ABSTRACT
The objectives of the present work were first to evaluate the sensitivity to cow raw milk of the population of Fez, and then to study the effect of heating and pepsin hydrolysis on the allergenicity of casein. A cross-sectional study was carried out in Fez Hospitals, in which 1000 patients were recruited to establish a sera bank used to evaluate specific IgE to cow milk and to casein. Then, we evaluated the reaction of human IgE to heated and pepsin-hydrolysed casein. The results showed that 11.5% of the population studied self-reported reactions to foods. From them, 3.6% reported allergy to milk. Evaluation of specific IgE to cow raw milk showed that 11.9% of patients presented higher specific IgE levels. The treatments of casein indicated that both heating and pepsin hydrolysis totally decreased its binding on the human IgE.
Introduction
Food allergy is taking an important part in common life; it is increasing at an alarming rate in the industrialized world affecting 4–8% of children and about 1–2% of the adult population (Baral & Hourihane, Citation2005; Jansen et al., Citation1994). Cow’s milk proteins are among the most common food allergens and the prevalence of cow’s milk allergy ranges from 0.3% to 7.5% in the general population (Høst, Citation1994; Høst & Halken, Citation1998). For children, cow’s milk allergy was about 11.9% in France (Rance, Grandmottet, & Grandjean, Citation2005), 10% in Turkey (Orhan et al., Citation2009) and 2.2% in Canada (Soller et al., Citation2012). In Morocco, the self-reported food allergy in the general population was estimated about 9.5% (Ouahidi, El Hamsas, & Aarab, Citation2011). Another study based on the determination of specific IgE levels indicated that food allergy was about 2.5% (Bouhsain et al., Citation2008). For cow milk, a prospective study including 160 atopic children in Marrakech showed that 2% of this population suffered from allergy to cow milk (Ghadi, Dutau, & Rancé, Citation2007).
Cow milk contains large allergenic proteins. Most important of them are casein, β-lactoglobulin, α-lactalbumin and bovine serum albumin (Bahna, Citation2002; Cocco, Järvinen, Sampson, & Beyer, Citation2003; Docena, Fernandez, Chirdo, & Fossati, Citation1996; El-Agamy, Citation2007; Peñas, Snel, Floris, Préstamo, & Gomez, Citation2006). Various food processing techniques, such as heating, enzymatic digestion and fermentation, can influence the allergenic potential of food proteins. It can either increase or decrease the allergenicity of the food by modification of epitopes reacting with antibodies (Davis, Smales, & James, Citation2001; Ouahidi et al., Citation2011; Takagi, Teshima, Okunuki, & Sawada, Citation2003; Thomas et al., Citation2007). For milk allergens, it has been indicated in previous data that food processing, such as heat treatment and hydrolysis processes, could even reduce or increase the allergenicity of milk proteins (Dupont et al., Citation2010; Ehn, Ekstrand, Bengtsson, & Ahlstedt, Citation2004; Ehn et al., Citation2005; Jedrychowski, Citation1999; Morisawa et al., Citation2009).
Thus, the aim of our study was to evaluate the milk sensitivity of Moroccan population in Fez city then to study the variation of the IgE sensitivity to casein cow’s milk under heating and pepsin hydrolysis.
Materials and methods
Patients
The work was conducted with a questionnaire completed with a sera bank, obtained from 1000 volunteers, recruited from the Ibn El Khatib Hospital and the Hospital University Center of Fez in a period of 9 months (June 2014 to April 2015). This studied population was represented by 20.5% (n = 205) of a young person aged between 2 and 20 years, and 79.5% (n = 795) of adults aged between 20 and 60 years. The study was based on the questionnaire fulfilled by volunteers. Patients were asked about their allergic history to food, especially to milk. After formal consent of each patient and of children’s parents, a serum sample was collected.
After centrifugation at 3000 rpm/5 min, the collected sera were stored at −20°C until use. The patients recruited had not been sensitized to milk or challenged orally but they were visiting the hospital for several medical analysis.
