an allergen (Sun, Fu, & Wang, Citation2015). However, Beyer et al. have suggested that alterations in main lymphocyte populations are not useful in monitoring food challenges, although the authors have not assessed T cell subpopulations (Beyer, Renz, Wahn, & Niggemann, Citation1998). Our study revealed some changes within T cell subsets in children with a CM allergy. A significantly lower percentage of CD3+CD4+ cells with CD152+ (CTLA-4) expression, the negative regulator of T cell activation, was found in children with a persistent CMA, both prior to and following the CM challenge in comparison with the control group. Animal model studies have demonstrated that CD152 expression is required for the induction of peripheral T cell tolerance to food (Samoilova et al., Citation1998; van Wijk et al., Citation2007). Moreover, in our study, the same group with a persistent CMA demonstrated a significantly higher level of CD3+CD4+CD28+CD152+ T cells before the CM challenge compared to control. Samochocki et al. reported a marked elevation of CD152 expression alone as well as the co-expression of both CD152 and CD28 markers in CD4+ and CD8+ cell populations in individuals with AD as compared to controls (Samochocki et al., Citation2012). Cells bearing both CD28 and CD152 are the transient subpopulation of T lymphocytes (Samochocki et al., Citation2012). Little is still known about their role in immune regulation, especially in allergy or immune tolerance to food. Depending on costimulatory signals mediated by the interaction of ligands CD80/CD86, they may transform into active lymphocytes through binding to CD28 or, after binding to CD152, may generate inhibitory signals that “‘switch off’” T cell activation, proliferation and IL-2 production (Gardner, Jeffery, & Sansom, Citation2014). CD28 expression on the T cell surface is required for CD152 upregulation on activated T cells (Walunas & Bluestone, Citation1998). However, the affinity of CD80/CD86 for CD152 is higher than for CD28 (Linsley et al., Citation1991). Our results showed a higher percentage of CD3+CD8+CD28+CD152+ cells in children who developed tolerance to CMP, especially with a previous IgE-mediated allergy, in comparison with the subjects with a persistent CMA. To the best of our knowledge, no reports about the co-expression of CD28+CD152+ on CD3+CD4+ or CD3+CD8+ T cells in patients with food allergies have been published to date. Moreover, we found that an increased percentage of CD3+CD8+CD28+CD152+ might discriminate patients who outgrew their allergy from those who remained allergic to CM with a specificity of 75% at a cut-off level of 1.59%. Similar results were obtained in regard to the expression of CD3+CD4+CD25+CD62L+, the marker of central memory on regulatory T cells. Its increased expression characterized patients with tolerance to CMP with a specificity of 71% and a cut-off level of 21.3%. An enhanced number of CD4+CD25+ T cells with a regulatory function in peripheral blood 1 week after a milk challenge in children who had outgrown their allergy was reported by Karlsson et al. (Citation2004). In humans, more than 95% of CD4+CD25high Treg have been found to be CD62L-positive (Baecher-Allan & Hafler, Citation2005). Ermann et al. (Citation2005) showed that CD4+CD25+CD62L+ T cells inhibited the expansion of alloreactive CD4+CD25− T cells more efficiently than CD4+CD25+CD62L− T cells. Treg cells suppress antigen-specific immune responses not only by direct cell contact with CD152, but also by secreting soluble factors such as IL-10 and transforming growth factor-beta (TGF-β) (Oberg, Juricke, Kabelitz, & Wesch, Citation2011). Frischmeyer-Guerrerio et al. evaluated allergy development in patients with Loeys-Dietz syndrome, characterized by mutations in the genes encoding receptor subunits for TGF-β. The authors suggested that enhanced TGF-β signalling might predispose individuals to allergic phenotypes through the induction of Th2 cytokine production (Frischmeyer-Guerrerio et al., Citation2013). Since TGF-β modulates inflammatory response, the increased serum level of this cytokine in children with a persistent CMA found in our study might be associated with its regulatory function. On the other hand, it has been reported previously that the decreased synthesis of TGF-β in duodenal mucosa, a consequence of a decreased number of mucosal Th3 lymphocytes, might be one of the reasons for cow’s milk hypersensitivity development (Pérez-Machado et al., Citation2003).
Immune response in allergic inflammation involves also B lymphocytes producing allergen-specific IgE or IgA, which are important in food tolerance development. Ghosh et al. confirmed in an animal model that CD19+CD23+ B lymphocyte numbers are increased in the allergic lung and suggested a role of CD19+CD23+ lymphocytes in immunoglobulin secretion in the context of a fungal allergy (Ghosh, Hoselton, & Schuh, Citation2012). Krogulska et al. analysed CD19+CD23+ lymphocytes in children with asthma and food allergies, revealing their significant increase 24 hours after an oral food challenge. Moreover, the CD19+CD23+ increase was greater in individuals with severe reactions than in those with moderate/mild manifestation (Krogulska, Wasowska-Królikowska, Polakowska, & Chrul, Citation2009). In our analysis, a significant difference in the B cell number was found only in children who developed tolerance to CM, showing a decrease in the percentage of CD19+ cells expressing CD23+, the “low-affinity” receptor for IgE, after the CM challenge.
