?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The effects of various intake levels of soybean oil on carbohydrate metabolism and inflammation in mice were studied. Mice were supplied with different diets, in which soybean oil provided 5, 10, 15, 20, 25, and 30% of total energy. Blood glucose indices, blood fat indices, inflammatory factors, and gene expression of insulin receptor substrates 1 and 2 Insulin receptor substrate 1 (IRS1) and Insulin receptor substrate 2 (IRS2) were analyzed. The results showed that higher intake of soybean oil can lead to excessive weight gain, higher levels of fasting blood glucose and serum insulin, increased levels of tumor necrosis factor-α, interleukin 6, hyper-sensitive C-reactive protein, and reduced expression of IRS1 and IRS2 mRNA. Primarily, we found that reduced intake of soybean oil led to a similar trend to that of a high soybean oil diet. The optimal intake of soybean oil, as supplied in the diet, was about 15–20%.
1. Introduction
Metabolic syndrome comprises a mix of symptoms of interrelated risk factors of metabolic origin that have been proven to predict a higher risk of obesity, high blood pressure, atherosclerotic cardiovascular disease, as well as type II diabetes mellitus (Goulart et al., Citation2017; Imran et al., Citation2017). In comparison to people without metabolic syndrome, the risk of chronic diseases, such as cancer, cardiovascular disease, type II diabetes, and chronic inflammation is increased significantly in individuals affected by this condition (Uzunlulu, Caklili, & Oguz, Citation2016). Dietary habits are among the most important independent factors in the development of metabolic syndrome (Steffen et al., Citation2014). Oil is an important component of Chinese diets and it plays an important role in the onset of metabolic syndrome.
The effects of oil on the body are affected by the types and sources of fatty acids. The risk of developing diabetes and cardiovascular disease increases after long-term consumption of saturated fats (SFAs). However, this risk is reduced if the diet includes a certain amount of unsaturated fat (De Souza et al., Citation2015). A diet rich in ω-3 polyunsaturated fatty acids helps to reduce glucose oxidation, increase lipid oxidation, and increase glycogen storage in the liver (Jump, Depner, Tripathy, & Lytle, Citation2015). The family of ω-3 polyunsaturated fatty acids is mainly composed of alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) (Dyall, Citation2015). Inflammation contributes to all non-traumatic musculoskeletal diseases, thereby causing pain and disability in millions of people worldwide (Klein-Wieringa et al., Citation2016). The ω-3 polyunsaturated fatty acids are reportedly beneficial in wound healing and immune functioning (Draganescu et al., Citation2015). The supplementation of flaxseed oil, which is rich in ALA, suppresses endotoxin-triggered inflammation (Wang et al., Citation2016). In addition, flaxseed oil that is rich in ALA might reduce inflammatory and oxidative stress responses and improve wound healing in burn patients.
Overall health is also affected by the levels of dietary fat intake. Excessive fat intake can induce abdominal obesity, hypertension, dyslipidemia, insulin resistance, and liver lesions (Briand, Thiéblemont, Muzotte, & Sulpice, Citation2012; Narasimhan et al., Citation2016). Excessive fat intake can also increase the levels of free fatty acids in the blood and promote insulin secretion. High levels of free fatty acids in the blood can reduce insulin receptor binding to insulin, accelerate insulin degradation, reduce sugar utilization in the liver, and cause peripheral hyperinsulinemia and insulin resistance (Chow et al., Citation2017). Drake verified that a high-fat diet induced hepatic lipid accumulation, accompanied by delayed fat absorption and metabolism in the liver (Burak et al., Citation2017). A high-fat diet can eventually lead to systemic glucose intolerance.
The fatty acid ALA is abundant in plant oils, such as perilla seed, walnut, flaxseed, canola, olive, and soybean oils (Burak et al., Citation2017). Soybean oil is the most important source of dietary fat for Chinese people. Although soybean oil contains high levels of ALA that might be beneficial in the control of inflammation, blood glucose, and blood lipids, a large number of Chinese people in modern society tend to overeat.
The aim of this study was to determine the influence of different intake levels of soybean oil on carbohydrate metabolism, lipid metabolism, and the immune system. Our findings are expected to provide guidance for important scientific recommendations on the diets of Chinese people.