Extraction of proteins and treatments
A volume (100 ml) of cow milk was skimmed, and a concentration of 0.5 mg/ml of cow raw milk was prepared. In order to extract casein, a volume (100 ml) of cow milk was skimmed and its pH was adjusted to 4.6 by HCl (3 mol/l) and centrifuged at 5000 rpm/20 min (Wal et al., Citation1995). Casein extracted in the pellet was washed, and then treated in three different ways: (1) heated at different temperatures (70°C, 80°C and 90°C) for 30, 60 and 120 min, (2) digested by pepsin (hog stomach, 3354 U/mg) at a concentration of 50 μg/ml in an acidic medium (pH = 2) during 30, 60 and 120 min at 37°C or (3) processed by a combination of the two treatments, heating and pepsin hydrolysis.
Production of polyclonal anti-casein antibodies
Anti-casein antibodies were prepared by immunizing rabbits against the native casein using Freund adjuvant. Later, animals were sacrificed according to National Ethical Laws and blood was collected in dry tubes. After centrifugation of 5 min at 3000 rpm at 4°C, serum was separated and frozen at −20°C until use.
Specific IgE determination
In order to determine the levels of specific IgE to cow raw milk and to casein, indirect ELISA was used as described before (Bousfiha & Aarab, Citation2013; Ouahidi et al., Citation2011). Briefly, 100 µl of casein or skimmed raw milk (0.5 mg/ml) in PBS (phosphate buffered saline, pH 7.4) was deposited on the wells of the micro-titration plate (100 μl/well). Thereafter, wells were saturated by BBS (borate buffered saline, pH 8.4) containing 2.5% Tween 20, and 100 µl of the human serum added. The revelation was made by adding the anti-human IgE conjugated to peroxidase followed by the addition of the orthophenylenediamine (OPD 0.05%) substrate. After incubation at 37°C during 20 min, the reaction was stopped, by adding 50 μl of HCl (3 mol/l) and the absorbance was measured at 490 nm by an ELISA reader.
This study was conducted with the approval of the ethic committee of the University Hospital Center of Fez.
SDS-PAGE and immunoblotting
SDS-PAGE was performed in 15% polyacrylamide gel under denaturation conditions. Casein samples (100 μl) were mixed with loading buffer (10% SDS, 10% glycerol, 10% β-mercaptoethanol and 2.5% bromophenol blue). Then, gels were fixed and stained with Coomassie blue R250 0.1%.
The separated proteins were electrotransferred to nitrocellulose membranes under a current of 400 mA. The membranes were then blocked with BBS containing 2.5% Tween and cut into strips. Each strip was incubated with a patient serum or with rabbit serum anti-casein as a positive control for all night at 4°C. After washing, the strips were incubated with anti-IgE peroxidase labelled, then, the reaction is revealed by the incubation of strips in a solution containing 0.05% of diaminobenzidine (DAB) in BBS tampon.
Statistical analysis
Statistical analysis was based on Student’s t-test taking p < 0.05 as the limit of significant value.
Results
Reported allergy
A total of 1000 patients were recruited for this study including 88.5% (n = 885) women and 11.5% (n = 115) men. This population visited hospitals for several health reasons and were aged between 2 and 60 years. It was represented by 20.5% of young persons (n = 205) and 79.5% (n = 795) of adults.
The self-reported allergy recorded according to the questionnaire was 11.5% for all foods, distributed as follows: 8% to fish; 3.3% to eggs and 3.6% to milk. Milk allergy was mostly caused by cow milk (3.3%), and it was slightly more reported by men (4.3%) than women (3.2%).
Specific IgE to raw milk
Measurement of specific IgE levels to cow raw milk was realized for all the patients recruited. The results showed that 19.2% presented positive values (>2 IU/ml), and 11.9% presented values more than 30 IU/ml. The average of positive values of IgE was 107.9 IU/ml.
Regarding age, the specific IgE levels for children ranged from 2.7 to 453 IU/ml with 2.9% (n = 6) presenting values higher than 200 IU/ml. Whereas, for adults, the average of positive values of IgE was 112.3 IU/ml with 3.7% (n = 30) presenting values more than 200 IU/ml ().