The limitations of our study were the small number of patients with CMA, various clinical manifestations of CMA and a lack of in vitro analysis concerning the functional response of lymphocytes before and after the CMP challenge. However, the prevalence of CMA in the region of our centre is not high, so it would take years to collect an adequate number of patients with single phenotype manifestation of CMA. The in vitro experiments require the acquisition of larger blood samples from the studied patients, which is ethically unacceptable in studies on paediatric subjects.
In conclusion, our results confirmed the previously reported involvement of immune cells in the acquisition of CMP tolerance. In particular, low levels of CD19+CD23+ B cells and high levels of CD3+CD8+CD28+CD152+ and CD3+CD4+CD25+CD62L+ T cells might suggest the stratification of patients who will develop tolerance. More studies are needed to evaluate the usefulness of these markers as predictors of CMP tolerance development.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Urszula Daniluk http://orcid.org/0000-0002-1671-6578
Additional information
Funding
References
- Baecher-Allan, C. M., & Hafler, D. A. (2005). Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+CD25+ T cells. Clinical Immunology, 117(2), 192; discussion 193. doi: https://doi.org/10.1016/j.clim.2005.08.008
- Beyer, K., Renz, H., Wahn, U., & Niggemann, B. (1998). Changes in blood leukocyte distribution during double-blind, placebo-controlled food challenges in children with atopic dermatitis and suspected food allergy. International Archives of Allergy and Immunology, 116(2), 110–115. doi: https://doi.org/10.1159/000023933
- Ermann, J., Hoffmann, P., Edinger, M., Dutt, S., Blankenberg, F. G., Higgins, J. P., & Strober, S. (2005). Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood, 105(5), 2220–2226. doi: https://doi.org/10.1182/blood-2004-05-2044
- Frischmeyer-Guerrerio, P. A., Guerrerio, A. L., Oswald, G., Chichester, K., Myers, L., Halushka, M. K., & Dietz, H. C. (2013). TGFβ receptor mutations impose a strong predisposition for human allergic disease. Science Translational Medicine, 5(195), 195ra194. doi: https://doi.org/10.1126/scitranslmed.3006448
- Gardner, D., Jeffery, L. E., & Sansom, D. M. (2014). Understanding the CD28/CTLA-4 (CD152) pathway and its implications for costimulatory blockade. American Journal of Transplantation, 14(9), 1985–1991. doi: https://doi.org/10.1111/ajt.12834
- Ghosh, S., Hoselton, S. A., & Schuh, J. M. (2012). Characterization of CD19(+)CD23(+)B2 lymphocytes in the allergic airways of BALB/c mice in response to the inhalation of Aspergillus fumigatus conidia. The Open Immunology Journal, 5, 46–54. doi: https://doi.org/10.2174/1874226201205010046
- Hughes, D. A. (1999). Diet and the maturation of the immune system. Food and Agricultural Immunology, 11(4), 279–285. doi: https://doi.org/10.1080/09540109999663
- Jo, J., Garssen, J., Knippels, L., & Sandalova, E. (2014). Role of cellular immunity in cow’s milk allergy: Pathogenesis, tolerance induction, and beyond. Mediators of Inflammation, 2014, 249784. doi: https://doi.org/10.1155/2014/249784
- Karlsson, M. R., Rugtveit, J., & Brandtzaeg, P. (2004). Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. The Journal of Experimental Medicine, 199(12), 1679–1688. doi: https://doi.org/10.1084/jem.20032121
- Krogulska, A., Wasowska-Królikowska, K., Polakowska, E., & Chrul, S. (2009). Evaluation of receptor expression on immune system cells in the peripheral blood of asthmatic children undergoing food challenges. International Archives of Allergy and Immunology, 150(4), 377–388. doi: https://doi.org/10.1159/000226239
- Linsley, P. S., Brady, W., Urnes, M., Grosmaire, L. S., Damle, N. K., & Ledbetter, J. A. (1991). CTLA-4 is a second receptor for the B cell activation antigen B7. Journal of Experimental Medicine, 174(3), 561–569. doi: https://doi.org/10.1084/jem.174.3.561