2. Materials and methods
2.1. Reagents
The assay kits for the determination of fasting plasma glucose (FPG), insulin, free fatty acids (FFAs), and low density lipoprotein cholesterol (LDL-C) were supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu Province, China). The ELISA kits for high-sensitive C-reactive protein (HsCRP), tumor necrosis factor (TNF-α), and interleukin-6 (IL-6) were acquired from Wuhan Boster Biological Technology Co. (Wuhan, Hubei Province, China).
2.2. Experimental animals
ICR mice were obtained from Suzhou Xinuosai Biological Technology Co., Ltd (Suzhou, Jiangsu Province, China). The mice were aged 9–11 weeks, weighed 37 ± 2 g, and were housed at 24 ± 2 °C, with 60 ± 10% humidity, under a 12 h light/dark cycle. The mice had access to feed and water ad libitum.
2.3. Diets and experimental design
The study was approved by the Animal Care Ethics Committee of Jiangnan University. After a 1-week adaptation period, the mice were randomly divided into six groups consisting of 15 animals each: Y5 (soybean oil provided 5% of the total energy of the feed); Y10 (soybean oil provided 10% of the total energy of the feed); Y15 (soybean oil provided 15% of the total energy fed); Y20 (soybean oil provided 20% of the total energy fed); Y25 (soybean oil provided 25% of total energy fed); and Y30 (soybean oil provided 30% of the total energy fed). The diets were based on AIN-93M (Reeves, Nielsen, & Fahey, Citation1993). The mice had ad libitum access to their respective diets for 10 weeks. The nutrient composition of the diets is presented in . Weight gain and food consumption were monitored every week.
Table 1. Ingredient composition of mice diets.
2.4. Blood and tissue sample preparation
At the end of the feeding trial, the mice were fasted overnight. Procedures such as retro-orbital bleeding and euthanasia were performed under anesthesia, induced by continuous inhalation of 2% isoflurane via a nose cone. The blood samples were collected and centrifuged at 3000 × g at 4 °C for 15 min, and the supernatants were stored at −80 °C for further biochemical analyses.
2.5. RNA extraction and analysis of gene expression
Total RNA was extracted from the liver and epididymal fat samples, using an RNA extraction kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China). Total RNA from liver samples was used for gene expression analysis of insulin receptor substrates 1 and 2 (IRS1 and IRS2). Total RNA (3.0 µg) was reverse-transcribed into cDNA, using the Revert Aid First Strand cDNA synthesis kit (TaKaRa Bio Inc., Tokyo, Japan). Real-time quantitative PCR was performed using the SYBR Premix Ex Taq™ (TaKaRa Bio Inc.) in a QuantStudio™ 6 Flex Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The primers are shown in . The ΔΔCt method was used for the relative quantification of gene expression. The ΔΔCt value of each sample was determined by calculating the difference between the Ct value of the target genes and the Ct value of the β-actin reference gene.
Table 2. Primers used for real-time RT-PCR.
2.6. Statistical analysis
Data were expressed as mean ± SD and analyzed using SPSS software (version 20; SPSS Inc., Chicago, IL, USA). The Duncan’s multiple range test and Student t-test were used to detect differences among groups. Statistical significance was set at p < 0.05.