Table 1. Levels of specific IgE (IU/ml) to raw milk in human sera.
Effect of treatments
To study the effect of temperature and pepsin hydrolysis on allergenicity of the casein, three sets of experimentation were conducted. We have studied the action of the temperature, the pepsin hydrolysis and the two effects combined. The detection of treated casein was evaluated by determining the binding of rabbit anti-casein IgG and human IgE by both ELISA and immunoblot assay.
Effect of heating and pepsin hydrolysis on the antigenicity of casein
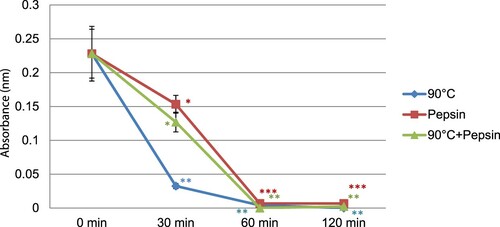
Our results showed that the detection of casein, by rabbit IgG, was reduced after heating within 30 min at different temperatures and was slightly modified for more heating time. Temperature of 80°C and 90°C highly changed the liaison to IgG more than 70°C. Maximal reduction of IgG binding observed was 60% at 70°C, 87% at 80°C and completely blocked at 90°C. Under pepsin hydrolysis, we noticed that this treatment completely blocked the detection, by rabbit IgG, of casein after 60 min of hydrolysis. In the same conditions, we noticed that the combination of heating and pepsin decreased the detection of casein by rabbit IgG in the same manner like pepsin alone ().
Effect of heating and pepsin hydrolysis on the allergenicity of casein
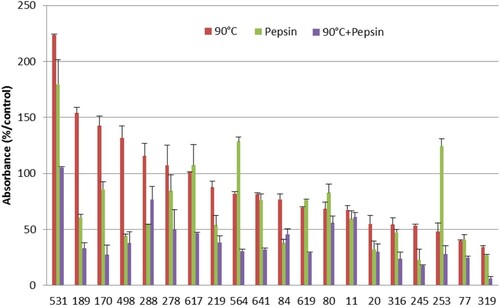
In a second part, we studied the variation of the binding of the treated casein to human IgE. After heating of casein at 90°C, we noticed that 65% (n = 13 from 20) of patients showed an important decrease in the recognition of treated casein with a maximum reduction of 66%, and an average of 24%. However, 7 of 20 patients showed an increase in reactivity to casein heated. Under pepsin hydrolysis, the results showed that 80% of patients presented a reduction in the recognition of treated casein. This reduction was varying from 19% to 67%. Furthermore, we noticed that 4 of 20 patients have shown an increase in the recognition of casein after pepsin action. By a combination of heating and pepsin hydrolysis, we remarked that 95% of patients showed a decrease in the recognition of treated casein by IgE reaching a maximum reduction of 82%, only one person showed resistance to this combined treatment ().
SDS-PAGE and immunoblotting
Effect of heating and pepsin hydrolysis on casein protein using SDS-PAGE
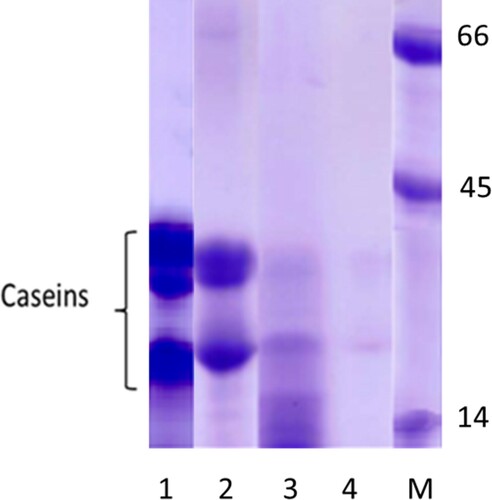
Casein extracted quality was analysed by SDS-PAGE as shown in . The effect of different treatments showed that heat treatment slightly reduced casein bands, whereas under pepsin hydrolysis, the bands disappeared and we observed very slight bands of hydrolysed casein. With the combination of the two treatments, we observed an absence of casein bands.