- Nowak-Wegrzyn, A., Assa’ad, A. H., Bahna, S. L., Bock, S. A., Sicherer, S. H., Teuber, S. S., & Adverse Reactions to Food Committee of American Academy of Allergy, A. t. I. (2009). Work group report: Oral food challenge testing. Journal of Allergy and Clinical Immunology, 123(6 Suppl.), S365–S383. doi: https://doi.org/10.1016/j.jaci.2009.03.042
- Oberg, H. H., Juricke, M., Kabelitz, D., & Wesch, D. (2011). Regulation of T cell activation by TLR ligands. European Journal of Cell Biology, 90(6–7), 582–592. doi: https://doi.org/10.1016/j.ejcb.2010.11.012
- Pérez-Machado, M. A., Ashwood, P., Thomson, M. A., Latcham, F., Sim, R., Walker-Smith, J. A., & Murch, S. H. (2003). Reduced transforming growth factor-beta1-producing T cells in the duodenal mucosa of children with food allergy. European Journal of Immunology, 33(8), 2307–2315. doi: https://doi.org/10.1002/eji.200323308
- Samochocki, Z., Alifier, M., Bodera, P., Jeziorkowska, R., Rosiak, E., Jurkiewicz, B., & Stankiewicz, W. (2012). T-regulatory cells in severe atopic dermatitis: Alterations related to cytokines and other lymphocyte subpopulations. Archives of Dermatological Research, 304(10), 795–801. doi: https://doi.org/10.1007/s00403-012-1290-9
- Samoilova, E. B., Horton, J. L., Zhang, H., Khoury, S. J., Weiner, H. L., & Chen, Y. (1998). CTLA-4 is required for the induction of high dose oral tolerance. International Immunology, 10(4), 491–498. doi: https://doi.org/10.1093/intimm/10.4.491
- Savilahti, E. M., Karinen, S., Salo, H. M., Klemetti, P., Saarinen, K. M., Klemola, T., & Vaarala, O. (2010). Combined T regulatory cell and Th2 expression profile identifies children with cow’s milk allergy. Clinical Immunology, 136(1), 16–20. doi: https://doi.org/10.1016/j.clim.2010.02.011
- Savilahti, E. M., & Savilahti, E. (2013). Development of natural tolerance and induced desensitization in cow’s milk allergy. Pediatric Allergy and Immunology, 24(2), 114–121. doi: https://doi.org/10.1111/pai.12004
- Sicherer, S. H. (2011). Epidemiology of food allergy. Journal of Allergy and Clinical Immunology, 127(3), 594–602. doi: https://doi.org/10.1016/j.jaci.2010.11.044
- Sun, C., Fu, L.-L., & Wang, Y. (2015). IgE- and T-lymphocyte-dependent hypersensitivity responses induced in mice by exposure to shrimp (Fenneropenaeus chinensis) proteins. Food and Agricultural Immunology, 26(4), 577–589. doi: https://doi.org/10.1080/09540105.2014.998635
- Tiemessen, M. M., Van Ieperen-Van Dijk, A. G., Bruijnzeel-Koomen, C. A., Garssen, J., Knol, E. F., & Van Hoffen, E. (2004). Cow’s milk-specific T-cell reactivity of children with and without persistent cow’s milk allergy: Key role for IL-10. Journal of Allergy and Clinical Immunology, 113(5), 932–939. doi: https://doi.org/10.1016/j.jaci.2003.12.016
- van Wijk, F., & Knippels, L. (2007). Initiating mechanisms of food allergy: Oral tolerance versus allergic sensitization. Biomedicine and Pharmacotherapy, 61(1), 8–20. doi: https://doi.org/10.1016/j.biopha.2006.11.003
- van Wijk, F., Nierkens, S., de Jong, W., Wehrens, E. J., Boon, L., van Kooten, P., & Pieters, R. (2007). The CD28/CTLA-4-B7 signaling pathway is involved in both allergic sensitization and tolerance induction to orally administered peanut proteins. Journal of Immunology, 178(11), 6894–6900. doi: https://doi.org/10.4049/jimmunol.178.11.6894
- Walunas, T. L., & Bluestone, J. A. (1998). CTLA-4 regulates tolerance induction and T cell differentiation in vivo. Journal of Immunology, 160(8), 3855–3860.
- Wan, K.-S., Wu, H.-L., Yang, W., Wu, K.-G., Wu, T.-C., & Hwang, B. (2012). The critical role of allergen-specific Ige, Igg4 and Iga antibodies In the tolerance of Ige-mediated food sensitisation In primary school children. Food and Agricultural Immunology, 23(2), 93–98. doi: https://doi.org/10.1080/09540105.2011.604772
- Weiner, H. L. (2001). Oral tolerance: Immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes and Infection, 3(11), 947–954. doi: https://doi.org/10.1016/S1286-4579(01)01456-3
- Wood, R. A., Sicherer, S. H., Vickery, B. P., Jones, S. M., Liu, A. H., Fleischer, D. M., & Sampson, H. A. (2013). The natural history of milk allergy in an observational cohort. Journal of Allergy and Clinical Immunology, 131(3), 805–812. doi: https://doi.org/10.1016/j.jaci.2012.10.060