3. Results and discussion
3.1. Effects of soybean oil intake on body weight
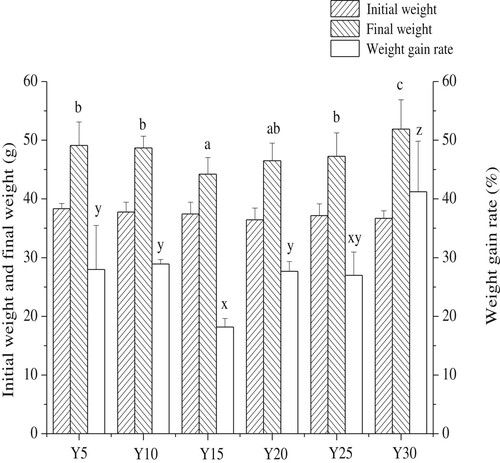
The changes observed in the weight of mice are shown in . The final weight and weight gain of the Y30 group were both significantly higher than those of the other groups (p < 0.05). Sundaram, Bukowski, and Lie (Citation2016) reported that regardless of the type of oil consumed, a high-fat diet can cause significant increase in body weight compared to a low-fat diet. However, the final weight and weight gain rate of the Y15 group were significantly lower than that of the Y5, Y10, Y25, and Y30 groups of mice, respectively (p < 0.05), but at a similar level as that of the Y20 group. Furthermore, the results showed that the mice showed the lowest weight gain, when soybean oil provided 15% of the total energy of the feed. There were no significant differences in weight among the Y5, Y10, Y20, and Y25 groups. The findings of (Park, Ahn, & Lee, Citation2016) are consistent, and they report that a low-fat diet can also have adverse effects on overall health. Thus, the present results show that both higher and lower levels of energy intake derived from soybean oil can lead to excessive weight gain. However, the body weight of mice can be effectively controlled when energy intake from soybean oil is maintained between 15 and 20% of the total energy of the feed.
3.2. Effects of soybean oil intake on blood glucose
Daily intakes of feed of each group are shown in the . The feed intake of Y5 group was indeed higher than that of other groups except the Y10 group, and the Y15, Y20 and Y25 group had no significant differences. Y30 group had the least feed intake of the six groups and was significantly lower than Y5, Y10 and Y20 group. The effects of soybean oil on serum blood glucose is shown in A. No significant differences were noted in serum fasting blood glucose levels in mice of the Y5, Y10, Y20, and Y25 groups. The serum fasting blood glucose levels in the Y15 group were significantly lower than those in the other groups, with the exception of the Y20 group (p < 0.05); whereas the serum fasting blood glucose levels in the Y30 group were significantly higher than those in the other groups (p < 0.05).
Figure 2. The amount of daily intake in each group (g, weekly average, n = 15 mice per group); Values are mean ± S.D; Means with different letters (a–d) differ significantly (P<0.05).
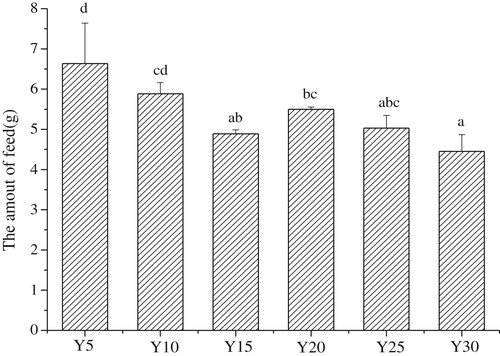
Figure 3. The serum levels of fasting blood glucose and insulin in each group (n = 15 mice per group); A, Concentration of fasting blood glucose (FPG); B, Concentration of insulin (INS); Values are mean ± S.D; Means within a row with different letters are significantly different. (P<0.05).
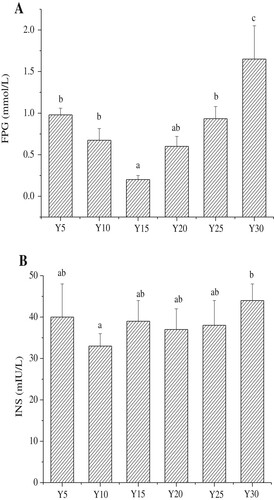
According to , the feed intake of Y5 group was higher than those of other groups. The serum fasting blood glucose level of Y5, however, was similar to those of Y10, Y20, and Y25 groups. Therefore, we can deduce that there was no relationship between serum fasting blood glucose level and feed intake of mice. But serum fasting blood glucose level was related to intake of soybean oil. From A, the fasting blood glucose of mice showed a significant increase when energy intake from soybean oil was 30% of the total energy of the feed. Furthermore, the results show that the lowest intake of soybean oil also increased the fasting blood glucose level. The optimal level of soybean oil that induces the lowest fasting blood glucose levels is about 15–20%.