Effect of heating and pepsin hydrolysis on the allergenicity of casein using immunoblot
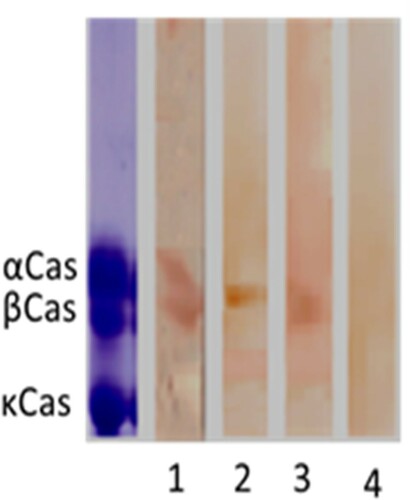
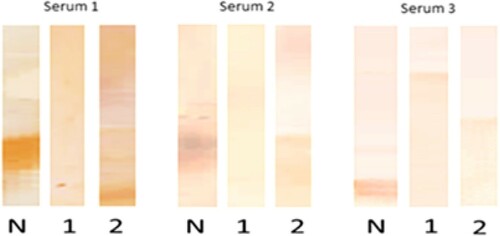
Immunoblotting analysis of casein is shown in . We observed that the rabbit IgG prepared in our laboratory reacted with α and β-casein but not with κ-casein. After the action of treatments, the rabbit IgG reacted slightly with heated and hydrolysed casein. However, no reaction was observed when these two treatments were combined. Regarding the reactivity of native and treated casein with human IgE, we analysed the sera of three patients presenting high levels of specific IgE to casein (). The results showed that two patients of them (Serum 1 and Serum 2) reacted to native α and β-casein, whereas the third patient (Serum 3) reacted to κ-casein. This IgE reactivity disappeared with treated casein either by heat or by pepsin hydrolysis.
Discussion
The aim of this work was to evaluate the reported reactions to milk of a Moroccan population based on the study of IgE sensitivity. Then, we investigated the effect of thermal and enzymatic digestion on the allergenicity of casein in this population. The population studied was recruited from Ibn El Khatib Hospital and the University Hospital Center of Fez. The patients recruited has not been challenged or sensitized by any allergens.
Our results showed that 3.6% of the population studied reported allergy to cow’s milk proteins. This value still less than that reported in sensitive population like in Saudi Arabia (El-Rab, Citation1998), Egypt (Hossny, Gad, Shehab, & El-Haddad, Citation2011) and Tunisia (Ben Ameur et al., Citation2014). Other European countries reported allergy with higher levels as in Spain with 4.7% in children (Venter et al., Citation2006) and in France with 8% in general population reported in 2001 (Kanny et al., Citation2001) and 11.9% in children reported in 2005 (Rance et al., Citation2005). These data indicate that this Moroccan population was less sensitive to milk than European countries probably related to the low consumption of milk in Moroccan food habits. In fact, the average annual consumption of milk products, in Morocco, is relatively low (38 kg/person/year), whereas in Europe it is about 150 kg/person/year.
By measuring specific IgE to raw milk, we found that 11.9% of our population presented high levels of specific IgE to whole milk. A slight correlation was found between self-reporting allergy to milk and levels of specific IgE. In fact, 3% of adults presenting high levels of specific IgE to milk reported milk allergy.
Concerning the effect of treatments, heating of casein totally decreased its binding on rabbit IgG and on the human IgE. Majority of the studied patients recognized conformational epitopes which were denaturized by heating. This result was confirmed by immunoblot and indicated that these sensitive persons might tolerate heated and baked milk products. This conclusion has been observed previously by Nowak-Wegrzyn et al. (Citation2008) who showed that 75% of allergic children tolerating heated milk had lower specific casein IgE. This finding was confirmed later by Bloom, Huang, Bencharitiwong, Nowak-Wegrzyn, and Sampson (Citation2009) and Kim et al. (Citation2011). Besides, SDS-PAGE results showed that casein is slightly affected by heating since the casein bands persist but in a denatured form. On the other hand, 35% of patients showed an increase in IgE recognition of heated casein indicating that the temperature was not sufficient to decrease the sensitivity of these patients to casein. This suggests that this sensitivity implicated sequential epitopes. While, upon pepsin digestion, the casein bands disappeared completely and the binding of human IgE to hydrolysed casein was highly reduced in 80% of patients as observed by ELISA and by immunoblot assay. These findings were in accordance with works of Do, Williams, and Toomer (Citation2016), who found that the immunoreactivity of casein was greatly diminished upon pepsin hydrolysis, and with Benedé et al. (Citation2014), who showed that the IgE binding to pepsin-hydrolysed casein was reduced as the digestion proceeded. This conclusion was confirmed by the reduction of milk allergenicity by using hydrolysed milk formulas for children (Duan, Yang, Li, Zhao, & Huo, Citation2014; Sabadin, Villas-Boas, de Lima Zollner, & Netto, Citation2012).
By the combination of heating and pepsin hydrolysis, we remarked that 95% showed a reduction in IgE binding to treated casein. It has been shown that the combination of pepsin hydrolysis with preceding heat treatment considerably enhanced the tryptic and peptic action of the major milk protein and thereby reduced the allergenicity of milk (Bertrand-Harb, Baday, Dalgalarrondo, Chobert, & Haertle, Citation2002; Peyron, Mouécoucou, Frémont, Sanchez, & Gontard, Citation2006). This high decrease might be explained by the fact that when heating denaturation abolished conformational epitopes, pepsin action became more efficient on sequential epitopes which were then hydrolysed. These treatments together abolished totally all epitopes recognized by IgE of this Moroccan population.
Concerning the interaction of native casein to the IgE of the three studied patients, two of them reacted to α- and β-casein, whereas one patient reacted to κ-casein. Several studies demonstrate that most patients with cow’s milk allergy synthesize antibodies principally against α-casein and β-casein (Ametani et al., Citation2003; Bevilacqua et al., Citation2001; Restani et al., Citation1999).
Conclusion
In conclusion, we have shown that this Moroccan population, from Fez, was sensitive to cow’s milk as self-reported and revealed by IgE evaluation. The sensitivity to casein could be highly reduced by heat treatment followed by enzymatic digestion. This indicates that heating denaturation of casein followed by enzymatic digestion was sufficient to abolish most conformational and sequential epitopes of casein in this studied population.
Acknowledgements
This work was supported by grants of the Moroccan National Center for Scientific and Technical Research (CNRST) to Ouarda AZDAD. We would like to thank the Local authorities of Health Ministry of Fez as well as all patients participating in this work for their assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ametani, A., Sakurai, T., Katakura, Y., Kuhara, S., Hirakawa, H., Hosoi, T., … Kaminogawa, S. (2003). Amino acid residue substitution at T-cell determinant-flanking sites in β-lactoglobulin modulates antigen presentation to T cells through subtle conformational change. Bioscience, Biotechnology, and Biochemistry, 67(7), 1507–1514. doi: https://doi.org/10.1271/bbb.67.1507
- Bahna, S. L. (2002). Cow’s milk allergy versus cow milk intolerance. Annals of Allergy, Asthma & Immunology, 89(6), 56–60. doi: https://doi.org/10.1016/S1081-1206(10)62124-2
- Baral, V. R., & Hourihane, J. O. B. (2005). Food allergy in children. Postgraduate Medical Journal, 81(961), 693–701. doi: https://doi.org/10.1136/pgmj.2004.030288
- Ben Ameur, S., Alibi, S., Loukili, S., Telmoudhi, J., Aloulou, H., & Kamoun, T. H. (2014). Food allergy in south of Tunisia. Archives of Disease in Childhood, 99, A577.