According to B, the serum insulin levels of the Y10 group were the lowest, and the serum insulin levels of the Y30 group were the highest. Significant differences were noted between these two groups, but no significant differences in serum insulin levels were observed among the other groups. Serum insulin is a protein hormone that is secreted by beta cells of the pancreas, and it is the only hormone that can reduce blood glucose levels (Amiri et al., Citation2015). When normal doses of insulin cannot achieve the expected physiological effect, or the body becomes less sensitive to the hormone, this leads to a condition called insulin resistance (IR) (Friedrich et al., Citation2015). The initial morbidity of IR includes a repeated flood of compensatory insulin secretion to maintain normal blood glucose levels. Eventually, diabetes develops when the compensatory ability exceeds the body’s limits (Tahrani, Citation2017). The present results show that the effects of a diet comprising 10% energy intake provided by soybean oil on serum insulin were more favorable than those of a diet comprising 30% energy intake provided by soybean oil.
3.3. Effects of soybean oil intake on serum lipid levels
The serum lipid levels in mice at 10 weeks are presented in . The level of serum FFAs in the Y10 group was lowest among all groups, but at a similar level in the Y15. Y5, Y20, Y25, and Y30 groups, all of which showed higher FFA levels than the Y10 group (p < 0.05). The FFA level stimulates the body to produce more reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Fratantonio et al., Citation2015), both of which can induce the oxidative stress mechanism. These reactive molecules can directly oxidize and damage DNA, proteins, and lipids, and activate some signaling pathways that are closely associated with IR (Di et al., Citation2015), thereby eventually leading to diabetes.
Figure 4. The serum levels of free fatty acids and LDL-C in each group (n = 15 mice per group); A, Concentration of free fatty acids (FFAs); B, Concentration of low density lipoprotein cholesterol (LDL-C); Values are mean ± S.D; Means within a row with different letters are significantly different. (P<0.05).
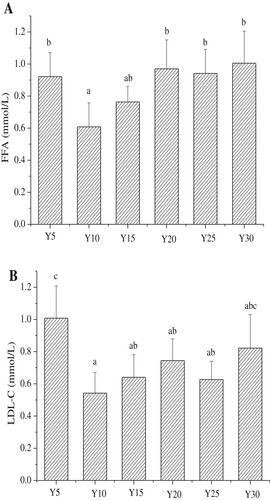
The LDLs comprise a major class of lipoproteins. The LDL particles can deposit cholesterol in the arteries that leads to atherosclerosis (Hazarika, Kalita, & Kalita, Citation2017). The LDL-C is commonly used to estimate LDL levels. Changes in LDL-C levels showed a similar trend to those in FFAs. The serum LDL-C in the Y10 group showed the lowest levels, and no significant differences were noted among the Y15, Y20, and Y25 groups. Compared to Y10, the serum LDL-C levels in the Y5 and Y30 groups were significantly increased.
The results indicate that the optimal energy provided by soybean oil in the diet should be about 10–15%, as these levels induce the lowest levels of FFAs and LDL-C.
3.4. Effects of soybean oil intake on serum inflammatory factors
When body tissues are invaded by pathogens or damaged, macrophages and peripheral mononuclear cells produce a series of cytokines, such as tumor necrosis factor (TNF-α), interleukin 6 (IL-6), and hyper-sensitive C-reactive protein (Hs-CRP). These inflammatory factors can cause fever and other effects that trigger additional inflammatory cytokines (Hartati, Widjanarko, Widyaningsih, & Rifa’I, Citation2017). The release of inflammatory factors can enhance immune defenses; however, high levels of inflammatory factors can lead to a series of chronic diseases, such as pregnancy eclampsia, atherosclerosis, and rheumatoid arthritis, among others (Del et al., Citation2015). Insulin resistance can also induce pro-inflammatory factors and improve levels of inflammatory factors.
The effects of soybean oil on serum inflammatory factors in mice are shown in . Serum levels of HsCRP and TNF-α in the Y30 group were significantly higher than those of the groups fed lower levels of soybean oil (p < 0.05). This suggests that high levels of soybean oil meal can promote the body’s inflammatory response, and increase levels of HsCRP and TNF-α. The IL-6 levels were lowest in the Y15 and Y20 groups. In contrast, the Y5, Y10, Y25, and Y30 groups showed the highest IL-6 levels.
Figure 5. The serum levels of Tumor Necrosis Factor-α, Interleukin 6 and hyper-sensitive C-reactive protein in each group (n = 15 mice per group); A, Concentration of Tumor Necrosis Factor-α (TNF-α); B, Concentration of Interleukin 6 (IL-6); C, Concentration of hyper-sensitive C-reactive protein (HsCRP);Values are mean ± S.D; Means within a row with different letters are significantly different. (P<0.05).
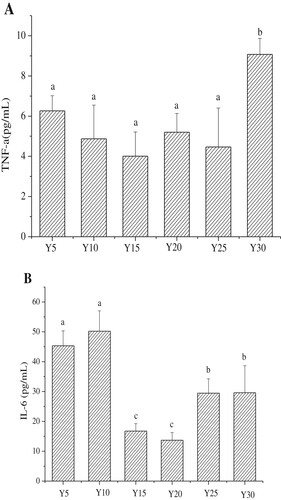
The inflammatory factors, IL - 6 and TNF-α are secreted by fat cells, and are associated with fat intake and obesity (Chen et al., Citation2016). Recognized as an inflammatory marker for atherosclerosis (Antonino et al., Citation2016), TNF-α can significantly increase LDL-C levels and accelerate the development of atherosclerosis (Silvan, Mingo, & Martinez-Rodriguez, Citation2017). As shown in and , changes in TNF-α levels were consistent with those of LDL-C. The inflammatory factor, IL-6 can stimulate the central nervous system at the early stages of acute inflammation (Sun et al., Citation2017). In addition, IL-6 is associated with increased mortality from cardiovascular disease (Pengli, Geli, & Ying, Citation2015). Moreover, TNF-α can induce increased production of IL-6 and lead to the formation of atherosclerotic plaques in blood vessels (Huo, Li, Kong, Wu, & Li, Citation2014). The HsCRP levels are closely linked to vascular diseases and play a significant role in the diagnosis of clinical atherosclerosis (Assadpour, Citation2014). A major determinant of Hs-CRP in the liver is IL-6.
A similar trend in the various levels of soybean oil in the diet was evident in the levels of inflammatory factors. Inflammatory factor levels in the Y15 and Y20 groups were slightly low. However, the inflammatory factor levels in the Y30 experimental group showed a sharp increase. The results indicate that both high and extremely low soybean oil diets are capable of increasing the risk of inflammatory reactions, especially that of an increase in Il-6 levels.
3.5. Effects of soybean oil intake on transcription of IRS1 and IRS2
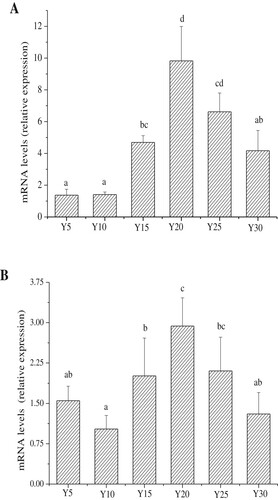
The liver is the main target organ of insulin action. The insulin receptor substrate proteins IRS1 and IRS2 are key targets of the insulin receptor tyrosine kinase and are required for hormonal control of metabolism (Copps & White, Citation2012). Insulin activates tyrosine kinases by linking insulin receptors on the surface of tissue cells and causes phosphorylation of insulin receptor tyrosine residues. At this point, the insulin receptor protein binds to and activates phosphatidylinositol-3-kinase (P13 K), its downstream protein kinase B (AKT), forkhead protein 01 (FOXOl 1), mitogen- activated protein kinase and other signal paths in order to elicit cellular responses such as glucose uptake, lipid metabolism and cell proliferation(Lavin, White, & Brazil, Citation2016). When IR occurs, the combined signal of insulin and receptor leads to a series of metabolic blocks in the liver cell that eventually lead to a reduced capacity to respond to insulin (Imran et al., Citation2017a). Gene expression of IRS is reduced when IR occurs in the cell (Qi et al., Citation2013). Therefore, it is important to study IRS gene expression in the liver to evaluate glucose metabolism more comprehensively. As shown in , transcription levels of IRS1 and IRS2 showed similar trends. When soybean oil provided between 15% and 25% of the energy in the diet, IRS mRNA expression in the liver was increased. In contrast, IRS mRNA expression was relatively low in the Y5, Y10, and Y30 groups. The Y20 group showed the highest IRS mRNA expression among all experimental groups. These results indicate that diets that are either high or low in soybean oil could reduce IRS mRNA expression. This could have an adverse effect on normal glucose metabolism.