- Benedé, S., López-Expósito, I., Giménez, G., Grishina, G., Bardina, L., Sampson, H. A., … López-Fandiño, R. (2014). In vitro digestibility of bovine β-casein with simulated and human oral and gastrointestinal fluids. Identification and IgE-reactivity of the resultant peptides. Food Chemistry, 143, 514–521. doi: https://doi.org/10.1016/j.foodchem.2013.07.110
- Bertrand-Harb, C., Baday, A., Dalgalarrondo, M., Chobert, J. M., & Haertle, T. (2002). Thermal modifications of structure and codenaturation of α-lactalbumin and β-lactoglobulin induce changes of solubility and susceptibility to proteases. Nahrung-Food, 46(4), 283–289. doi: https://doi.org/10.1002/1521-3803(20020701)46:4<283::AID-FOOD283>3.0.CO;2-A
- Bevilacqua, C., Martin, P., Candalh, C., Fauquant, J., Piot, M., Roucayrol, A. M., … Heyman, M. (2001). Goat’s milk of defective αs1-casein genotype decreases intestinal and systemic sensitization to β-lactoglobulin in Guinea pigs. Journal of Dairy Research, 68(2), 217–227. doi: https://doi.org/10.1017/S0022029901004861
- Bloom, K. A., Huang, F. R., Bencharitiwong, R., Nowak-Wegrzyn, A., & Sampson, H. A. (2009). Effect of heating on cow’s milk and differences in immunoblot reactivity to incrementally heated milk among cow’s milk-allergic children. Journal of Allergy and Clinical Immunology, 123(2), S182. doi: https://doi.org/10.1016/j.jaci.2008.12.688
- Bouhsain, S., Kamouni, Y., Dami, A., Zrara, A., Mechtani, S., Ouzzif, Z., … Derouiche, M. (2008). Profil biologique des allergies de type I chez les consultants de l’hôpital mohamed V de Rabat. Annales de Biologie Clinique, 66(6), 643–646.
- Bousfiha, A., & Aarab, L. (2013). Effect of heat and enzymatic treatments on human IgE and rabbit IgG sensitivity to white bean allergens. Iranian Journal of Allergy, Asthma and Immunology, 12(4), 304.
- Cocco, R. R., Järvinen, K. M., Sampson, H. A., & Beyer, K. (2003). Mutational analysis of major, sequential IgE-binding epitopes in α s1-casein, a major cow’s milk allergen. Journal of Allergy and Clinical Immunology, 112(2), 433–437. doi: https://doi.org/10.1067/mai.2003.1617
- Davis, P. J., Smales, C. M., & James, D. C. (2001). How can thermal processing modify the antigenicity of proteins? Allergy, 56(67), 56–60. doi: https://doi.org/10.1034/j.1398-9995.2001.00918.x
- Do, A. B., Williams, K., & Toomer, O. T. (2016). In vitro digestibility and immunoreactivity of bovine milk proteins. Food Chemistry, 190, 581–587. doi: https://doi.org/10.1016/j.foodchem.2015.05.113
- Docena, G. H., Fernandez, R., Chirdo, F. G., & Fossati, C. A. (1996). Identification of casein as the major allergenic and antigenic protein of cow’s milk. Allergy, 51(6), 412–416. doi: https://doi.org/10.1111/j.1398-9995.1996.tb04639.x
- Duan, C. C., Yang, L. J., Li, A. L., Zhao, R., & Huo, G. C. (2014). Effects of enzymatic hydrolysis on the allergenicity of whey protein concentrates. Iranian Journal of Allergy, Asthma and Immunology, 13(4), 231.