4. Conclusion
Soybean oil is the most important source of dietary fat for Chinese people, and over-consumption of soybean oil is very common in China. The effects of various intake levels of soybean oil on carbohydrate metabolism and inflammation in mice were studied. The results indicated that diets that are high in soybean oil might have adverse effects on blood glucose levels, and increase the risk of inflammation. However, diets that are extremely low in soybean oil could also increase the risk of disturbances in carbohydrate metabolism and inflammation. We recommend that the optimal level of soybean oil supplied in the diet should be about 15–20%.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amiri, L., John, A., Shafarin, J., Adeghate, E., Jayaprakash, P., Yasin, J., … Raza, H. (2015). Enhanced glucose tolerance and pancreatic Beta cell function by Low dose aspirin in hyperglycemic insulin-resistant type 2 diabetic goto-kakizaki (GK) rats. Cellular Physiology & Biochemistry International Journal of Experimental Cellular Physiology Biochemistry & Pharmacology, 36(5), 1939–1950. doi: https://doi.org/10.1159/000430162
- Antonino, T., Di, R. D., Rosaria, P., Carlo, M., Valentina, A., Della, C. V., … Rosario, M. (2016). Early high-dosage atorvastatin treatment improved Serum immune-inflammatory markers and functional outcome in acute ischemic strokes classified as large artery atherosclerotic stroke:A randomized trial. Medicine, 95(13), e3186. doi:10.1097/MD.0000000000003186
- Assadpour, P. M. (2014). The correlation between high-sensitivity C-reactive protein (hsCRP) Serum levels and severity of coronary atherosclerosis. International Cardiovascular Research Journal, 8(1), 6–8.
- Briand, F., Thiéblemont, Q., Muzotte, E., & Sulpice, T. (2012). High-fat and fructose intake induces insulin resistance, dyslipidemia, and liver steatosis and alters in vivo macrophage-to-feces reverse cholesterol transport in hamsters. Journal of Nutrition, 142(4), 704–709. doi: https://doi.org/10.3945/jn.111.153197
- Burak, C., Wolffram, S., Zur, B., Langguth, P., Fimmers, R., Alteheld, B., … Egert, S. (2017). Effects of the flavonol quercetin and α-linolenic acid on n-3 PUFA status in metabolically healthy men and women: A randomised, double- blinded, placebo-controlled, crossover trial. British Journal of Nutrition, 117(5), 698–711. doi: https://doi.org/10.1017/S0007114517000241
- Chen, X., Gong, Q., Wang, C. Y., Zhang, K., Ji, X., Chen, Y. X., & Yu, X. J. (2016). High-Fat diet induces distinct metabolic response in interleukin-6 and tumor necrosis factor-α knockout mice. Journal of Interferon & Cytokine Research the Official Journal of the International Society for Interferon & Cytokine Research, 36(10), 580–588. doi: https://doi.org/10.1089/jir.2016.0022
- Chow, L. S., Mashek, D. G., Wang, Q., Shepherd, S. O., Goodpaster, B. H., & Dube, J. J. (2017). Effect of acute physiological free fatty acid elevation in the context of hyperinsulinemia on fiber type specific IMCL accumulation. Journal of Applied Physiology, 123(1). doi:jap.00209.02017 doi: https://doi.org/10.1152/japplphysiol.00209.2017
- Copps, K. D., & White, M. F. (2012). Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin rece ptor substrate proteins IRS1 and IRS2. Diabetologia, 55(10), 2565–2582. doi: https://doi.org/10.1007/s00125-012-2644-8
- Del, R. I., Polak, J. F., O’Leary, D. H., Battafarano, D. F., Erikson, J. M., Restrepo, J. F., … Escalante, A. (2015). Systemic inflammation and cardiovascular risk factors predict rapid progression of atherosclerosis in rheumatoid arthritis. Annals of the Rheumatic Diseases, 74(6), 1118–1123. doi: https://doi.org/10.1136/annrheumdis-2013-205058
- De Souza, R. J., Mente, A., Maroleanu, A., Cozma, A. I., Ha, V., Kishibe, T., … Beyene, J. (2015). Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. Bmj, 351, h3978. doi.org/10.1136/bmj.h3978
- Di, M. E., Jha, J. C., Sharma, A., Wilkinsonberka, J. L., Jandeleitdahm, K. A., & de Haan, J. B. (2015). Are reactive oxygen species still the basis for diabetic complications? Clinical Science, 129(2), 199–216. doi: https://doi.org/10.1042/CS20150093
- Draganescu, D., Ibanescu, C., Tamba, B. I., Andritoiu, C. V., Dodi, G., & Popa, M. I. (2015). Flaxseed lignan wound healing formulation: Characterization and in vivo therapeutic evaluation. International Journal of Biological Macromolecules, 72, 614–623. doi: https://doi.org/10.1016/j.ijbiomac.2014.09.012
- Dyall, S. C. (2015). Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Frontiers in Aging Neuroscience, 7. doi: https://doi.org/10.3389/fnagi.2015.00052
- Fratantonio, D., Speciale, A., Ferrari, D., Cristani, M., Saija, A., & Cimino, F. (2015). Palmitate-induced endothelial dys– function is attenuated by cyanidin-3-O-glucoside through modulation of Nrf2/Bach1 and NF-κB pathways. Toxicology Letters, 239(3), 152–160. doi: https://doi.org/10.1016/j.toxlet.2015.09.020
- Friedrich, K., Üstünel, B. E., Wang, X., Jones, A., Rohm, M., Diaz, M. B., … Herzig, S. (2015). Transforming growth factor beta-like stimulated clone 22 D4 promotes diabetic hyperglycemia and insulin resistance. Diabetologie und Stoffwechsel, 10(S 01), FV12. doi:10.1055/s-0035-1549518
- Goulart, A., Varejão, A., Nogueira, F., Martins, S., Mesquitarodrigues, A., Sousa, N., & Leão, P. (2017). The influence of metabolic syndrome in the outcomes of colorectal cancer patients. Diabetes & Metabolic Syndrome. doi: https://doi.org/10.1016/j.dsx.2017.07.007
- Hartati, F. K., Widjanarko, S. B., Widyaningsih, T. D., & Rifa’I, M. (2017). Anti-Inflammatory evaluation of black rice extract inhibits TNF-α, IFN-γ and IL-6 cytokines produced by immunocompetent cells. Food and Agricultural Immunology, 8(3), 1–10. doi: https://doi.org/10.1080/09540105.2017.1332006
- Hazarika, A., Kalita, H., & Kalita, M. C. (2017). Withdrawal from high-carbohydrate high-saturated-fat diet changes saturated-fat distribution and improves hepatic low-density-lipoprotein-receptor expression to ameliorate metabolic syndrome in rats. Nutrition, 38, 95–101. doi: https://doi.org/10.1016/j.nut.2017.01.005
- Huo, Q., Li, X., Kong, Y., Wu, Z., & Li, Q. (2014). Rivaroxaban restrain IL-6inverted commas TNF-α and MMP9’s expression in apoE-/- mouse’s carotid atherosclerotic plaques. Experimental and Clinical Cardiology, 20(7), 1571–1576.