- Dupont, D., Mandalari, G., Molle, D., Jardin, J., Léonil, J., Faulks, R. M., … Mackie, A. R. (2010). Comparative resistance of food proteins to adult and infant in vitro digestion models. Molecular Nutrition & Food Research, 54(6), 767–780. doi: https://doi.org/10.1002/mnfr.200900142
- Ehn, B. M., Ekstrand, B., Bengtsson, U., & Ahlstedt, S. (2004). Modification of IgE binding during heat processing of the cow's milk allergen β-lactoglobulin. Journal of Agricultural and Food Chemistry, 52(5), 1398–1403. doi: https://doi.org/10.1021/jf0304371
- Ehn, B. M., Allmere, T., Telemo, E., Bengtsson, U., & Ekstrand, B. O. (2005). Modification of IgE binding to β- lactoglobulin by fermentation and proteolysis of cow's milk. Journal of Agricultural and Food Chemistry, 53(9), 3743–3748. doi: https://doi.org/10.1021/jf048121w
- El-Agamy, E. I. (2007). The challenge of cow milk protein allergy. Small Ruminant Research, 68(1), 64–72. doi: https://doi.org/10.1016/j.smallrumres.2006.09.016
- El-Rab, M. O. G. (1998). Foods and food allergy: The prevalence of IgE antibodies specific for food allergens in Saudi patients. Saudi Journal of Gastroenterology, 4(1), 25.
- Ghadi, A., Dutau, G., & Rancé, F. (2007). Étude des sensibilisations chez l’enfant atopique à marrakech. Étude prospective chez 160 enfants entre 2002 et 2005. Revue Française D’allergologie et D’immunologie Clinique, 47(6), 409–415. doi: https://doi.org/10.1016/j.allerg.2007.03.006
- Hossny, E., Gad, G., Shehab, A., & El-Haddad, A. (2011). Peanut sensitization in a group of allergic Egyptian children. Allergy, Asthma and Clinical Immunology, 7(1), 11. doi: https://doi.org/10.1186/1710-1492-7-11
- Høst, A. (1994). Cow’s milk protein allergy and intolerance in infancy: Some clinical, epidemiological and immunological aspects. Pediatric Allergy and Immunology, 5(6), 5–36. doi: https://doi.org/10.1111/j.1399-3038.1994.tb00352.x
- Høst, A., & Halken, S. (1998). Epidemiology and prevention of cow’s milk allergy. Allergy, 53(46), 111–113. doi: https://doi.org/10.1111/j.1398-9995.1998.tb04978.x
- Jansen, J. J. N., Kardinaal, A. F., Huijbers, G., Vlieg-Boerstra, B. J., Martens, B. P., & Ockhuizen, T. (1994). Prevalence of food allergy and intolerance in the adult Dutch population. Journal of Allergy and Clinical Immunology, 93(2), 446–456. doi: https://doi.org/10.1016/0091-6749(94)90353-0
- Jedrychowski, L. (1999). Reduction of the antigenicity of whey proteins by lactic acid fermentation. Food and Agricultural Immunology, 11(1), 91–99. doi: https://doi.org/10.1080/09540109999951
- Kanny, G., Moneret-Vautrin, D. A., Flabbee, J., Beaudouin, E., Morisset, M., & Thevenin, F. (2001). Population study of food allergy in France. Journal of Allergy and Clinical Immunology, 108(1), 133–140. doi: https://doi.org/10.1067/mai.2001.116427
- Kim, J. S., Nowak-Węgrzyn, A., Sicherer, S. H., Noone, S., Moshier, E. L., & Sampson, H. A. (2011). Dietary baked milk accelerates the resolution of cow’s milk allergy in children. Journal of Allergy and Clinical Immunology, 128(1), 125–131. doi: https://doi.org/10.1016/j.jaci.2011.04.036
- Morisawa, Y., Kitamura, A., Ujihara, T., Zushi, N., Kuzume, K., Shimanouchi, Y. , … Matsumoto, K. (2009). Effect of heat treatment and enzymatic digestion on the B cell epitopes of cow's milk proteins. Clinical & Experimental Allergy, 39(6), 918–925. doi: https://doi.org/10.1111/j.1365-2222.2009.03203.x