- Imran, M., Nadeem, M., Saeed, F., Imran, A., Khan, M. R., Khan, M. A. … Rauf, A. (2017). Immunomodulatory perspectives of potential biological spices with special reference to cancer and diabetes. Food and Agricultural Immunology, 28(1), 1–30. doi: https://doi.org/10.1080/09540105.2016.1202206
- Jump, D. B., Depner, C. M., Tripathy, S., & Lytle, K. A. (2015). Potential for dietary ω-3 fatty acids to prevent nonalcoholic fatty liver disease and reduce the risk of primary liver cancer. Advances in Nutrition: An International Review Journal, 6(6), 694–702. doi: https://doi.org/10.3945/an.115.009423
- Klein-Wieringa, I. R., de Lange-Brokaar, B. J., Yusuf, E., Andersen, S. N., Kwekkeboom, J. C., Kroon, H. M., … Nelissen, R. G. (2016). Inflammatory cells in patients with endstage knee osteoarthritis: A comparison between the synovium and the infrapatellar Fat Pad. The Journal of Rheumatology, 43(4), 771–778. doi: https://doi.org/10.3899/jrheum.151068
- Lavin, D. P., White, M. F., & Brazil, D. P. (2016). IRS proteins and diabetic complications. Diabetologia, 59(11), 2280–2291. doi: https://doi.org/10.1007/s00125-016-4072-7
- Narasimhan, S., Nagarajan, L., Vaidya, R., Gunasekaran, G., Rajagopal, G., Parthasarathy, V., … Sudha, V. (2016). Dietary fat intake and its association with risk of selected components of the metabolic syndrome among rural south Indians. Indian Journal of Endocrinology and Metabolism, 20(1), 47–54. doi: https://doi.org/10.4103/2230-8210.172248
- Park, S., Ahn, J., & Lee, B. K. (2016). Very-low-fat diets may be associated with increased risk of metabolic syndrome in the adult population. Clinical Nutrition, 35(5), 1159–1167. doi: https://doi.org/10.1016/j.clnu.2015.09.010
- Pengli, B., Geli, L., & Ying, W. (2015). Association between IL-6 and related risk factors of metabolic syndrome and cardiovascular disease in young rats. International Journal of Clinical & Experimental Medicine, 8(8), 13491–13499.
- Qi, Y., Xu, Z., Zhu, Q., Thomas, C., Kumar, R., Feng, H., … Guo, S. (2013). Myocardial loss of IRS1 and IRS2 causes heart failure and Is controlled by p38α MAPK during insulin resistance. Diabetes, 62(11), 3887–3900. doi: https://doi.org/10.2337/db13-0095
- Reeves, P. G., Nielsen, F. H., & Fahey Jr, G. C. (1993). AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. Journal of Nutrition, 123(11), 1939–1951. doi: https://doi.org/10.1093/jn/123.11.1939
- Silvan, J. M., Mingo, E., & Martinez-Rodriguez, A. J. (2017). Grape seed extract (GSE) modulates campylobacter pro-inflammatory response in human intestinal epithelial cell lines. Food and Agricultural Immunology, 18, 1–15. doi: https://doi.org/10.1080/09540105.2017.1350637
- Steffen, L. M., Van Horn, L., Daviglus, M. L., Zhou, X., Reis, J. P., Loria, C. M., … Duffey, K. J. (2014). A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: The CARDIA (coronary artery risk development in young adults) study. British Journal of Nutrition, 112(10), 1654–1661. doi: https://doi.org/10.1017/S0007114514002633
- Sun, L., Li, Y., Jia, X., Wang, Q., Li, Y., Hu, M., … Zhang, W. (2017). Neuroprotection by IFN-γviaastrocyte-secreted IL-6 in acute neuroinflammation. Oncotarget, 8(25), 40065–40078.
- Sundaram, S., Bukowski, M. R., & Lie, W. R. (2016). High-fat diets containing different amounts of n3 and n6 polyunsaturated fatty acids modulate inflammatory cytokine production in Mice. High-Fat Diets Containing Different Amounts of n3 and n6 Polyunsaturated Fatty Acids Modulate Inflammatory, 51, 1–12.
- Tahrani, A. A. (2017). Novel therapies in type 2 diabetes: Insulin resistance. Practical Diabetes, 34(5), 161–166a. doi: https://doi.org/10.1002/pdi.2109
- Uzunlulu, M., Caklili, O. T., & Oguz, A. (2016). Association between metabolic syndrome and cancer. Annals of Nutrition and Metabolism, 68(3), 173–179. doi: https://doi.org/10.1159/000443743
- Wang, M., Zhang, X. J., Yan, C., He, C., Li, P., Chen, M., … Wan, J. B. (2016). Preventive effect of α-linolenic acid-rich flaxseed oil against ethanol-induced liver injury is associated with ameliorating gut-derived endotoxin-mediated inflammation in mice. Journal of Functional Foods, 23, 532–541. doi: https://doi.org/10.1016/j.jff.2016.03.012