- Nowak-Wegrzyn, A., Bloom, K. A., Sicherer, S. H., Shreffler, W. G., Noone, S., Wanich, N., & Sampson, H. A. (2008). Tolerance to extensively heated milk in children with cow’s milk allergy. Journal of Allergy and Clinical Immunology, 122(2), 342–347. doi: https://doi.org/10.1016/j.jaci.2008.05.043
- Orhan, F., Karakas, T., Cakir, M., Aksoy, A., Baki, A., & Gedik, Y. (2009). Prevalence of immunoglobulin E-mediated food allergy in 6–9-year-old urban schoolchildren in the eastern Black Sea region of Turkey. Clinical and Experimental Allergy, 39(7), 1027–1035. doi: https://doi.org/10.1111/j.1365-2222.2009.03263.x
- Ouahidi, I., El Hamsas, A. E. Y., & Aarab, L. (2011). Modulation of egg white protein allergenicity under physical and chemical treatments. Food and Agricultural Immunology, 22(1), 57–68. doi: https://doi.org/10.1080/09540105.2010.526202
- Peñas, E., Snel, H., Floris, R., Préstamo, G., & Gomez, R. (2006). High pressure can reduce the antigenicity of bovine whey protein hydrolysates. International Dairy Journal, 16(9), 969–975. doi: https://doi.org/10.1016/j.idairyj.2005.09.010
- Peyron, S., Mouécoucou, J., Frémont, S., Sanchez, C., & Gontard, N. (2006). Effects of heat treatment and pectin addition on β-lactoglobulin allergenicity. Journal of Agricultural and Food Chemistry, 54(15), 5643–5650. doi: https://doi.org/10.1021/jf053178j
- Rance, F., Grandmottet, X., & Grandjean, H. (2005). Prevalence and main characteristics of schoolchildren diagnosed with food allergies in France. Clinical and Experimental Allergy, 35(2), 167–172. doi: https://doi.org/10.1111/j.1365-2222.2005.02162.x
- Restani, P., Gaiaschi, A., Plebani, A., Beretta, B., Cavagni, G., Fiocchi, A., … Galli, C. L. (1999). Cross-reactivity between milk proteins from different animal species. Clinical and Experimental Allergy, 29(7), 997–1004. doi: https://doi.org/10.1046/j.1365-2222.1999.00563.x
- Sabadin, I. S., Villas-Boas, M. B., de Lima Zollner, R., & Netto, F. M. (2012). Effect of combined treatment of hydrolysis and polymerization with transglutaminase on β-lactoglobulin antigenicity. European Food Research and Technology, 235(5), 801–809. doi: https://doi.org/10.1007/s00217-012-1802-z
- Soller, L., Ben-Shoshan, M., Harrington, D. W., Fragapane, J., Joseph, L., Pierre, Y. S., … Clarke, A. E. (2012). Overall prevalence of self-reported food allergy in Canada. Journal of Allergy and Clinical Immunology, 130(4), 986–988. doi: https://doi.org/10.1016/j.jaci.2012.06.029
- Takagi, K., Teshima, R., Okunuki, H., & Sawada, J. I. (2003). Comparative study of in vitro digestibility of food proteins and effect of preheating on the digestion. Biological and Pharmaceutical Bulletin, 26(7), 969–973. doi: https://doi.org/10.1248/bpb.26.969
- Thomas, K., Herouet-Guicheney, C., Ladics, G., Bannon, G., Cockburn, A., Crevel, R., … Vieths, S. (2007). Evaluating the effect of food processing on the potential human allergenicity of novel proteins: International workshop report. Food and Chemical Toxicology, 45(7), 1116–1122. doi: https://doi.org/10.1016/j.fct.2006.12.016
- Venter, C., Pereira, B., Grundy, J., Clayton, C. B., Arshad, S. H., & Dean, T. (2006). Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: A population-based study. Pediatric Allergy and Immunology, 17(5), 356–363. doi: https://doi.org/10.1111/j.1399-3038.2006.00428.x
- Wal, J. M., Bernard, H., Yvon, M., Peltre, G., David, B., Creminon, C., … Grassi, J. (1995). Enzyme immunoassay of specific human IgE to purified cows’ milk allergens. Food and Agricultural Immunology, 7(2), 175–187. doi: https://doi.org/10.1080/09540109509354876